Cost-Effectiveness Analysis of Sequential Treatment Strategies for Advanced Melanoma in Real Life in France
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment Sequence Modelling
2.2. Efficacy
2.3. Data
2.4. Costs of Care
2.5. Utility Estimates
2.6. Analyses
3. Results
3.1. Cohort Characteristics
3.2. Cost and Utility per Line
3.3. Survival, QALY and Cost over 10 Years
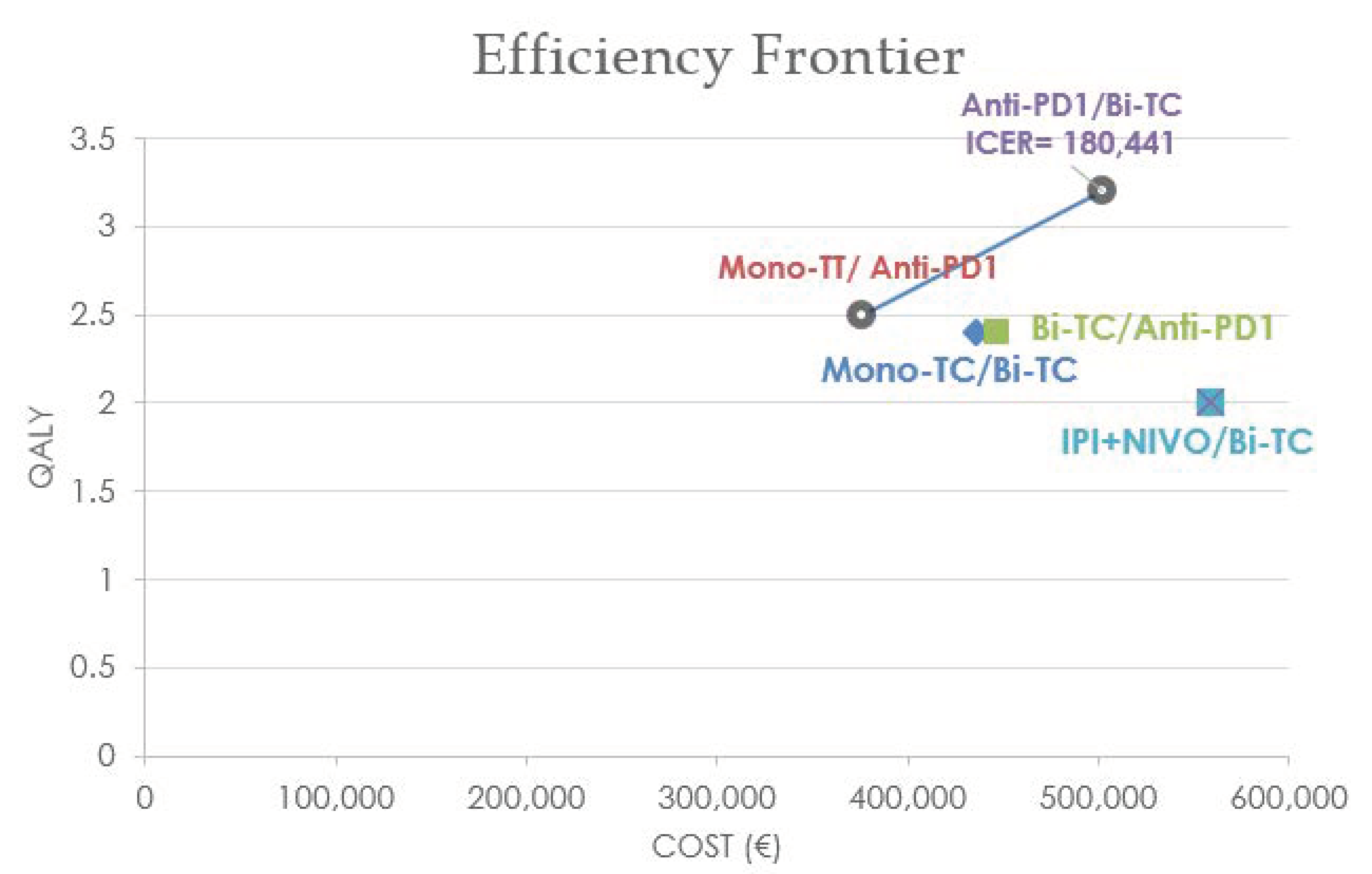
3.4. Cost-Effectiveness Analysis
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; McArthur, G.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Di Giacomo, A.M.; Mandal, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016, 17, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Dreno, B.; Ascierto, P.A.; Atkinson, V. Health-related quality of life impact of cobimetinib in combination with vemurafenib in patients with advanced or metastatic BRAF V600 mutation—Positive melanoma. Br. J. Cancer 2018, 118, 777–784. [Google Scholar] [CrossRef]
- Grob, J.J.; Amonkar, M.M.; Karaszewska, B.; Schachter, J.; Dummer, R.; Mackiewicz, A.; Stroyakovskiy, D.; Drucis, K.; Grange, F.; Chiarion-Sileni, V.; et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): Results of a phase 3, open-l. Lancet Oncol. 2015, 16, 1389–1398. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Ascierto, P.A.; Robert, C.; Hassel, J.C.; Rutkowski, P.; Savage, K.J.; Taylor, F.; Coon, C.; Gilloteau, I.; et al. Effect of nivolumab on health-related quality of life in patients with treatment-naïve advanced melanoma: Results from the phase III CheckMate 066 study. Ann. Oncol. 2016, 27, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Petrella, T.M.; Robert, C.; Richtig, E.; Miller, W.H.; Masucci, G.V.; Walpole, E.; Lebbe, C.; Steven, N.; Middleton, M.R.; Hille, D.; et al. Patient-reported outcomes in KEYNOTE-006, a randomised study of pembrolizumab versus ipilimumab in patients with advanced melanoma. Eur. J. Cancer 2017, 86, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Revicki, D.A.; van den Eertwegh, A.J.M.; Lorigan, P.; Lebbe, C.; Linette, G.; Ottensmeier, C.H.; Safikhani, S.; Messina, M.; Hoos, A.; Wagner, S.; et al. Health related quality of life outcomes for unresectable stage III or IV melanoma patients receiving ipilimumab treatment. Health Qual. Life Outcomes 2012, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Amonkar, M.M.; Milhem, M.; Grotzinger, K.; Demidov, L.V.; Rutkowski, P.; Garbe, C.; Dummer, R.; Hassel, J.C.; Wolter, P.; et al. Functional and symptom impact of trametinib versus chemotherapy in BRAF V600E advanced or metastatic melanoma: Quality-of-life analyses of the METRIC study. Ann. Oncol. 