A Novel, Simple, and Low-Cost Approach for Machine Learning Screening of Kidney Cancer: An Eight-Indicator Blood Test Panel with Predictive Value for Early Diagnosis
Abstract
1. Introduction
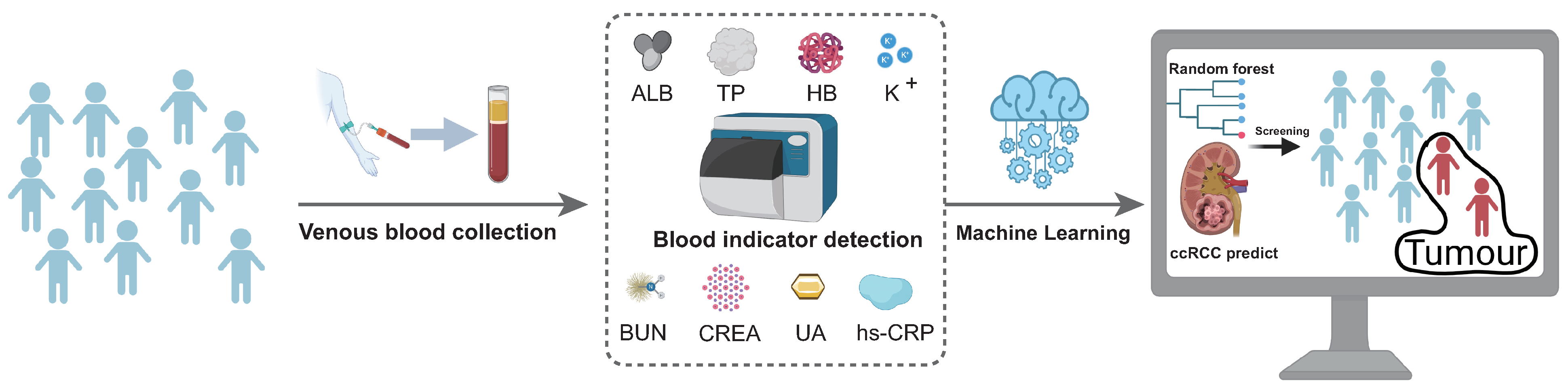
2. Materials and Methods
2.1. Patients and Samples
2.2. Statistical Analyses
2.3. Modelling of Early Screening Models
2.4. Data Visualisation
3. Results
3.1. The Demographic Characteristics of All Samples
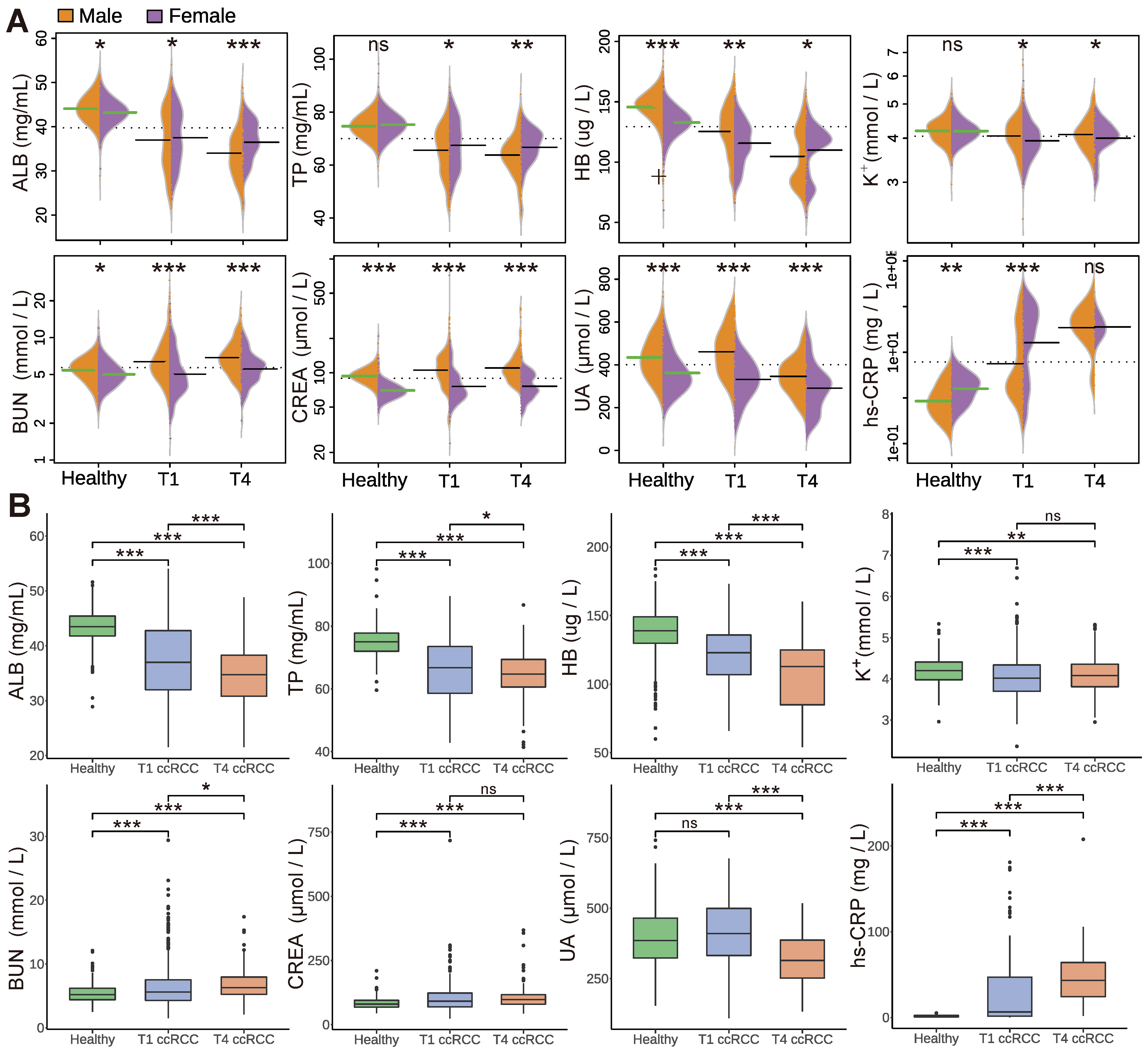
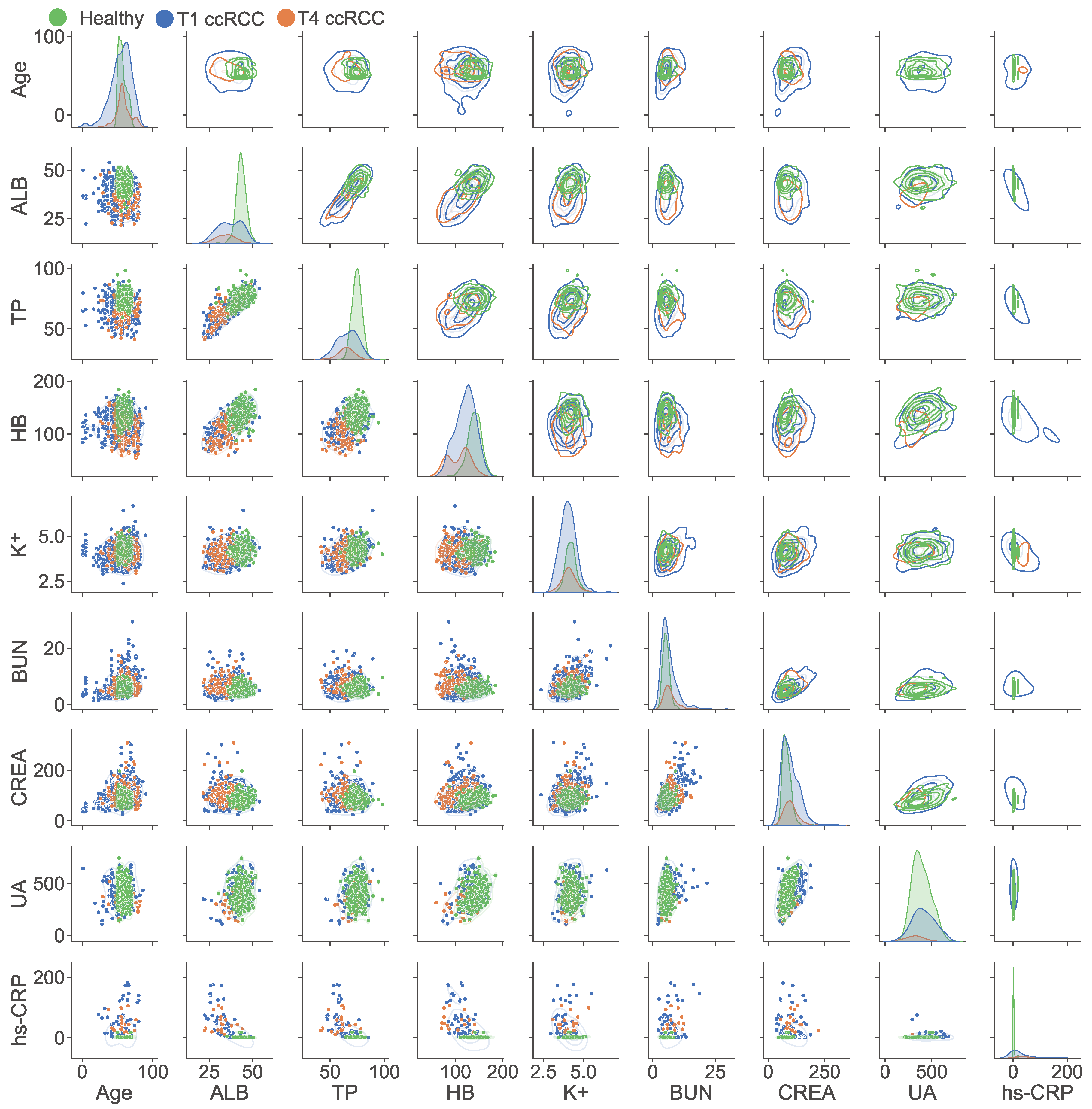
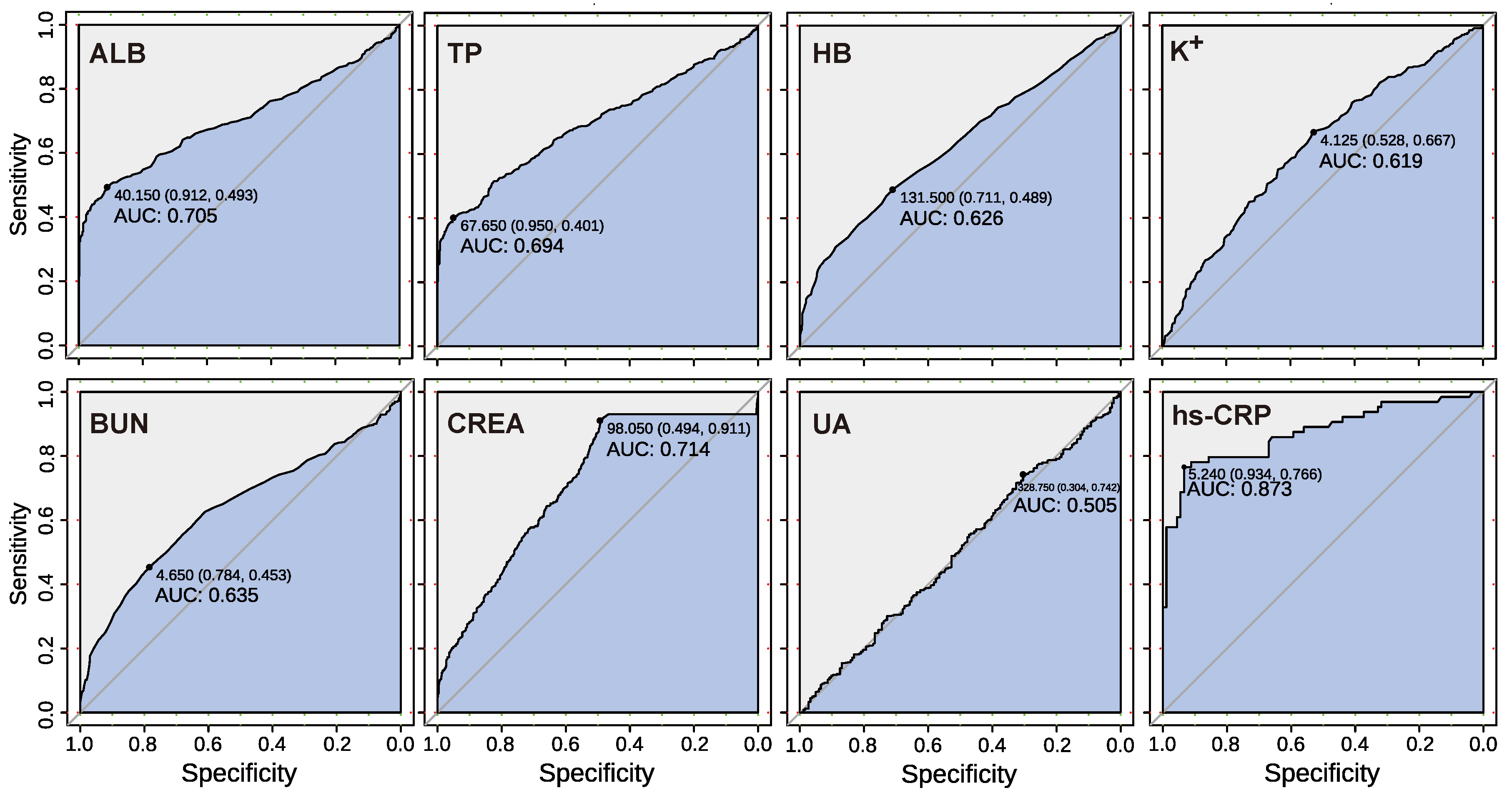
3.2. Analysis of Blood Biochemical Indicators
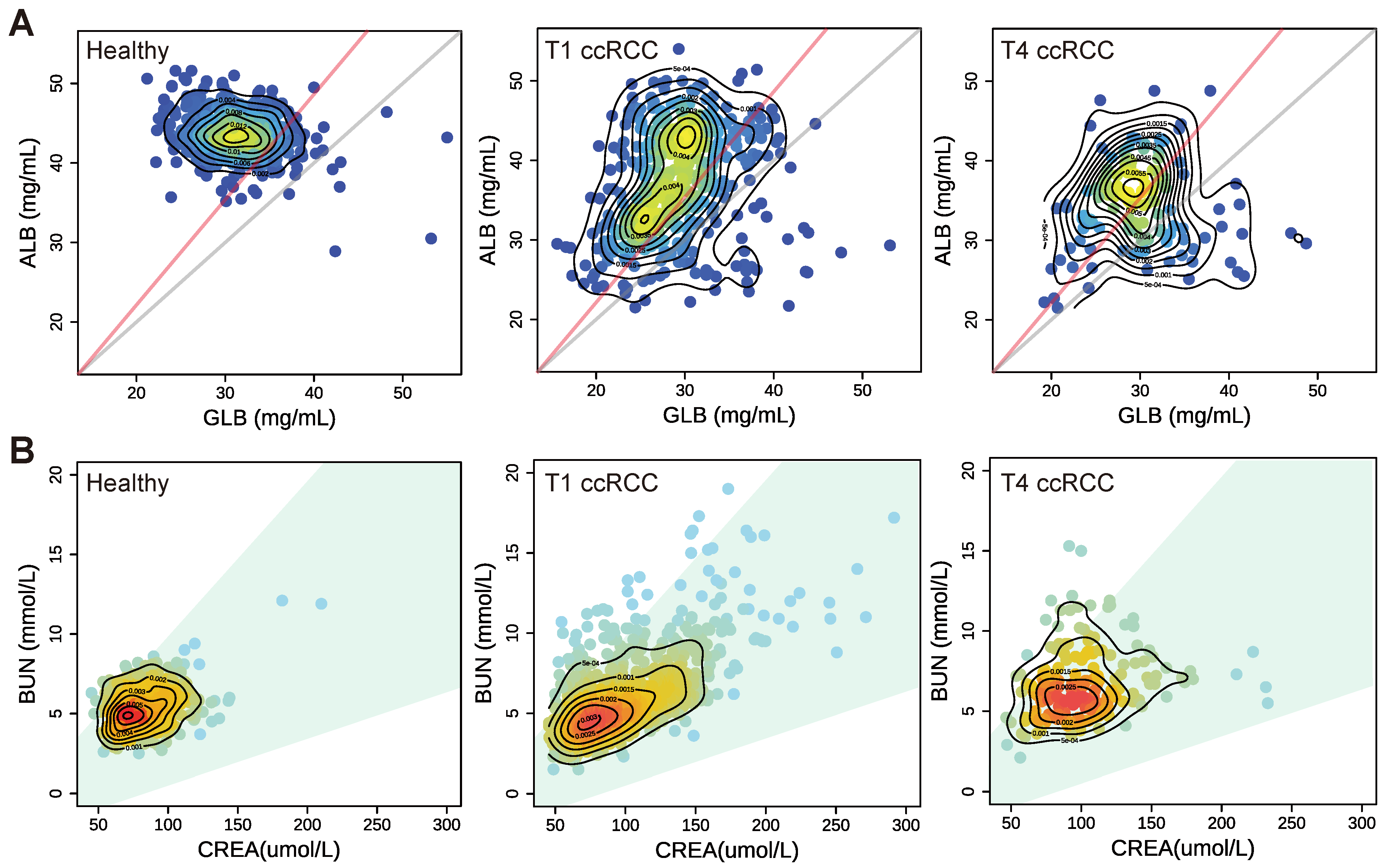
3.3. The Clinical Significance of the Blood Indicator Ratio
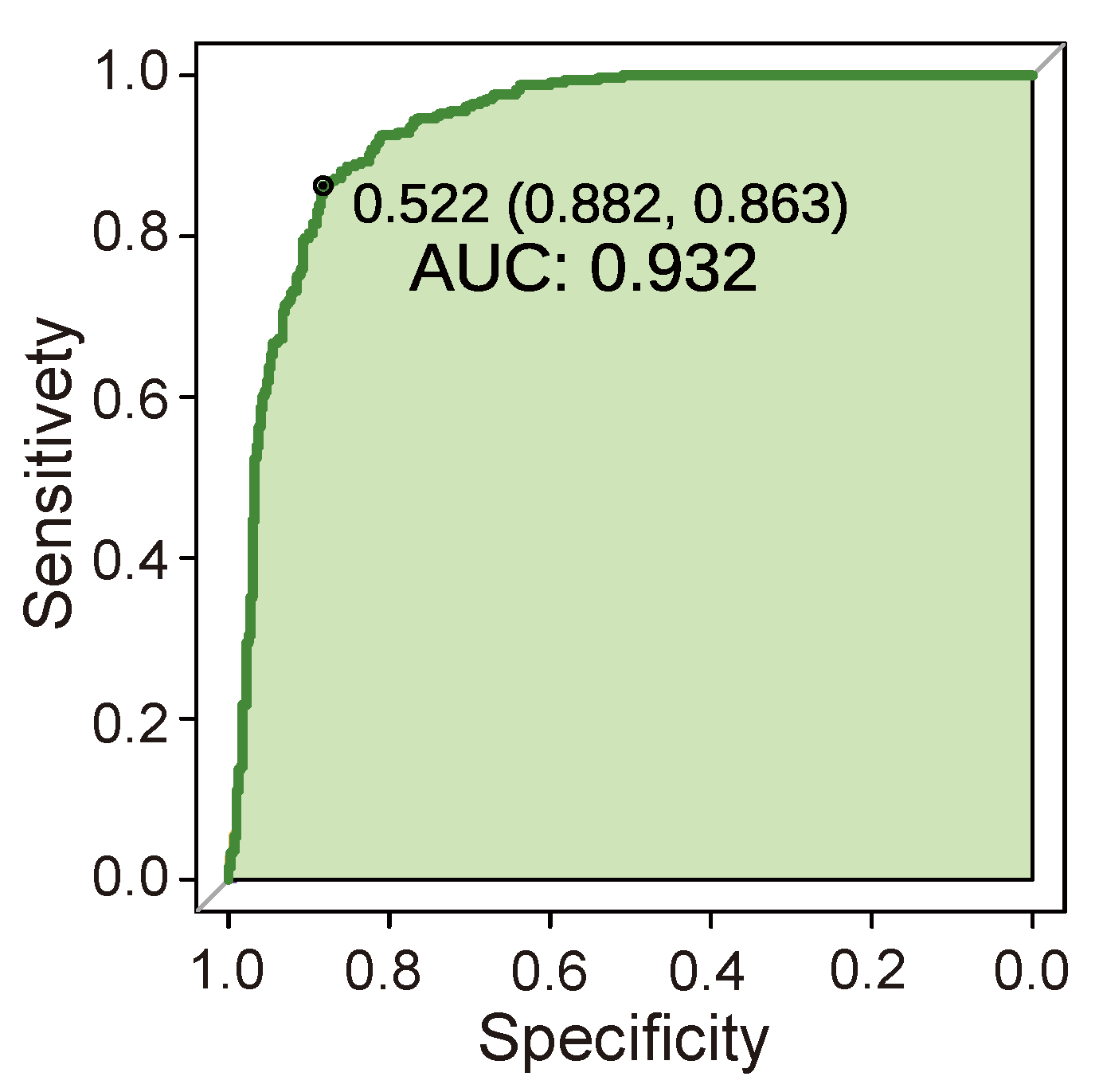
3.4. Performance Test of Cancer Prediction Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hancock, S.B.; Georgiades, C.S. Kidney cancer. Cancer J. 2016, 22, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Cai, G.; Li, J.; Chen, X. Cisplatin-induced renal toxicity in elderly people. Ther. Adv. Med. Oncol. 2020, 12, 1758835920923430. [Google Scholar] [CrossRef] [PubMed]
- Grundy, E. Ageing and vulnerable elderly people: European perspectives. Ageing Soc. 2006, 26, 105–134. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Bensalah, K.; Bex, A.; Giles, R.; Hora, M.; Kuczyk, M.; Lam, T.; Marconi, L.; Merseburger, A.; et al. EAU guidelines on renal cell carcinoma. Eur. Assoc. Urol. 2017, 58, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, J.P.; Constâncio, V.; Lobo, J.; Henrique, R.; Jerónimo, C. Unveiling the World of Circulating and Exosomal microRNAs in Renal Cell Carcinoma. Cancers 2021, 13, 5252. [Google Scholar] [CrossRef]
