Characteristics of Interval Colorectal Cancer: A Canadian Retrospective Population-Level Analysis from Newfoundland and Labrador
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
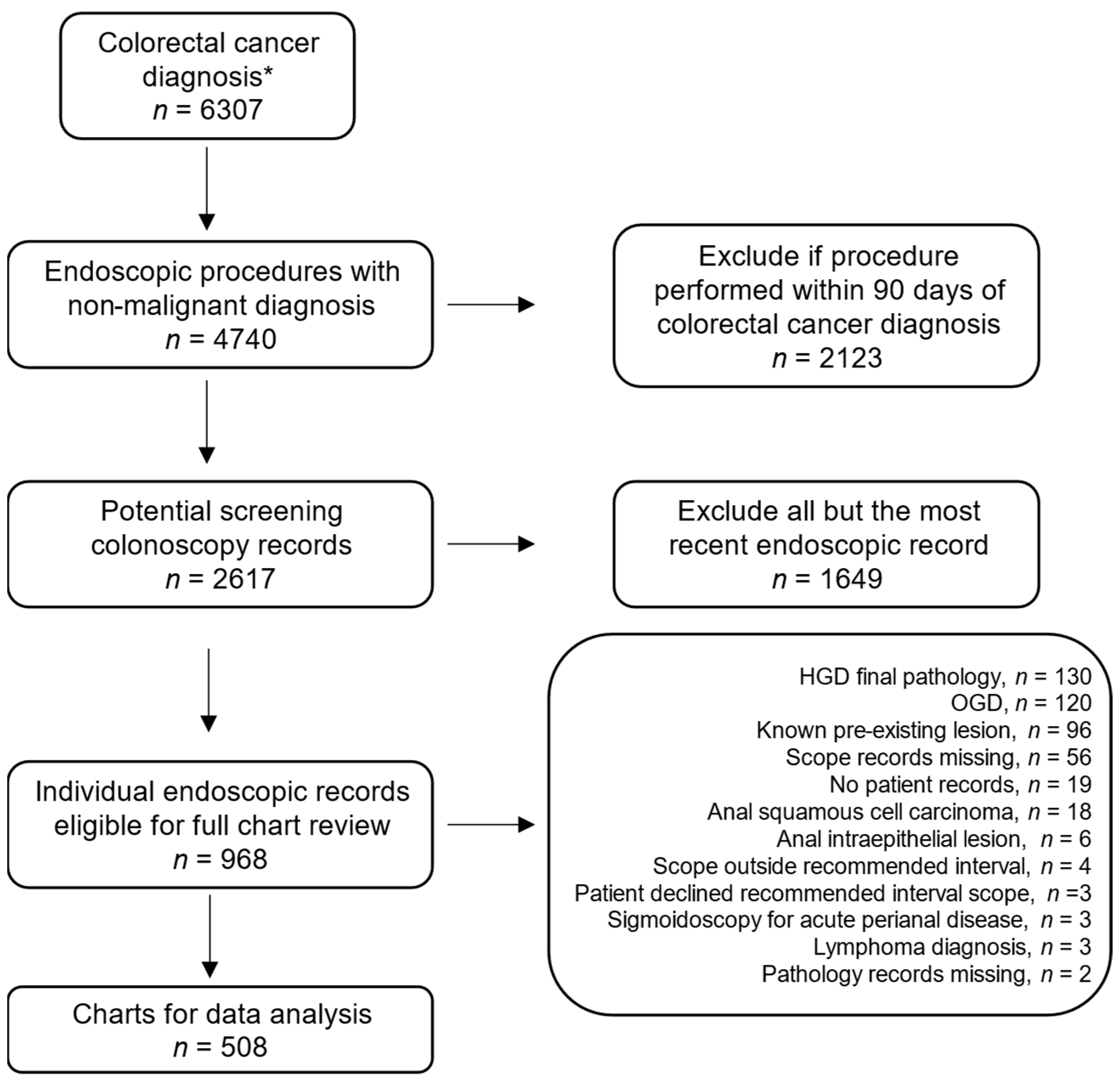
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Force, U.P.S.T. Screening for colorectal cancer: Us preventive services task force recommendation statement. JAMA 2021, 325, 1965–1977. [Google Scholar]
- Recommendations on screening for colorectal cancer in primary care. Can. Med. Assoc. J. 2016, 188, 340–348. [CrossRef]
- Leddin, D.; Lieberman, D.A.; Tse, F.; Barkun, A.N.; Abou-Setta, A.M.; Marshall, J.K.; Samadder, N.J.; Singh, H.; Telford, J.J.; Tinmouth, J.; et al. Clinical practice guideline on screening for colorectal cancer in individuals with a family history of nonhereditary colorectal cancer or adenoma: The canadian association of gastroenterology banff consensus. Gastroenterology 2018, 155, 1325–1347.e1323. [Google Scholar] [CrossRef] [PubMed]
- Ertem, F.U.; Ladabaum, U.; Mehrotra, A.; Tehranian, S.; Shi, Z.; Saul, M.; Morris, M.; Crockett, S.D.; Schoen, R.E. Incidence of interval colorectal cancer attributable to an endoscopist in clinical practice. Gastrointest. Endosc. 2018, 88, 705–711.e701. [Google Scholar] [CrossRef]
- Samadder, N.J.; Neklason, D.; Snow, A.; Samowitz, W.; Cessna, M.H.; Rowe, K.; Sandhu, I.; Boucher, K.; Pappas, L.; Smith, K.R.; et al. Clinical and molecular features of post-colonoscopy colorectal cancers. Clin. Gastroenterol. Hepatol. 2019, 17, 2731–2739.e2732. [Google Scholar] [CrossRef] [PubMed]
- Sanduleanu, S.; le Clercq, C.M.C.; Dekker, E.; Meijer, G.A.; Rabeneck, L.; Rutter, M.D.; Valori, R.; Young, G.P.; Schoen, R.E. Definition and taxonomy of interval colorectal cancers: A proposal for standardising nomenclature. Gut 2015, 64, 1257–1267. [Google Scholar] [CrossRef]
- Yang, K.; Cao, Y.; Gurjao, C.; Liu, Y.; Guo, C.G.; Lo, C.H.; Zong, X.; Drew, D.; Geraghty, C.; Prezioso, E.; et al. Clinical and genomic characterization of interval colorectal cancer in 3 prospective cohorts. Gastroenterology 2022, 163, 1522–1530.e5. [Google Scholar] [CrossRef] [PubMed]
- le Clercq, C.M.C.; Bouwens, M.W.E.; Rondagh, E.J.A.; Bakker, C.M.; Keulen, E.T.P.; de Ridder, R.J.; Winkens, B.; Masclee, A.A.M.; Sanduleanu, S. Postcolonoscopy colorectal cancers are preventable: A population-based study. Gut 2014, 63, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.J.; Lieberman, D.A.; Winawer, S.J.; Ahnen, D.J.; Baron, J.A.; Schatzkin, A.; Cross, A.J.; Zauber, A.G.; Church, T.R.; Lance, P.; et