2014, 25, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Larkin, J.; Wolchok, J.; Hodi, F.S.; Chiarion-sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.; Cowey, C.L.; Lao, C.; et al. Health-related quality of life results from the phase III CheckMate 067 study. Eur. J. Cancer 2017, 82, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chmielowski, B.; Pellissier, J.; Xu, R.; Stevinson, K.; Liu, F.X. Cost-Effectiveness of Pembrolizumab Versus Ipilimumab in Ipilimumab-Naïve Patients with Advanced Melanoma in the United States. J. Manag. Care Spec. Pharm. 2017, 23, 184–194. [Google Scholar] [CrossRef]
- Kohn, C.G.; Zeichner, S.B.; Chen, Q.; Montero, A.J.; Goldstein, D.A.; Christopher, R. Cost-Effectiveness of Immune Checkpoint Inhibition in BRAF Wild-Type Advanced Melanoma. J. Clin. Oncol. 2017, 35, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Hertel, N.; Ellis, J.; Morais, E.; Johnson, H.; Philips, Z.; Roskell, N.; Walker, A.; Lee, D. The cost-effectiveness of nivolumab monotherapy for the treatment of advanced melanoma patients in England. Eur. J. Health Econ. 2017, 19, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.; Tran, D.M.; McDowell, L.C.; Keyvani, D.; Barcelon, J.A.; Merino, O.; Wilson, L. Cost-Effectiveness of Nivolumab-Ipilimumab Combination Therapy Compared with Monotherapy for First-Line Treatment of Metastatic Melanoma in the United States. J. Manag. Care Spec. Pharm. 2017, 23, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Bohensky, M.A.; Pasupathi, K.; Gorelik, A.; Kim, H.; Harrison, J.P.; Liew, D. A Cost-Effectiveness Analysis of Nivolumab Compared with Ipilimumab for the Treatment of BRAF Wild-Type Advanced Melanoma in Australia. Value Health 2016, 19, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Miguel, L.S.; Lopes, F.V.; Pinheiro, B.; Wang, J.; Xu, R.; Pellissier, J.; Laires, P.A. Cost Effectiveness of Pembrolizumab for Advanced Melanoma Treatment in Portugal. Value Health 2017, 20, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Matter-Walstra, K.; Braun, R.; Kolb, C.; Ademi, Z.; Dummer, R.; Pestalozzi, B.C.; Schwenkglenks, M. A cost-effectiveness analysis of trametinib plus dabrafenib as first-line therapy for metastatic BRAF V600-mutated melanoma in the Swiss setting. Br. J. Dermatol. 2015, 173, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Curl, P.; Vujic, I.; Van’t Veer, L.J.; Ortiz-Urda, S.; Kahn, J.G. Cost-effectiveness of treatment strategies for BRAF-mutated metastatic melanoma. PLoS ONE 2014, 9, e107255. [Google Scholar] [CrossRef] [PubMed]