- Abu-Ghanem, Y.; Powles, T.; Capitanio, U.; Beisland, C.; Järvinen, P.; Stewart, G.D.; Gudmundsson, E.O.; Lam, T.B.; Marconi, L.; Fernandéz-Pello, S.; et al. The impact of histological subtype on the incidence, timing, and patterns of recurrence in patients with renal cell carcinoma after surgery—results from RECUR Consortium. Eur. Urol. Oncol. 2021, 4, 473–482. [Google Scholar] [CrossRef]
- Mancini, M.; Zazzara, M.; Zattoni, F. Stem cells, biomarkers and genetic profiling: Approaching future challenges in urology. Urol. J. 2016, 83, 4–13. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, L.; Liao, N.; Gao, P.; Chen, L.; Li, X.; Fan, M. Specific computed tomography imaging characteristics of congenital mesoblastic nephroma and correlation with ultrasound and pathology. J. Pediatr. Urol. 2018, 14, 571.e1–571.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, R.; Li, G.; Jin, L.; Shi, Q.; Du, L. Value of Contrast-Enhanced Ultrasound in the Diagnosis of Renal Cancer and in Comparison With Contrast-Enhanced Computed Tomography: A Meta-analysis. J. Ultrasound Med. 2019, 38, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Franco, M.M.; Leon-Rodriguez, E. Delays in breast cancer detection and treatment in developing countries. Breast Cancer Basic Clin. Res. 2018, 12, 1178223417752677. [Google Scholar] [CrossRef] [PubMed]