al. Colorectal cancers soon after colonoscopy: A pooled multicohort analysis. Gut 2014, 63, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Erichsen, R.; Frøslev, T.; Pedersen, L.; Vyberg, M.; Koeppe, E.; Crockett, S.D.; Hamilton, S.R.; Sørensen, H.T.; Baron, J.A. Clinical and molecular characteristics of post-colonoscopy colorectal cancer: A population-based study. Gastroenterology 2016, 151, 870–878.e873. [Google Scholar] [CrossRef] [PubMed]
- Cisyk, A.L.; Penner-Goeke, S.; Lichtensztejn, Z.; Nugent, Z.; Wightman, R.H.; Singh, H.; McManus, K.J. Characterizing the prevalence of chromosome instability in interval colorectal cancer. Neoplasia 2015, 17, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.-M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in canada in 2022. Can. Med. Assoc. J. 2022, 194, E601–E607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Halfyard, B.; Roebothan, B.; West, R.; Buehler, S.; Sun, Z.; Squires, J.; McLaughlin, J.R.; Parfrey, P.S.; Wang, P.P. Tobacco smoking and colorectal cancer: A population-based case-control study in newfoundland and labrador. Can J. Public Health 2010, 101, 281–289. [Google Scholar] [CrossRef]
- Sikdar, K.C.; Walsh, S.J.; Roche, M.; Jiang, Y.; Syrowatka, A.; Collins, K.D. Diabetes and sex-specific colorectal cancer risks in newfoundland and labrador: A population-based retrospective cohort study. Can J. Public Health 2013, 104, e101–e107. [Google Scholar] [CrossRef]
- Sharma, I.; Zhu, Y.; Woodrow, J.R.; Mulay, S.; Parfrey, P.S.; McLaughlin, J.R.; Hebert, J.R.; Shivappa, N.; Li, Y.; Zhou, X.; et al. Inflammatory diet and risk for colorectal cancer: A population-based case-control study in newfoundland, canada. Nutrition 2017, 42, 69–74. [Google Scholar] [CrossRef]
- Green, J.; O’Driscoll, M.; Barnes, A.; Maher, E.R.; Bridge, P.; Shields, K.; Parfrey, P.S. Impact of gender and parent of origin on the phenotypic expression of hereditary nonpolyposis colorectal cancer in a large newfoundland kindred with a common msh2 mutation. Dis. Colon. Rectum. 2002, 45, 1223–1232. [Google Scholar] [CrossRef]
- Green, R.C.; Green, J.S.; Buehler, S.K.; Robb, J.D.; Daftary, D.; Gallinger, S.; McLaughlin, J.R.; Parfrey, P.S.; Younghusband, H.B. Very high incidence of familial colorectal cancer in newfoundland: A comparison with ontario and 13 other population-based studies. Fam. Cancer 2007, 6, 53–62. [Google Scholar] [CrossRef]