- Delea, T.E.; Amdahl, J.; Wang, A.; Amonkar, M.M.; Thabane, M. Cost Effectiveness of Dabrafenib as a First-Line Treatment in Patients with BRAF V600 Mutation-Positive Unresectable or Metastatic Melanoma in Canada. Pharmacoeconomics 2014, 33, 367–380. [Google Scholar] [CrossRef]
- Shih, V.; Ten Ham, R.M.; Bui, C.T.; Tran, D.N.; Ting, J.; Wilson, L. Targeted therapies compared to dacarbazine for treatment of brafv600e metastatic melanoma: A cost-effectiveness analysis. J. Skin Cancer 2015, 2015, 505302. [Google Scholar] [CrossRef]
- Tarhini, A.; McDermott, D.; Ambavane, A.; Gupte-Singh, K.; Aponte-Ribero, V.; Ritchings, C.; Benedict, A.; Rao, S.; Regan, M.M.; Atkins, M. Clinical and economic outcomes associated with treatment sequences in patients with BRAF-mutant advanced melanoma. Immunotherapy 2019, 11, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.; Benedict, A.; McDermott, D.; Rao, S.; Ambavane, A.; Gupte-Singh, K.; Sabater, J.; Ritchings, C.; Aponte-Ribero, V.; Regan, M.M.; et al. Sequential treatment approaches in the management of BRAF wild-type advanced melanoma: A cost-effectiveness analysis. Immunotherapy 2018, 10, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.; Kimper-Karl, M.L.; Høyer, K.L.; Bastholt, L.; Schmidt, H.; Svane, I.M. The majority of patients with metastatic melanoma are not represented in pivotal phase III immunotherapy trials. Eur. J. Cancer 2017, 74, 89–95. [Google Scholar] [CrossRef]
- Wu, B.; Shi, L. Frontline braf testing-guided treatment for advanced melanoma in the era of immunotherapies a cost-utility analysis based on long-term survival data. JAMA Dermatol. 2020, 156, 1177–1184. [Google Scholar] [CrossRef]
- Putter, H.; Fiocco, M.; Geskus, R.B. Tutorial in biostatistics: Competing risks and multi-state models. Stat. Med. 2007, 26, 2389–2430. [Google Scholar] [CrossRef] [PubMed]