- Yicheng, S. Clinical Significance and Differentiation of Hematuria and Hemoglobinuria. Front. Med. Sci. Res. 2020, 2, 43–46. [Google Scholar]
- Namdari, F.; Nazar Pour, M.J.; Behzadi, A.; Akbari, M.; Moradi, H.; Dialameh, H. Primary renal osteosarcoma: A case report and review of literature. Clin. Case Rep. 2022, 10, e5957. [Google Scholar] [CrossRef]
- Arneth, B. Tumor microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- He, X.; Guo, S.; Chen, D.; Yang, G.; Chen, X.; Zhang, Y.; He, Q.; Qin, Z.; Liu, Z.; Xue, Y.; et al. Preoperative albumin to globulin ratio (AGR) as prognostic factor in renal cell carcinoma. J. Cancer 2017, 8, 258–265. [Google Scholar] [CrossRef]
- Shah, A.; Molnar, M.Z.; Lukowsky, L.R.; Zaritsky, J.J.; Kovesdy, C.P.; Kalantar-Zadeh, K. Hemoglobin level and survival in hemodialysis patients with polycystic kidney disease and the role of administered erythropoietin. Am. J. Hematol. 2012, 87, 833–836. [Google Scholar] [CrossRef][Green Version]
- Checheriţă, I.; David, C.; Diaconu, V.; Ciocâlteu, A.; Lascăr, I. Potassium level changes—Arrhythmia contributing factor in chronic kidney disease patients. Rom. J. Morphol. Embryol. 2011, 52, 1047–1050. [Google Scholar]
- Lazich, I.; Bakris, G.L. Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. In Seminars in Nephrology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 34, pp. 333–339. [Google Scholar]
- Dwinnell, B.G.; Anderson, R.J. Diagnostic evaluation of the patient with acute renal failure. In Atlas of Diseases of Kidney; Current Medicine Inc.: Philadelphia, PA, USA, 1999; p. 12. [Google Scholar]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function–measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar]