- Morris, E.J.; Rutter, M.D.; Finan, P.J.; Thomas, J.D.; Valori, R. Post-colonoscopy colorectal cancer (pccrc) rates vary considerably depending on the method used to calculate them: A retrospective observational population-based study of pccrc in the english national health service. Gut 2015, 64, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Bressler, B.; Paszat, L.F.; Chen, Z.; Rothwell, D.M.; Vinden, C.; Rabeneck, L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: A population-based analysis. Gastroenterology 2007, 132, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.N.; Sutradhar, R.; Forbes, S.S.; Paszat, L.F.; Saskin, R.; Rabeneck, L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011, 140, 65–72. [Google Scholar] [CrossRef]
- Singh, H.; Nugent, Z.; Demers, A.A.; Bernstein, C.N. Rate and predictors of early/missed colorectal cancers after colonoscopy in manitoba: A population-based study. Off. J. Am. Coll. Gastroenterol. ACG 2010, 105, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- Samadder, N.J.; Curtin, K.; Tuohy, T.M.; Pappas, L.; Boucher, K.; Provenzale, D.; Rowe, K.G.; Mineau, G.P.; Smith, K.; Pimentel, R.; et al. Characteristics of missed or interval colorectal cancer and patient survival: A population-based study. Gastroenterology 2014, 146, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef]
- Arain, M.A.; Sawhney, M.; Sheikh, S.; Anway, R.; Thyagarajan, B.; Bond, J.H.; Shaukat, A. Cimp status of interval colon cancers: Another piece to the puzzle. Am. J. Gastroenterol. 2010, 105, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.P.; Murad, M.H.; Singh, H.; Samadder, J.N. Prevalence, risk factors, and outcomes of interval colorectal cancers: A systematic review and meta-analysis. Off. J. Am. Coll. Gastroenterol. ACG 2014, 109, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.F.; Regula, J.; Kraszewska, E.; Polkowski, M.; Wojciechowska, U.; Didkowska, J.; Zwierko, M.; Rupinski, M.; Nowacki, M.P.; Butruk, E. Quality indicators for colonoscopy and the risk of interval cancer. N. Engl. J. Med. 2010, 362, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Dalal, Z.; Raw, E.; Roberts, C.; Lyndon, P.J. Anatomical distribution of colorectal cancer over a 10 year period in a district general hospital: Is there a true “rightward shift”? Postgrad. Med. J. 2004, 80, 667–669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahn, S.B.; Han, D.S.; Bae, J.H.; Byun, T.J.; Kim, J.P.; Eun, C.S. The miss rate for colorectal adenoma determined by quality-adjusted, back-to-back colonoscopies. Gut Liver 2012, 6, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Hernández, J.; Hwang, J.; Kandel, G. Seeing better-evidence based recommendations on optimizing colonoscopy adenoma detection rate. World J. Gastroenterol. 2016, 22, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.; Pace, D.; Borgaonkar, M.; Harnett, J.; Miné-Goldring, M.; Ge, M.M.; Brodie, J.; Boone, D.; McGrath, J. Effect of an educational intervention on colonoscopy quality outcomes. Surg. Endosc. 2020, 34, 5142–5147. [Google Scholar] [CrossRef]