- Kandel, M.; Allayous, C.; Dalle, S.; Mortier, L.; Dalac, C.; Dutriaux, C.; Leccia, M.T.; Guillot, B.; Saiag, P.; Lacour, J.P.; et al. Update of survival and cost of metastatic melanoma with new drugs: Estimations from the MelBase cohort. Eur. J. Cancer 2018, 105, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Allayous, C.; Da Meda, L.; Barthelemy, M.; Benessiano, J.; Liegey, E.; Demerville, L.; Dutriaux, C.; Dalle, S.; Mortier, L.; Lesimple, T.; et al. MelBase, constitution et suivi d’une cohorte nationale de patients atteints de mélanome stade III inopérable ou stade IV avec collection d’une base de données clinico-biologiques. Ann. Dermatol. Venereol. 2014, 141, S396. [Google Scholar] [CrossRef]
- Mccaffrey, D.F.; Griffin, A.; Almirall, D.; Slaughter, M.E.; Burgette, L.F. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat. Med. 2013, 32, 3388–3414. [Google Scholar] [CrossRef] [PubMed]
- Ridgeway, A.G.; Mccaffrey, D.; Morral, A.; Griffin, B.A.; Burgette, L. Package ‘twang’. Available online: https://cran.r-project.org/web/packages/twang/vignettes/twang.pdf (accessed on 1 November 2022).
- EuroQol Group EQ-5D-3L User Guide Basic Information on How to Use the EQ-5D-3L Instrument; French Health Autority: Paris, France, 2015; Volume 30, pp. 1–67.
- Chevalier, J.; De Pouvourville, G. Valuing EQ-5D using Time Trade-Off in France. Eur. J. Health Econ. 2013, 14, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kandel, M.; Dalle, S.; Bardet, A.; Allayous, C.; Mortier, L.; Dutriaux, C.; Guillot, B.; Leccia, M.T.; Dalac, S.; Legoupil, D.; et al. Quality-of-life assessment in French patients with metastatic melanoma in real life. Cancer 2020, 126, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Haute autorité de santé. Choices in Methods for Economic Evolution. 2011. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2020-11/choices_in_methods_for_economic_evaluation_-_2011.pdf (accessed on 29 October 2019).
- Williams, C.; Lewsey, J.D.; Mackay, D.F.; Briggs, A.H. Estimation of Survival Probabilities for Use in Cost-Effectiveness Analyses: A Comparison of a Multi-State Modeling Survival Analysis Approach with Partitioned Survival and Markov Decision-Analytic Modeling. Med. Decis. Mak. 2016, 37, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Lewsey, J.D.; Briggs, A.H.; Mackay, D.F. Cost-Effectiveness Analysis in R Using a Multi-state Modeling Survival Analysis Framework: A Tutorial. Med. Decis. Mak. 2016, 37, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.Z.; Mangla, A. Scrambling for BRAF Mutation Status in patients with melanoma: Is it really necessary to initiate treatment? JCO Oncol. Pract. 2022, 27, 679–680. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | MelBase Patients (n = 1518), No. (%) or Mean (Range) | BRAF-Mutated Patients (n = 639), No. (%) or Mean (Range) | BRAF-Wild-Type Patients (n = 879), No. (%) or Mean (Range) |
