Association of Surgical Margin Status with Oncologic Outcome in Patients Treated with Breast-Conserving Surgery
Abstract
1. Introduction
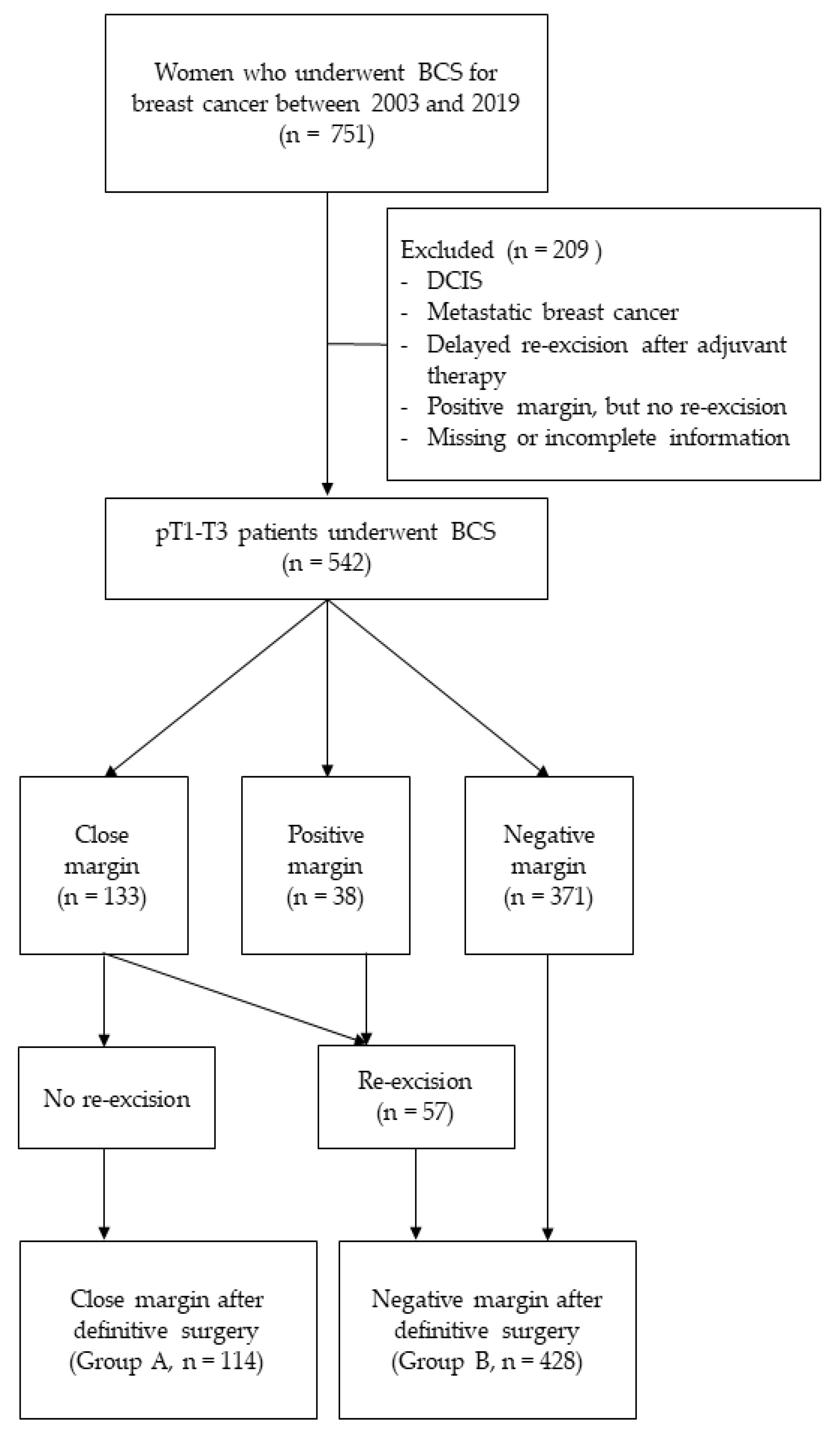
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, K.; Zhang, J.; Beeraka, N.M.; Sinelnikov, M.Y.; Zhang, X.; Cao, Y.; Lu, P. Robot-Assisted Minimally Invasive Breast Surgery: Recent Evidence with Comparative Clinical Outcomes. J. Clin. Med. 2022, 11, 1827. [Google Scholar] [CrossRef]
- Chen, K.; Beeraka, N.M.; Zhang, J.; Reshetov, I.V.; Nikolenko, V.N.; Sinelnikov, M.Y.; Mikhaleva, L.M. Efficacy of da Vinci robot-assisted lymph node surgery than conventional axillary lymph node dissection in breast cancer—A comparative study. Int. J. Med. Robot. 2021, 17, e2307. [Google Scholar] [CrossRef]
- Chen, K.; Beeraka, N.M.; Sinelnikov, M.Y.; Zhang, J.; Song, D.; Gu, Y.; Li, J.; Reshetov, I.V.; Startseva, O.I.; Liu, J.; et al. Patient Management Strategies in Perioperative, Intraoperative, and Postoperative Period in Breast Reconstruction with DIEP-Flap: Clinical Recommendations. Front. Surg. 2022, 9, 729181. [Google Scholar] [CrossRef]
- Arriagada, R.; Le, M.G.; Rochard, F.; Contesso, G. Conservative treatment versus mastectomy in early breast cancer: Patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J. Clin. Oncol. 1996, 14, 1558–1564. [Google Scholar] [CrossRef]
- Lichter, A.S.; Lippman, M.E.; Danforth, D.N., Jr.; d’Angelo, T.; Steinberg, S.M.; deMoss, E.; MacDonald, H.D.; Reichert, C.M.; Merino, M.; Swain, S.M.; et al. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: A randomized trial at the National Cancer Institute. J. Clin. Oncol. 1992, 10, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, D.; Lê, M.G.; Arriagada, R.; Contesso, G.; Fontaine, F.; Spielmann, M.; Rochard, F.; Le Chevalier, T.; Lacour, J. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother. Oncol. 1989, 14, 177–184. [Google Scholar] [CrossRef]
- Nayyar, A.; Gallagher, K.K.; McGuire, K.P. Definition and management of oositive margins for invasive breast cancer. Surg. Clin. N. Am. 2018, 98, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Corsi, F.; Sorrentino, L.; Bossi, D.; Sartani, A.; Foschi, D. Preoperative localization and surgical margins in conservative breast surgery. Int. J. Surg. Oncol. 2013, 2013, 793819. [Google Scholar] [CrossRef] [PubMed]
- Cabioglu, N.; Hunt, K.K.; Sahin, A.A.; Kuerer, H.M.; Babiera, G.V.; Singletary, S.E.; Whitman, G.J.; Ross, M.I.; Ames, F.C.; Feig, B.W.; et al. Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann. Surg. Oncol. 2007, 14, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.S.; Schnitt, S.J.; Giuliano, A.E.; Harris, J.R.; Khan, S.A.; Horton, J.; Klimberg, S.; Chavez-MacGregor, M.; Freedman, G.; Houssami, N.; et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J. Clin. Oncol. 2014, 32, 1507–1515. [Google Scholar] [CrossRef]