- Clark, B.T.; Rustagi, T.; Laine, L. What level of bowel prep quality requires early repeat colonoscopy: Systematic review and meta-analysis of the impact of preparation quality on adenoma detection rate. Am. J. Gastroenterol. 2014, 109, 1714–1723, quiz 1724. [Google Scholar] [CrossRef] [PubMed]
- Jover, R.; Zapater, P.; Polanía, E.; Bujanda, L.; Lanas, A.; Hermo, J.A.; Cubiella, J.; Ono, A.; González-Méndez, Y.; Peris, A.; et al. Modifiable endoscopic factors that influence the adenoma detection rate in colorectal cancer screening colonoscopies. Gastrointest. Endosc. 2013, 77, 381–389.e381. [Google Scholar] [CrossRef]
- Anderson, J.C.; Butterly, L.F.; Robinson, C.M.; Goodrich, M.; Weiss, J.E. Impact of fair bowel preparation quality on adenoma and serrated polyp detection: Data from the new hampshire colonoscopy registry by using a standardized preparation-quality rating. Gastrointest. Endosc. 2014, 80, 463–470. [Google Scholar] [CrossRef]
- Sherer, E.A.; Imler, T.D.; Imperiale, T.F. The effect of colonoscopy preparation quality on adenoma detection rates. Gastrointest. Endosc. 2012, 75, 545–553. [Google Scholar] [CrossRef]
- Cardoso, R.; Guo, F.; Heisser, T.; De Schutter, H.; Van Damme, N.; Nilbert, M.C.; Christensen, J.; Bouvier, A.-M.; Bouvier, V.; Launoy, G.; et al. Overall and stage-specific survival of patients with screen-detected colorectal cancer in european countries: A population-based study in 9 countries. Lancet Reg. Health–Eur. 2022, 21, 100458. [Google Scholar] [CrossRef]
| N | 508 |
|---|---|
| Sex (%) | |
| Male | 255 (50.2) |
| Female | 253 (49.8) |
| Age at colonoscopy (Years) | |
| Mean (SD) | 66.4 (11.5) |
| Median | 67.1 |
| Range (IQR) | 34.2–86.3 (59.1–75.1) |
| Interval time (Years) | |
| Mean (SD) | 2.9 (1.4) |
| Median | 2.9 |
| Range (IQR) | 0.3–5.0 (1.4–4.0) |
| Age at CRC diagnosis (Years) | |
| Mean (SD) | 69.1 (11.4) |
| Median | 69.6 |
| Range (IQR) | 35.3–90.0 (61.7–77.8) |
| Indication for scope (%) | |
| Rectal/acute bleed | 94 (18.5) |
| History of polyps | 91 (17.9) |
| Family history CRC/polyps | 73 (14.4) |
| Anemia/FOBT+/FIT+ | 63 (12.4) |
| Altered bowel habits | 48 (9.5) |
| Abdominal pain | 32 (6.3) |
| History/Suspected IBD (screening) | 22 (4.3) |