|---|---|---|---|
| Age (years) | 64 (18–98) | 58.3 (18–94) | 67.6 (25–98) |
| <65 years | 719 (47.4) | 406 (63.5) | 313 (35.6) |
| ≥65 years | 799 (52.6) | 233 (36.5) | 566 (64.4) |
| Gender | |||
| Male | 879 (57.9) | 368 (57.6) | 511 (58.1) |
| Female | 639 (42.1) | 271 (42.4) | 368 (41.9) |
| Years of inclusion | |||
| 2013 | 132 (8.7) | 50 (7.8) | 82 (9.3) |
| 2014 | 267 (17.6) | 115 (18.0) | 152 (17.3) |
| 2015 | 338 (22.2) | 147 (23.0) | 191 (21.7) |
| 2016 | 332 (21.9) | 160 (25.0) | 172 (19.6) |
| 2017 | 305 (20.1) | 111 (17.4) | 194 (22.1) |
| 2018 1 | 144 (9.5) | 56 (8.8) | 88 (10.0) |
| BRAF Mutated | 639 (42.1) | 639 (100) | 0 |
| V600E | 498 (78.3) | 498 (78.3) | |
| Wild-type | 879 (57.9) | 0 | 879 (100) |
| Elevated LDH > 1 ULN 2 | 461 (30.4) | 197 (30.9) | 264 (30.1) |
| High neutrophil to lymphocyte ratio | 430 (28.3) | 196 (30.7) | 234 (27.1) |
| Presence of metastases | |||
| Brain | 272 (17.9) | 146 (22.9) | 126 (14.3) |
| Liver | 413 (27.2) | 182 (28.5) | 231(26.3) |
| ECOG performance status | |||
| 0,1 | 1248 (82.2) | 520 (81.4) | 728 (82.8) |
| 2,3,4 | 116 (7.6) | 49 (7.7) | 67 (7.6) |
| Unknown | 154 (10.1) | 70 (11.0) | 84 (9.6) |
| Melanoma Metastatic status | |||
| Unresectable stage IIIC | 154 (10.1) | 46 (7.2) | 108 (12.3) |
| M1a | 145 (9.6) | 64 (10.0) | 81 (9.2) |
| M1b | 251 (16.5) | 75 (11.7) | 176 (20.0) |
| M1c | 891 (58.7) | 422 (66.0) | 469 (53.4) |
| Unknown | 77 (5.1%) | 32 (5.0) | 45 (5.1) |
| Anatomic location of melanoma | |||
| Upper and lower extremities | 440 (29.0) | 171 (26.8) | 269 (3.1) |
| Trunk | 461 (30.4) | 276 (43.2) | 185 (21.0) |
| Head and Neck | 210 (13.8) | 85 (13.3) | 125 (14.2) |
| Acral lentiginous | 103 (6.8) | 14 (2.2) | 89 (10.1) |
| Mucosa | 100 (6.6) | 9 (1.4) | 91 (10.4) |
| Unknown | 204 (13.4) | 84 (13.1) | 120 (13.7) |
| BMI 3 | |||
| <30 | 1215 (80.0) | 521 (81.5) | 694 (79.0) |
| >30 | 303 (20.0) | 118 (18.5) | 185 (21.0) |
| First line of treatment | |||
| Cytotoxic chemotherapy | 88 (5.8) | 5 (0.8) | 83 (9.4) |
| Ipilimumab | 202 (13.3) | 16 (2.5) | 186 (21.2) |
| Ipilimumab + Nivolumab | 163 (10.7) | 51 (8.0) | 112 (12.7) |
| Anti-PD1 | 540 (35.6) | 78 (12.2) | 462 (52.6) |
| Mono-targeted therapy | 182 (12.0) | 155 (24.3) | 27 (3.1) |