- Windgassen, E.B.; Funtowicz, L.; Lunsford, T.N.; Harris, L.A.; Mulvagh, S.L. C-reactive protein and high-sensitivity C-reactive protein: An update for clinicians. Postgrad. Med. 2011, 123, 114–119. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Engel, J.M.; Stankowski, R.V.; Liang, H.; Berg, R.L.; Doi, S.A.R. High-sensitivity C-reactive protein (hs-CRP) as a biomarker for trastuzumab-induced cardiotoxicity in HER2-positive early-stage breast cancer: A pilot study. Breast Cancer Res. Treat. 2012, 134, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, D.D.; Whiting, K.; Curry, M.; Lavery, J.A.; Larmarange, J. Reproducible Summary Tables with the gtsummary Package. R J. 2021, 13, 570–580. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Sellke, T.; Bayarri, M.; Berger, J.O. Calibration of ρ values for testing precise null hypotheses. Am. Stat. 2001, 55, 62–71. [Google Scholar] [CrossRef]
- Rish, I. An empirical study of the naive Bayes classifier. In Proceedings of the IJCAI 2001 Workshop on Empirical Methods in Artificial Intelligence, Seattle, WA, USA, 4–10 August 2001; Volume 3, pp. 41–46. [Google Scholar]
- Taheri, S.; Mammadov, M.; Bagirov, A.M. Improving Naive Bayes Classifier Using Conditional Probabilities; Deakin University: Geelong, Australia, 2010. [Google Scholar]
- Kumar, R.; Indrayan, A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011, 48, 277–287. [Google Scholar] [CrossRef]