| History of CRC | 25 (4.9) |
| Abnormal colonic imaging | 17 (3.4) |
| CRC genetic syndrome | 15 (3.0) |
| Asymptomatic screening | 11 (2.2) |
| Diverticular flare (follow-up) | 4 (0.8) |
| Not stated | 13 (2.6) |
| Specialty (%) | |
| Surgeon | 286 (56.3) |
| Gastroenterologist | 148 (29.1) |
| Internist | 58 (11.4) |
| Not stated | 16 (3.2) |
| Trainee present (%) | |
| Yes | 15 (3.0) |
| No | 48 (9.5) |
| Not stated | 445 (87.5) |
| Full colonoscopy completed (%) | |
| Yes | 340 (66.9) |
| No | 163 (32.1) |
| Flexible sigmoidoscopy | 70 (13.8) |
| Incomplete | 93 (18.3) |
| Not stated | 5 (1.0) |
| Rectal retroflex performed (%) | |
| Yes | 73 (14.4) |
| No | 435 (85.6) |
| Withdrawal time recorded (%) | |
| Yes | 53 (10.4) |
| No | 455 (89.6) |
| Adverse effects (%) | |
| Yes | 17 (3.3) |
| No | 264 (52.0) |
| Not stated | 227 (44.7) |
| Bowel prep regimen recorded (%) | |
| Yes | 174 (34.3) |
| No | 334 (65.7) |
| Bowel prep quality (%) | |
| Excellent/very good | 34 (6.7) |
| Good | 73 (14.4) |
| Fair/poor | 47 (9.2) |
| Very poor/bad | 22 (4.3) |
| Not stated | 332 (65.3) |
| Antispasmodic used (%) | |
| Yes | 22 (4.3) |
| No | 193 (38.0) |
| Not stated | 293 (57.7) |
| Non-polyp findings (%) | |
| Normal | 258 (50.8) |
| Benign (diverticulosis, angiodysplasia) | 207 (40.7) |
| Inflammatory bowel disease | 23 (4.5) |
| Bleeding | 1 (0.2) |
| Not stated | 19 (3.7) |
| Polyps identified (Number identified) (%) | |
| Yes | 255 (50.2) |
| 1–2 | 163 (32.1) |
| 3–4 | 43 (8.5) |
| 5–10 | 22 (4.3) |
| >10 | 27 (5.3) |
| No | 234 (46.1) |
| Not stated | 19 (3.7) |
| Polyps removed or biopsied (%) | |
| Yes | 239 (47.0) |
| No | 262 (51.6) |
| Not stated | 7 (1.4) |
| Largest polyp size (%) | |
| 1–5 mm | 46 (9.1) |
| 6–10 mm | 36 (7.1) |
| 11–20 mm | 26 (5.1) |
| >20 mm | 13 (2.6) |
| Not stated | 387 (76.2) |
| Polyp morphology (%) | |
| No polyp | 252 (49.6) |
| Sessile | 67 (13.2) |
| Pedunculated | 22 (4.3) |
| Flat | 20 (3.9) |
| Not stated | 147 (28.9) |
| Polyp histology (%) | |
| No polyp | 252 (49.6) |
| Adenoma | 161 (31.7) |
| Hyperplastic | 49 (9.6) |
| Serrated | 16 (3.2) |
| Inflammatory | 5 (1.0) |
| Lymphoid | 1 (0.2) |
| Normal mucosa | 3 (0.6) |
| Not determined (polyp not retrieved) | 21 (4.1) |
| Polyp location (largest) (%) | |
| Cecum | 12 (4.7) |
| Right colon (not cecum) | 41 (16.0) |
| Transverse colon | 13 (5.1) |
| Left colon (not rectum) | 80 (31.2) |
| Rectum | 28 (10.9) |
| Pan colonic | 46 (18.0) |
| Not stated | 36 (14.1) |
| Interval cancer pathology (%) | |
| Adenocarcinoma | 484 (95.3) |
| Other | 19 (3.7) |