- Morrow, M.; Van Zee, K.J.; Solin, L.J.; Houssami, N.; Chavez-MacGregor, M.; Harris, J.R.; Horton, J.; Hwang, S.; Johnson, P.L.; Marinovich, M.L.; et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on Margins for Breast-Conserving Surgery with Whole-Breast Irradiation in Ductal Carcinoma In Situ. J. Clin. Oncol. 2016, 34, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Smitt, M.C.; Nowels, K.; Carlson, R.W.; Jeffrey, S.S. Predictors of reexcision findings and recurrence after breast conservation. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Chism, D.B.; Freedman, G.M.; Li, T.; Anderson, P.R. Re-excision of margins before breast radiation-diagnostic or therapeutic? Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.T.; Ahmed, M.; Kovacs, T.; Marco, V. Margins in breast conserving surgery: A practice-changing process. Eur. J. Surg. Oncol. 2016, 42, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Scopa, C.D.; Aroukatos, P.; Tsamandas, A.C.; Aletra, C. Evaluation of margin status in lumpectomy specimens and residual breast carcinoma. Breast. J. 2006, 12, 150–153. [Google Scholar] [CrossRef]
- Saarela, A.O.; Paloneva, T.K.; Rissanen, T.J.; Kiviniemi, H.O. Determinants of positive histologic margins and residual tumor after lumpectomy for early breast cancer: A prospective study with special reference to touch preparation cytology. J. Surg. Oncol. 1997, 66, 248–253. [Google Scholar] [CrossRef]
- Garvey, E.M.; Senior, D.A.; Pockaj, B.A.; Wasif, N.; Dueck, A.C.; McCullough, A.E.; Ocal, I.T.; Gray, R.J. Rates of residual disease with close but negative margins in breast cancer surgery. Breast 2015, 24, 413–417. [Google Scholar] [CrossRef]
- Vos, E.L.; Gaal, J.; Verhoef, C.; Brouwer, K.; van Deurzen, C.; Koppert, L.B. Focally positive margins in breast conserving surgery: Predictors, residual disease, and local recurrence. Eur. J. Surg. Oncol. 2017, 43, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Bundred, J.R.; Michael, S.; Stuart, B.; Cutress, R.I.; Beckmann, K.; Holleczek, B.; Dahlstrom, J.E.; Gath, J.; Dodwell, D.; Bundred, N.J. Margin status and survival outcomes after breast cancer conservation surgery: Prospectively registered systematic review and meta-analysis. BMJ 2022, 378, e070346. [Google Scholar] [CrossRef]
- Sabel, M.S.; Rogers, K.; Griffith, K.; Jagsi, R.; Kleer, C.G.; Diehl, K.A.; Breslin, T.M.; Cimmino, V.M.; Chang, A.E.; Newman, L.A. Residual disease after re-excision lumpectomy for close margins. J. Surg. Oncol. 2009, 99, 99–103. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Romanoff, A.; Cohen, A.; Schmidt, H.; Weltz, C.; Bleiweis, I.J.; Jaffer, S.; Port, E.R. Close and positive lumpectomy margins are associated with similar rates of residual disease with additional surgery. Ann. Surg. Oncol. 2016, 23, 4270–4276. [Google Scholar] [CrossRef]