- Kannan, R.; Vasanthi, V. Machine learning algorithms with ROC curve for predicting and diagnosing the heart disease. In Soft Computing and Medical Bioinformatics; Springer: Berlin/Heidelberg, Germany, 2019; pp. 63–72. [Google Scholar]
- Nachar, N. The Mann-Whitney U: A test for assessing whether two independent samples come from the same distribution. Tutor. Quant. Methods Psychol. 2008, 4, 13–20. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Criminisi, A.; Shotton, J.; Konukoglu, E. Decision forests: A unified framework for classification, regression, density estimation, manifold learning and semi-supervised learning. In Foundations and Trends® in Computer Graphics and Vision; Now Pub: Delft, The Netherlands, 2012; Volume 7, pp. 81–227. [Google Scholar]
- Fest, J.; Ruiter, R.; Ikram, M.A.; Voortman, T.; van Eijck, C.H.; Stricker, B.H. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci. Rep. 2018, 8, 10566. [Google Scholar] [CrossRef] [PubMed]
- Choe, H.; Kobayashi, N.; Abe, K.; Hieda, Y.; Tezuka, T.; Inaba, Y. Evaluation of Serum Albumin and Globulin in Combination With C-Reactive Protein Improves Serum Diagnostic Accuracy for Low-Grade Periprosthetic Joint Infection. J. Arthroplast. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xun, Y.; Yu, X.; Liu, Z.; Cui, L.; Zhang, J.; Li, C.; Wang, S. Albumin-globulin ratio: A novel predictor of sepsis after flexible ureteroscopy in patients with solitary proximal ureteral stones. Transl. Androl. Urol. 2020, 9, 1980–1989. [Google Scholar] [CrossRef]