| Not stated | 5 (1.0) |
| Interval cancer location (%) | |
| Cecum | 126 (24.8) |
| Right colon (not cecum) | 116 (22.8) |
| Transverse colon | 50 (9.8) |
| Left colon (not rectum) | 116 (22.8) |
| Rectum | 78 (15.4) |
| Multiple cancers | 7 (1.4) |
| Liver metastasis | 1 (0.2) |
| Not stated | 14 (2.8) |
| Interval cancer stage (%) | |
| Stage 0 (in situ) | 39 (7.7) |
| Stage 1 | 108 (21.3) |
| Stage 2 | 119 (23.4) |
| Stage 3 | 124 (24.4) |
| Stage 4 | 77 (15.2) |
| Not stated | 41 (8.1) |
| 2001–2009 | 2010–2018 | p-Value | |
|---|---|---|---|
| N | 320 | 188 | - |
| Sex (%) | |||
| Male | 153 (47.8) | 102 (54.3) | 0.16 |
| Female | 167 (52.2) | 86 (45.7) | |
| Age at colonoscopy (Years) | |||
| Mean (SD) | 65.7 (12.2) | 67.7 (10.2) | 0.06 |
| Median | 65.8 | 68.5 | |
| Range (IQR) | 30.8–86.3 (57.8–75.0) | 37.6–86.4 (60.5–74.4) | |
| Interval time (Years) | |||
| Mean (SD) | 2.7 (1.4) | 2.7 (1.4) | 0.6 |
| Median | 2.9 | 3 | |
| Range (IQR) | 0.3–5.0 (1.4–4.0) | 0.2–4.9 (1.4–3.9) | |
| Age at CRC diagnosis (Years) | |||
| Mean (SD) | 68.4 (12.1) | 70.3 (10.1) | 0.06 |
| Median | 68.9 | 70.4 | |
| Range (IQR) | 34.0–89.6 (61.0–77.9) | 40.9–90.5 (63.7–77.8) | |
| Specialty (%) | |||
| Surgeon | 99 (30.9) | 49 (26.1) | 0.5 |
| Gastroenterologist | 38 (11.9) | 20 (10.6) | |
| Internist | 172 (53.8) | 114 (60.6) | |
| Not stated | 11 (3.4) | 5 (2.7) | |
| Trainee present (%) | 8 (2.5) | ||
| Yes | 24 (7.5) | 7 (3.7) | 0.1 |
| No | 288 (90.0) | 24 (12.8) | |
| Not stated | 157 (83.5) | ||
| Full colonoscopy completed (%) | |||
| Yes | 194 (60.6) | 146 (77.7) | <0.001 |
| No | 123 (38.4) | 40 (21.3) | |
| Flexible sigmoidoscopy | 65 (20.3) | 16 (8.5) | |
| Incomplete | 58 (18.1) | 24 (12.8) | |
| Not stated | 3 (0.9) | 2 (1.1) | |
| Rectal retroflex performed (%) | |||
| Yes | 31 (9.7) | 42 (22.3) | <0.001 |
| No | 289 (90.3) | 146 (77.7) | |
| Withdrawal time recorded (%) | |||
| Yes | 12 (3.8) | 41 (21.8) | <0.001 |
| No | 308 (96.2) | 147 (78.2) | |
| Adverse effects (%) | |||
| Yes | 14 (4.4) | 3 (1.6) | <0.001 |
| No | 138 (43.1) | 126 (67.0) | |
| Not stated | 168 (52.5) | 59 (31.4) | |
| Bowel prep regimen recorded (%) | |||
| Yes | 71 (22.2) | 98 (52.1) | <0.001 |
| No | 249 (77.8) | 90 (47.9) | |
| Bowel prep quality (%) | |||
| Excellent/very good | 9 (2.8) | 25 (13.3) | <0.001 |
| Good | 25 (7.8) | 48 (25.5) | |
| Fair/poor | 30 (9.4) | 17 (9.0) | |
| Very poor/bad | 16 (5.0) | 6 (3.2) | |
| Not stated | 240 (75.0) | 92 (48.9) | |
| Antispasmodic used (%) | |||
| Yes | 14 (4.4) | 8 (4.3) | <0.001 |
| No | 90 (28.1) | 103 (54.8) | |