- Hadzikadic Gusic, L.; McGuire, K.P.; Ozmen, T.; Soran, A.; Thomas, C.R.; McAuliffe, P.F.; Diego, E.J.; Bonaventura, M.; Johnson, R.R.; Ahrendt, G.M. Margin width is not predictive of residual disease on re-excision in breast conserving therapy. J. Surg. Oncol. 2014, 109, 426–430. [Google Scholar] [CrossRef]
- Jaffré, I.; Campion, L.; Dejode, M.; Bordes, V.; Sagan, C.; Loussouarn, D.; Dravet, F.; Andrieux, N.; Classe, J.M. Margin width should not still enforce a systematic surgical re-excision in the conservative treatment of early breast infiltrative ductal carcinoma. Ann. Surg. Oncol. 2013, 20, 3831–3838. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.L.; Coopey, S.B.; Tang, R.; McEvoy, M.P.; Specht, M.C.; Hughes, K.S.; Gadd, M.A.; Smith, B.L. Implications of new lumpectomy margin guidelines for breast-conserving surgery: Changes in reexcision rates and predicted rates of residual tumor. Ann. Surg. Oncol. 2016, 23, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.F.; Hill, A.D.; Quinn, C.M.; McDermott, E.W.; O’Higgins, N. A pathologic assessment of adequate margin status in breast-conserving therapy. Ann. Surg. Oncol. 2006, 13, 333–339. [Google Scholar] [CrossRef]
- Cellini, C.; Hollenbeck, S.T.; Christos, P.; Martins, D.; Carson, J.; Kemper, S.; Lavigne, E.; Chan, E.; Simmons, R. Factors associated with residual breast cancer after re-excision for close or positive margins. Ann. Surg. Oncol. 2004, 11, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Fregatti, P.; Gipponi, M.; Atzori, G.; Rosa, R.; Diaz, R.; Cornacchia, C.; Sparavigna, M.; Garlaschi, A.; Belgioia, L.; Fozza, A.; et al. The Margins’ Challenge: Risk Factors of Residual Disease after Breast Conserving Surgery in Early-stage Breast Cancer. In Vivo 2022, 36, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Gurdal, S.O.; Karanlik, H.; Cabioglu, N.; Ozcinar, B.; Yavuz, E.; Tuzlali, S.; Ozmen, V. Positive or close margins in breast conserving surgery: Is re-excision always necessary? Eur. J. Surg. Oncol. 2012, 38, 399–406. [Google Scholar] [CrossRef]
- Neri, A.; Marrelli, D.; Megha, T.; Bettarini, F.; Tacchini, D.; De Franco, L.; Roviello, F. Clinical significance of multifocal and multicentric breast cancers and choice of surgical treatment: A retrospective study on a series of 1158 cases. BMC Surg. 2015, 15, 1. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Faverly, D.R.; Burgers, L.; Bult, P.; Holland, R. Three dimensional imaging of mammary ductal carcinoma in situ: Clinical implications. Semin. Diagn. Pathol. 1994, 11, 193–198. [Google Scholar] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [PubMed]