- Suh, B.; Park, S.; Shin, D.W.; Yun, J.; Keam, B.; Yang, H.K.; Ahn, E.; Lee, H.; Park, J.; Cho, B. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann. Oncol. 2014, 25, 2260–2266. [Google Scholar] [CrossRef]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Bellomo, R.; Goldsmith, D. The meaning of the blood urea nitrogen/creatinine ratio in acute kidney injury. Clin. Kidney J. 2012, 5, 187–191. [Google Scholar] [CrossRef]
- Gabriele, L.; Buoncervello, M.; Ascione, B.; Bellenghi, M.; Matarrese, P.; Carè, A. The gender perspective in cancer research and therapy: Novel insights and on-going hypotheses. Ann. Dell’istituto Super. Di Sanita 2016, 52, 213–222. [Google Scholar]
- Mancini, M.; Righetto, M.; Baggio, G. Gender-related approach to kidney cancer management: Moving forward. Int. J. Mol. Sci. 2020, 21, 3378. [Google Scholar] [CrossRef]
- Rivadeneira, D.E.; Evoy, D.; Fahey III, T.J.; Lieberman, M.D.; Daly, J.M. Nutritional support of the cancer patient. CA Cancer J. Clin. 1998, 48, 69–80. [Google Scholar] [CrossRef]
- Szkandera, J.; Stotz, M.; Absenger, G.; Stojakovic, T.; Samonigg, H.; Kornprat, P.; Schaberl-Moser, R.; Alzoughbi, W.; Lackner, C.; Ress, A.; et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br. J. Cancer 2014, 110, 183–188. [Google Scholar] [CrossRef]
- Yim, K.; Bindayi, A.; McKay, R.; Mehrazin, R.; Raheem, O.A.; Field, C.; Bloch, A.; Wake, R.; Ryan, S.; Patterson, A.; et al. Rising serum uric acid level is negatively associated with survival in renal cell carcinoma. Cancers 2019, 11, 536. [Google Scholar] [CrossRef]