| Not stated | 216 (67.5) | 77 (41.0) | |
| Polyps identified (Number Identified) (%) | |||
| Yes | 141 (44.1) | 114 (60.6) | <0.001 |
| 1–2 | 90 (28.1) | 73 (38.8) | 0.002 |
| 3–4 | 19 (5.9) | 24 (12.8) | |
| 5–10 | 12 (3.8) | 10 (5.3) | |
| >10 | 20 (6.2) | 7 (3.7) | |
| No | 163 (50.9) | 71 (37.8) | |
| Not stated | 16 (5.0) | 3 (1.6) | |
| Polyps removed or biopsied (%) | |||
| Yes | 129 (40.3) | 110 (58.5) | <0.001 |
| No | 186 (58.1) | 76 (40.4) | |
| Not stated | 5 (1.6) | 2 (1.1) | |
| Largest polyp size (%) | |||
| 1–5 mm | 20 (6.2) | 26 (13.8) | 0.05 |
| 6–10 mm | 23 (7.2) | 13 (6.9) | |
| 11–20 mm | 15 (4.7) | 11 (5.9) | |
| >20 mm | 7 (2.2) | 6 (3.2) | |
| Not stated | 255 (79.7) | 132 (70.2) | |
| Polyp morphology (%) | |||
| No polyp | 178 (55.6) | 74 (39.4) | <0.001 |
| Sessile | 34 (10.6) | 33 (17.6) | |
| Pedunculated | 16 (5.0) | 6 (3.2) | |
| Flat | 7 (2.2) | 14 (7.4) | |
| Not stated | 85 (26.6) | 61 (32.4) | |
| Polyp location (largest) (%) | |||
| Cecum | 6 (4.2) | 6 (5.3) | 0.06 |
| Right colon (not cecum) | 19 (13.4) | 22 (19.3) | |
| Transverse colon | 8 (5.6) | 5 (4.4) | |
| Left colon (not rectum) | 52 (36.6) | 28 (24.6) | |
| Rectum | 13 (9.2) | 15 (13.2) | |
| Pan colonic | 19 (13.4) | 27 (23.7) | |
| Not stated | 25 (17.6) | 11 (9.6) | |
| Interval cancer location (%) | |||
| Cecum | 85 (26.6) | 41 (21.8) | 0.85 |
| Right colon (not cecum) | 70 (21.9) | 46 (24.5) | |
| Transverse colon | 29 (9.1) | 21 (11.2) | |
| Left colon (not rectum) | 74 (23.1) | 42 (22.3) | |
| Rectum | 47 (14.7) | 31 (16.5) | |
| Multiple cancers | 4 (1.2) | 3 (1.6) | |
| Liver metastasis | 1 (0.3) | 0 (0.0) | |
| Not stated | 10 (3.1) | 4 (2.1) | |
| Interval cancer stage (%) | |||
| Stage 0 (in situ) | 30 (9.4) | 9 (4.8) | 0.26 |
| Stage 1 | 67 (20.9) | 41 (21.8) | |
| Stage 2 | 71 (22.2) | 48 (25.5) | |
| Stage 3 | 73 (22.8) | 51 (27.1) | |
| Stage 4 | 54 (16.9) | 23 (12.2) | |
| Not stated | 25 (7.8) | 16 (8.5) |
| Univariate | p-Value | Multivariable | p-Value | |
|---|---|---|---|---|
| Scope reason | ||||
| Polyp/CRC history | 1.26 (0.77–2.07) | 0.35 | 1.41 (0.83–2.41) | 0.21 |
| Bowel preparation | ||||
| Poor/bad | 1.75 (0.94–3.29) | 0.08 | 1.82 (0.74–4.44) | 0.19 |
| Not stated | 1.16 (0.75–1.80) | 0.5 | 1.15 (0.61–2.17) | 0.66 |
| Largest polyp (>1 cm) | 0.76 (0.40–1.47) | 0.42 | 0.71 (0.28–1.79) | 0.47 |
| Complete scope (No) | 0.98 (0.69–1.43) | 0.9 | 1.46 (0.83–2.28) | 0.21 |
| Stage (Advanced/not stated) | 1.17 (0.82–1.67) | 0.38 | 1.38 (0.84–2.28) | 0.21 |