- Houssami, N.; Macaskill, P.; Marinovich, M.L.; Morrow, M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: A meta-analysis. Ann. Surg. Oncol. 2014, 21, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Aziz, D.; Rawlinson, E.; Narod, S.A.; Sun, P.; Lickley, H.L.; McCready, D.R.; Holloway, C.M. The role of reexcision for positive margins in optimizing local disease control after breast-conserving surgery for cancer. Breast J. 2006, 12, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Freedman, G.; Fowble, B.; Hanlon, A.; Nicolaou, N.; Fein, D.; Hoffman, J.; Sigurdson, E.; Boraas, M.; Goldstein, L. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Anscher, M.S.; Jones, P.; Prosnitz, L.R.; Blackstock, W.; Hebert, M.; Reddick, R.; Tucker, A.; Dodge, R.; Leight, G., Jr.; Iglehart, J.D.; et al. Local failure and margin status in early-stage breast carcinoma treated with conservation surgery and radiation therapy. Ann. Surg. 1993, 218, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Obedian, E.; Haffty, B.G. Negative margin status improves local control in conservatively managed breast cancer patients. Cancer J. Sci. Am. 2000, 6, 28–33. [Google Scholar] [PubMed]
- Morrow, M.; Harris, J.R.; Schnitt, S.J. Surgical margins in lumpectomy for breast cancer—Bigger is not better. N. Engl. J. Med. 2012, 367, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Wapnir, I.L.; Dignam, J.J.; Fisher, B.; Mamounas, E.P.; Anderson, S.J.; Julian, T.B.; Land, S.R.; Margolese, R.G.; Swain, S.M.; Costantino, J.P.; et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J. Natl. Cancer Inst. 2011, 103, 478–488. [Google Scholar] [CrossRef] [PubMed]

| Variables | Values, n (%) |
|---|---|
| Age, years | |
| ≤40 | 52 (9.6%) |
| 41–60 | 343 (63.3%) |
| ≥61 | 147 (27.1%) |
| Margin status after initial BCS | |
| Positive | 38 (7.0%) |
| Close, ≤2 mm | 133 (24.5%) |
| Negative | 371 (68.5%) |
| Final margin status after definitive surgery | |
| Close, ≤2 mm | 114 (21.0%) |
| Negative | 428 (79.0%) |
| Tumor size | |
| ≤2 cm | 384 (70.8%) |
| >2 cm | 158 (29.2%) |
| Nodal status | |
| Negative | 424 (78.2%) |
| Positive | 118 (21.8%) |
| Histology | |
| IDC | 456 (84.1%) |
| ILC | 15 (2.8%) |
| Mixed/Other | 71 (13.1%) |
| Grade | |
| I | 150 (27.7%) |
| II | 201 (37.1%) |
| III | 154 (28.4%) |
| Unknown | 37 (6.8%) |
| Multifocality | |
| Absent | 469 (86.5%) |
| Present | 73 (13.5%) |
| EIC | |
| Absent | 426 (78.6%) |
| Present | 116 (21.4%) |
| LVI | |
| Absent | 437 (80.6%) |
| Present | 105 (19.4%) |
| Hormone receptor | |
| Negative | 107 (19.7%) |
| Positive | 435 (80.3%) |
| HER2 | |
| Negative | 414 (76.4%) |
| Positive | 96 (17.7%) |
| Unknown | 32 (5.9%) |
| Ki-67 | |
| <15 | 197 (36.3%) |
| ≥15 | 182 (33.6%) |
| Unknown | 163 (30.1%) |
| Neoadjuvant CTx | |
| No | 518 (95.6%) |
| Yes | 24 (4.4%) |
| Adjuvant CTx | |
| No | 264 (48.7%) |
| Yes | 278 (51.3%) |
| Adjuvant HTx | |
| No | 113 (20.8%) |
| Yes | 429 (79.2%) |
| Adjuvant HER2-targeted therapy | |