- Norberg, S.M.; Oros, M.; Birkenbach, M.; Bilusic, M. Spontaneous tumor lysis syndrome in renal cell carcinoma: A case report. Clin. Genitourin. Cancer 2014, 12, e225–e227. [Google Scholar] [CrossRef]
- Aktepe, O.H.; Güner, G.; Güven, D.C.; Taban, H.; Yıldırım, H.Ç.; Şahin, T.K.; Ardıç, F.S.; Yeter, H.H.; Yüce, D.; Erman, M. Impact of albumin to globulin ratio on survival outcomes of patients with metastatic renal cell carcinoma. Turk. J. Urol. 2021, 47, 113–119. [Google Scholar] [CrossRef]
- Koparal, M.Y.; Polat, F.; Çetin, S.; Bulut, E.C.; Sözen, T.S. Prognostic role of preoperative albumin to globulin ratio in predicting survival of clear cell renal cell carcinoma. Int. Braz. J. Urol. 2018, 44, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Iseki, Y.; Ikeya, T.; Sugano, K.; Hirakawa, K. The pretreatment albumin to globulin ratio predicts chemotherapeutic outcomes in patients with unresectable metastatic colorectal cancer. BMC Cancer 2015, 15, 347. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Pan, H.; Liang, W.; Xiao, D.; Chen, X.; Guo, M.; He, J. Prognostic effect of albumin-to-globulin ratio in patients with solid tumors: A systematic review and meta-analysis. J. Cancer 2017, 8, 4002–4010. [Google Scholar] [CrossRef]
- Elemento, O.; Leslie, C.; Lundin, J.; Tourassi, G. Artificial intelligence in cancer research, diagnosis and therapy. Nat. Rev. Cancer 2021, 21, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Zhao, H.; Lin, H.; Wang, J.; Wang, N.; Li, X.; Ding, D. ISPRF: A machine learning model to predict the immune subtype of kidney cancer samples by four genes. Transl. Androl. Urol. 2021, 10, 3773–3786. [Google Scholar] [CrossRef]