| Bi-targeted therapy | 326 (21.5) | 325 (50.9) | 1 (0.1) |
| Included in a clinical trial | 17 (1.1) | 9 (1.4) | 8 (0.9) |
| Second line of treatment | |||
| None, as still on first line of treatment at data cut-off | 318 (21.0) | 128 (20.0) | 190 (21.6) |
| None, as dead during the first line | 299 (19.7) | 88 (13.8) | 211 (24.0) |
| Off treatment | 108 (7.1) | 20 (3.1) | 88 (10.0) |
| Second line observed including: | 793 (52.2) | 403 (63.1) | 390 (44.4) |
| Chemotherapy | 107 (13.5) | 24 (3.8) | 83 (21.3) |
| Ipilimumab | 143 (18.0) | 15 (2.3) | 128 (32.8) |
| Ipilimumab + Nivolumab | 18 (2.3) | 11 (1.7) | 7 (1.8) |
| Anti-PD1 | 317 (40.0) | 180 (28.2) | 137 (35.1) |
| Mono-targeted therapy | 67 (8.4) | 51 (8.0) | 16 (4.1) |
| Bi-targeted therapy | 104 (13.1) | 103 (16.1) | 1 (0.3) |
| Included in a clinical trial | 37 (4.7) | 19 (3.0) | 18 (4.6) |
| BRAF-Mutated Patients | BRAF Wild-Type Patients | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First Line of Treatment (L1) | Anti-PD1 | Bi-Targeted Therapy | Ipilimumab + Nivolumab | Mono-Targeted Therapy | Mono-Targeted Therapy | Anti-PD1 | Ipilimumab + Nivolumab | Ipilimumab | Chemotherapy |
| Second Line of Treatment (L2) | Bi-Targeted Therapy | Anti-PD1 | Bi-Targeted Therapy | Bi-Targeted Therapy | Anti-PD1 | ||||
| Cost/month in L1 | 7723 ± 3701 | 13,050 ± 6867 | 26,966 ± 32,824 | 11,932 ± 11,962 | 8165 ± 5077 | 6769 ± 4433 | 11,412 ± 11,994 | 15,858 ± 29,563 | 3381 ± 6996 |
| (mean ± SD 1, EUR) | |||||||||
| Drug | 5730 ± 3194 | 12,107 ± 6518 | 22,023 ± 26,959 | 10,693 ± 11,675 | 7320 ± 4043 | 4839 ± 2567 | 9112 ± 10,111 | 13,708 ± 27,560 | 537 ± 5256 |
| Administration | 541 ± 159 | 0 | 1246 ± 947 | 0 | 0 | 546 ± 265 | 817 ± 498 | 401 ± 865 | 0 |
| Radiotherapy | 363 ± 1608 | 116 ± 358 | 331 ± 925 | 259 ± 944 | 123 ± 471 | 207 ± 1166 | 73 ± 240 | 231 ± 928 | 235 ± 1122 |
| Biological exam | 121 ± 70 | 54 ± 30 | 109 ± 68 | 84 ± 136 | 64 ± 55 | 119 ± 70 | 90 ± 62 | 146 ± 472 | 85 ± 104 |
| Radiological exam | 74 ± 50 | 76 ± 53 | 127 ± 74 | 150 ± 180 | 102 ± 100 | 73 ± 71 | 74 ± 79 | 130 ± 447 | 145 ± 215 |
| Hospitalization | 1334 ± 1925 | 1104 ± 1139 | 4768 ± 6278 | 954 ± 1109 | 1204 ± 1986 | 1444 ± 3032 | 1715 ± 3002 | 1653 ± 2453 | 3220 ± 7448 |
| Visit | 28 ± 12 | 15 ± 8 | 17 ± 12 | 25 ± 22 | 20 ± 15 | 32 ± 12 | 21 ± 12 | 22 ± 12 | 21 ± 25 |
| Cost/month in L2 | 11,127 ± 3361 | 8685 ± 5525 | 10,733 ± 3917 | 9566 ± 3731 | 9481 ± 13,063 | 7932 ± 5796 | 14,956 ± 40,275 | 4942 ± 2688 | 7551 ± 5256 |