| No | 477 (88.0%) |
| Yes | 65 (12.0%) |
| Adjuvant RTx | |
| No | 23 (4.2%) |
| Yes | 519 (95.8%) |
| Types of recurrence | |
| Locoregional | 24 (4.4%) |
| Distant | 29 (5.4%) |
| Any | 42 (7.7%) |
| Variables | Group A (%) n = 114 | Group B (%) n = 428 | p-Value 1 |
|---|---|---|---|
| Age, years | 0.011 | ||
| ≤40 | 4 (3.5%) | 48 (11.2%) | |
| 41–60 | 70 (61.4%) | 273 (63.8%) | |
| ≥61 | 40 (35.1%) | 107 (25.0%) | |
| Tumor size | 0.056 | ||
| ≤2 cm | 89 (78.1%) | 295 (68.9%) | |
| >2 cm | 25 (21.9%) | 133 (31.1%) | |
| Nodal status | 0.472 | ||
| Negative | 92 (80.7%) | 332 (77.6%) | |
| Positive | 22 (19.3%) | 96 (22.4%) | |
| Histology | 0.787 | ||
| IDC | 94 (82.5%) | 362 (84.6%) | |
| ILC | 4 (3.5%) | 11 (2.6%) | |
| Mixed/Other | 16 (14.0%) | 55 (12.8%) | |
| Grade | 0.627 | ||
| I | 35 (30.7%) | 115 (26.9%) | |
| II | 42 (36.8%) | 159 (37.1%) | |
| III | 32 (28.1%) | 122 (28.5%) | |
| Unknown | 5 (4.4%) | 32 (7.5%) | |
| Multifocality | 0.611 | ||
| Absent | 97 (85.1%) | 372 (86.9%) | |
| Present | 17 (14.9%) | 56 (13.1%) | |
| EIC | 0.877 | ||
| Absent | 89 (78.1%) | 337 (78.7%) | |
| Present | 25 (21.9%) | 91 (21.3%) | |
| LVI | 0.807 | ||
| Absent | 91 (79.8%) | 346 (80.8%) | |
| Present | 23 (20.2%) | 82 (19.2%) | |
| Hormone receptor | 0.146 | ||
| Negative | 28 (24.6%) | 79 (18.5%) | |
| Positive | 86 (75.4%) | 349 (81.5%) | |
| HER2 | 0.180 | ||
| Negative | 93 (81.6%) | 321 (75.0%) | |
| Positive | 18 (15.8%) | 78 (18.2%) | |
| Unknown | 3 (2.6%) | 29 (6.8%) | |
| Ki-67 | <0.001 | ||
| <15 | 46 (40.4%) | 151 (35.3%) | |
| ≥15 | 53 (46.5%) | 129 (30.1%) | |
| Unknown | 15 (13.2%) | 148 (34.6%) | |
| Neoadjuvant CTx | 1.000 2 | ||
| No | 109 (95.6%) | 409 (95.6%) | |
| Yes | 5 (4.4%) | 19 (4.4%) | |
| Adjuvant CTx | 0.602 | ||
| No | 58 (50.9%) | 206 (48.1%) | |
| Yes | 56 (49.1%) | 222 (51.9%) | |
| Adjuvant HTx | 0.061 | ||
| No | 31 (27.2%) | 82 (19.2%) | |
| Yes | 83 (72.8%) | 346 (80.8%) | |
| Adjuvant HER2-targeted therapy | 0.234 | ||
| No | 104 (91.2%) | 373 (87.1%) | |
| Yes | 10 (8.8%) | 55 (12.9%) | |
| Adjuvant RTx | 0.600 | ||
| No | 6 (5.3%) | 17 (4.0%) | |
| Yes | 108 (94.7%) | 411 (96.0%) |
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Age, years | ||||
| ≤40 | 1 | |||
| 41–60 | 0.454 | 0.141–1.461 | ||
| ≥61 | 1.330 | 0.391–4.522 | ||
| Tumor size | ||||
| ≤2 cm | 1 | 1 | ||
| >2 cm | 0.888 | 0.350–2.252 | 0.752 | 0.288–1.966 |
| Nodal status | ||||
| Negative | 1 | 1 | ||
| Positive | 1.258 | 0.498–3.177 | 1.911 | 0.715–5.106 |
| Histology | ||||
| IDC/ILC | 1 | |||
| Mixed/Other | 1.870 | 0.696–5.025 | ||
| Grade | ||||
| I | 1 | |||
| II | 0.713 | 0.250–2.034 | ||
| III | 0.985 | 0.345–2.810 | ||
| Multifocality | ||||
| Absent | 1 | 1 | ||
| Present | 0.273 | 0.037–2.023 | 0.298 | 0.039–2.286 |
| EIC | ||||
| Absent | 1 | |||
| Present | 1.225 | 0.484–3.096 | ||