| Univariate | p-Value | Multivariable | p-Value | Univariate | p-Value | Multivariable | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Scope indication | Bleeding/anemia | Personal history polyp/CRC | ||||||
| Bowel preparation | ||||||||
| Poor/bad | 1.83 (0.92–3.61) | 0.08 | 1.77 (0.87–3.57) | 0.11 | 0.68 (0.34–1.38) | 0.29 | 0.76 (0.36–1.60) | 0.47 |
| Not stated | 1.83 (1.10–3.07) | 0.02 | 1.77 (1.04–3.03) | 0.04 | 0.65 (0.40–1.07) | 0.09 | 0.72 (0.43–1.21) | 0.54 |
| Largest polyp (>1 cm) | 1.28 (0.64–2.53) | 0.48 | 1.38 (0.65–2.94) | 0.4 | 0.86 (0.39–1.93) | 0.72 | 0.83 (0.34–2.01) | 0.65 |
| Complete scope (No) | 1.92 (1.29–2.85) | 0.001 | 1.93 (1.28–2.93) | 0.2 | 0.25 (0.14–0.44) | <0.001 | 0.29 (0.16–0.52) | <0.001 |
| Tumor location (Right) | 0.95 (0.64–1.40) | 0.79 | 0.96 (0.64–1.44) | 0.86 | 1.28 (0.83–1.99) | 0.26 | 1.31 (0.83–2.06) | 0.25 |
| Stage (Advanced/not stated) | 1.13 (0.77–1.64) | 0.53 | 1.12 (0.75–1.66) | 0.59 | 0.60 (0.39–0.92) | 0.02 | 0.60 (0.38–0.94) | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanahan, J.J.; LeBlanc, D.M.; Courage, E.R.; Benesch, M.G.K.; Hickey, K.E.; Hartwig, K.A.; Armstrong, C.D.; Engelbrecht, R.; Fagan, M.G.; Borgaonkar, M.R.; et al. Characteristics of Interval Colorectal Cancer: A Canadian Retrospective Population-Level Analysis from Newfoundland and Labrador. Curr. Oncol. 2022, 29, 9150-9162. https://doi.org/10.3390/curroncol29120716
Shanahan JJ, LeBlanc DM, Courage ER, Benesch MGK, Hickey KE, Hartwig KA, Armstrong CD, Engelbrecht R, Fagan MG, Borgaonkar MR, et al. Characteristics of Interval Colorectal Cancer: A Canadian Retrospective Population-Level Analysis from Newfoundland and Labrador. Current Oncology. 2022; 29(12):9150-9162. https://doi.org/10.3390/curroncol29120716
Chicago/Turabian StyleShanahan, Jessica J., Danielle M. LeBlanc, Emily R. Courage, Matthew G. K. Benesch, Kala E. Hickey, Katia A. Hartwig, Casey D. Armstrong, Reniel Engelbrecht, Mitchell G. Fagan, Mark R. Borgaonkar, and et al. 2022. "Characteristics of Interval Colorectal Cancer: A Canadian Retrospective Population-Level Analysis from Newfoundland and Labrador" Current Oncology 29, no. 12: 9150-9162. https://doi.org/10.3390/curroncol29120716
APA StyleShanahan, J. J., LeBlanc, D. M., Courage, E. R., Benesch, M. G. K., Hickey, K. E., Hartwig, K. A., Armstrong, C. D., Engelbrecht, R., Fagan, M. G., Borgaonkar, M. R., & Pace, D. E. (2022). Characteristics of Interval Colorectal Cancer: A Canadian Retrospective Population-Level Analysis from Newfoundland and Labrador. Current Oncology, 29(12), 9150-9162. https://doi.org/10.3390/curroncol29120716