| (mean ± SD 1, EUR) | |||||||||
| Drug | 9804 ± 3484 | 6006 ± 4058 | 9498 ± 4163 | 8309 ± 3366 | 6004 ± 5099 | 6202 ± 5068 | 12,586 ± 37,246 | 3386 ± 2083 | 6458 ± 4915 |
| Administration | 209 ± 122 | 523 ± 338 | 140 ± 79 | 143 ± 97 | 433 ± 446 | 282 ± 193 | 2499 ± 4539 | 433 ± 236 | 357 ± 205 |
| Radiotherapy | 107 ± 347 | 193 ± 447 | 45 ± 99 | 53 ± 140 | 480 ± 1656 | 203 ± 518 | 142 ± 337 | 140 ± 304 | 35 ± 94 |
| Biological exam | 49 ± 22 | 101 ± 70 | 48 ± 24 | 45 ± 35 | 86 ± 48 | 88 ± 271 | 67 ± 51 | 100 ± 73 | 89 ± 55 |
| Radiologic exam | 46 ± 20 | 51 ± 41 | 53 ±38 | 50 ± 42 | 51 ± 28 | 44 ± 30 | 46 ± 21 | 57 ± 89 | 57 ± 51 |
| Hospitalization | 1275 ± 1621 | 2233 ± 3164 | 1084 ± 1184 | 1116 ± 1374 | 2638 ± 8473 | 1924 ± 2030 | 2884 ± 4543 | 1050 ± 1774 | 1068 ± 1145 |
| Visit | 22 ± 11 | 38 ± 20 | 18 ± 8 | 17 ± 9 | 34 ± 28 | 35 ± 84 | 75 ± 187 | 36 ± 36 | 29 ± 13 |
| Cost of palliative care (mean ± SD 1, EUR) | 2928 ± 5349 | 2477 ± 4329 | 1247 ± 2864 | 2652 ± 4922 | 2652 ± 4922 | 1713 ± 3037 | 1407 ± 3944 | 2900 ± 5562 | 2240 ± 3411 |
| Utility L1 (mean, CI95%2) | 0.75 (0.65–0.85) | 0.77 (0.72–0.81) | 0.68 (0.57–0.80) | 0.66 (0.57–0.75) | 0.76 (0.68–0.84) | 0.74 (0.71–0.76) | 0.75 (0.70–0.80) | 0.73 (0.68–0.78) | 0.67 (0.59–0.75) |
| Utility L2 (mean, CI95%2) | 0.81 (0.72–0.90) | 0.73 (0.69–0.77) | 0.70 (0.60–0.79) | 0.70 (0.62–0.78) | 0.72 (0.65–0.80) | 0.64 (0.60–0.68) | 0.65 (0.57–0.74) | 0.70 (0.65–0.74) | 0.65 (0.56–0.73) |
| Sequence |
Mean Time Spent in First Line ([CI95%], (Month, Years)) |
Mean Time Spent in Subsequent Lines ([CI95%], (Month, Years)) |
Mean Survival Time ([CI95%], (Month, Years)) |
Mean QALYs 3 [CI95%] | Mean Total Cost per Patient [CI95%], (EUR) | |
|---|---|---|---|---|---|---|
| BRAF-mutated patients | Anti-PD1 → Bi-T 2 | 15.6 (5.7–25.8) 1.3 (0.5–2.1) | 32.3 (13.4–59.7) 2.7 (1.1–5.0) | 47.9 (25.6–73.6) 4.0 (2.1–6.1) | 3.2 (1.7–4.9) | 502,045 (262,525–787,754) |
| Bi-TT 2 → Anti-PD1 | 21.4 (14.1–32.0) 1.8 (1.2–2.7) | 17.0 (7.3–26.4) 1.4 (0.6–2.2) | 38.4 (26.1–51.7) 3.2 (2.2–4.3) | 2.4 (1.6–3.2) | 446,766 (313,691–608,496) | |
| IPI+NIVO → Bi-TT 2 | 9.7 (4.4–16.5) 0.8 (0.4–1.4) | 25.7 (13.2–43.6) 2.1 (1.1–3.7) | 35.4 (20.9–54.9) 2.9 (1.7–4.6) | 2.0 (1.2–3.2) | 558,168 (343,439–813,136) | |
| MONO-TT 1 → Bi-TT 2 | 12.1 (7.0–25.6) 1.0 (0.6–2.1) | 29.4 (12.2–45.2) 2.5 (1.0–3.8) | 41.5 (23.1–62.4) 3.5 (1.9–5.2) | 2.4 (1.3–3.6) | 435,702 (246,112–663,460) | |