| LVI | ||||
| Absent | 1 | |||
| Present | 1.329 | 0.490–3.606 | ||
| Hormone receptor | ||||
| Negative | 1 | 1 | ||
| Positive | 0.505 | 0.207–1.232 | 0.535 | 0.203–1.412 |
| HER2 | ||||
| Negative | 1 | 1 | ||
| Positive | 1.506 | 0.541–4.191 | 1.645 | 0.572–4.728 |
| Ki-67 | ||||
| <15 | 1 | |||
| ≥15 | 1.367 | 0.417–4.481 | ||
| Adjuvant RTx | ||||
| No | 1 | 1 | ||
| Yes | 0.147 | 0.054–0.401 | 0.127 | 0.038–0.421 |
| Final margin status after definitive surgery | ||||
| Close | 1 | 1 | ||
| Negative | 0.262 | 0.109–0.633 | 0.215 | 0.086–0.543 |
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age, years | ||||
| ≤40 | 1 | |||
| 41–60 | 1.857 | 0.330–10.446 | ||
| ≥61 | 0.833 | 0.114–6.111 | ||
| Margin status after initial BCS | ||||
| Positive | 1 | |||
| Close | 0.648 | 0.212–1.979 | ||
| Histological type of positive or close margin | ||||
| DCIS component | 1 | 1 | ||
| Invasive component | 0.508 | 0.173–1.490 | 0.140 | 0.022–0.904 |
| Tumor size | ||||
| ≤2 cm | 1 | |||
| >2 cm | 1.181 | 0.360–3.871 | ||
| Nodal status | ||||
| Negative | 1 | |||
| Positive | 1.979 | 0.536–7.309 | ||
| Histology | ||||
| IDC/ILC | 1 | |||
| Mixed/Other | 0.788 | 0.506–1.227 | ||
| Grade | ||||
| I | 1 | |||
| II | 1.083 | 0.279–4.210 | ||
| III | 1.650 | 0.370–7.365 | ||
| Multifocality | ||||
| Absent | 1 | 1 | ||
| Present | 4.667 | 1.159–18.783 | 10.580 | 1.840–60.843 |
| EIC | ||||
| Absent | 1 | 1 | ||
| Present | 0.330 | 0.108–1.009 | 0.232 | 0.038–1.406 |
| LVI | ||||
| Absent | 1 | |||
| Present | 2.591 | 0.715–9.387 | ||
| Hormone receptor | ||||
| Negative | 1 | |||
| Positive | 2.083 | 0.494–8.782 | ||
| HER2 | ||||
| Negative | 1 | |||
| Positive | 1.333 | 0.223–7.980 | ||
| Ki-67 | ||||
| <15 | 1 | 1 | ||
| ≥15 | 4.407 | 1.074–18.092 | 4.815 | 0.976–23.756 |
| Neoadjuvant CTx | ||||
| No | 1 | |||
| Yes | 0.202 | 0.020–2.077 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, S.; Min, S.Y. Association of Surgical Margin Status with Oncologic Outcome in Patients Treated with Breast-Conserving Surgery. Curr. Oncol. 2022, 29, 9271-9283. https://doi.org/10.3390/curroncol29120726
Chae S, Min SY. Association of Surgical Margin Status with Oncologic Outcome in Patients Treated with Breast-Conserving Surgery. Current Oncology. 2022; 29(12):9271-9283. https://doi.org/10.3390/curroncol29120726
Chicago/Turabian StyleChae, Sumin, and Sun Young Min. 2022. "Association of Surgical Margin Status with Oncologic Outcome in Patients Treated with Breast-Conserving Surgery" Current Oncology 29, no. 12: 9271-9283. https://doi.org/10.3390/curroncol29120726
APA StyleChae, S., & Min, S. Y. (2022). Association of Surgical Margin Status with Oncologic Outcome in Patients Treated with Breast-Conserving Surgery. Current Oncology, 29(12), 9271-9283. https://doi.org/10.3390/curroncol29120726