| MONO-TT 1 → Anti-PD1 | 21.4 (13.2–38.6) 1.8 (1.1–3.2) | 18.9 (7.0–37.2) 1.6 (0.6–3.1) | 40.3 (26.1–64.8) 3.4 (2.2–5.4) | 2.5 (1.6–4.0) | 375,736 (240,740–604,066) | |
| BRAF Wild-Type patients | Anti-PD1 | 14.7 (10.6–17.9) 1.2 (0.9–1.5) | 8.0 (4.9–12.2) 0.7 (0.4–1.0) | 22.7 (17.0–27.5) 1.9 (1.4–2.3) | 1.3 (1.0–1.6) | 178,726 (132,270–218,287) |
| Ipi + Nivo | 21.3 (11.6–31.9) 1.8 (1.0–2.7) | 5.3 (2.6–12.1) 0.4 (0.2–1.0) | 26.6 (16.5–38.9) 2.2 (1.4–3.2) | 1.6 (1.0–2.4) | 351,590 (223,829–517,784) | |
| Ipilimumab | 5.6 (3.9–8.7) 0.5 (0.3–0.7) | 18.5 (10.0–27.1) 1.5 (0.8–2.3) | 24.1 (15.9–32.7) 2.0 (1.3–2.7) | 1.4 (0.9–1.9) | 190,322 (137,526–247,713) | |
| Chemotherapy | 4.1 (2.2–6.8) 0.3 (0.2–0.6) | 16.3 (8.0–27.0) 1.4 (0.7–2.3) | 20.4 (11.9–31.1) 1.7 (0.6–2.3) | 1.1 (0.7–1.7) | 151,475 (84,039–239,747) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandel, M.; Bardet, A.; Dalle, S.; Allayous, C.; Mortier, L.; Guillot, B.; Dutriaux, C.; Leccia, M.-T.; Dalac, S.; Montaudie, H.; et al. Cost-Effectiveness Analysis of Sequential Treatment Strategies for Advanced Melanoma in Real Life in France. Curr. Oncol. 2022, 29, 9255-9270. https://doi.org/10.3390/curroncol29120725
Kandel M, Bardet A, Dalle S, Allayous C, Mortier L, Guillot B, Dutriaux C, Leccia M-T, Dalac S, Montaudie H, et al. Cost-Effectiveness Analysis of Sequential Treatment Strategies for Advanced Melanoma in Real Life in France. Current Oncology. 2022; 29(12):9255-9270. https://doi.org/10.3390/curroncol29120725
Chicago/Turabian StyleKandel, Marguerite, Aurélie Bardet, Stéphane Dalle, Clara Allayous, Laurent Mortier, Bernard Guillot, Caroline Dutriaux, Marie-Thérèse Leccia, Sophie Dalac, Henri Montaudie, and et al. 2022. "Cost-Effectiveness Analysis of Sequential Treatment Strategies for Advanced Melanoma in Real Life in France" Current Oncology 29, no. 12: 9255-9270. https://doi.org/10.3390/curroncol29120725
APA StyleKandel, M., Bardet, A., Dalle, S., Allayous, C., Mortier, L., Guillot, B., Dutriaux, C., Leccia, M.-T., Dalac, S., Montaudie, H., Saiag, P., Legoupil, D., Brunet-Possenti, F., Arnault, J.-P., Quatrebarbes, J. D., Beylot-Barry, M., Maubec, E., Lesimple, T., Aubin, F., ... Borget, I. (2022). Cost-Effectiveness Analysis of Sequential Treatment Strategies for Advanced Melanoma in Real Life in France. Current Oncology, 29(12), 9255-9270. https://doi.org/10.3390/curroncol29120725