- Erdim, C.; Yardimci, A.H.; Bektas, C.T.; Kocak, B.; Koca, S.B.; Demir, H.; Kilickesmez, O. Prediction of benign and malignant solid renal masses: Machine learning-based CT texture analysis. Acad. Radiol. 2020, 27, 1422–1429. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, F.; Huang, W. A Novel, Simple, and Low-Cost Approach for Machine Learning Screening of Kidney Cancer: An Eight-Indicator Blood Test Panel with Predictive Value for Early Diagnosis. Curr. Oncol. 2022, 29, 9135-9149. https://doi.org/10.3390/curroncol29120715
Li H, Wang F, Huang W. A Novel, Simple, and Low-Cost Approach for Machine Learning Screening of Kidney Cancer: An Eight-Indicator Blood Test Panel with Predictive Value for Early Diagnosis. Current Oncology. 2022; 29(12):9135-9149. https://doi.org/10.3390/curroncol29120715
Chicago/Turabian StyleLi, Haiyang, Fei Wang, and Weini Huang. 2022. "A Novel, Simple, and Low-Cost Approach for Machine Learning Screening of Kidney Cancer: An Eight-Indicator Blood Test Panel with Predictive Value for Early Diagnosis" Current Oncology 29, no. 12: 9135-9149. https://doi.org/10.3390/curroncol29120715
APA StyleLi, H., Wang, F., & Huang, W. (2022). A Novel, Simple, and Low-Cost Approach for Machine Learning Screening of Kidney Cancer: An Eight-Indicator Blood Test Panel with Predictive Value for Early Diagnosis. Current Oncology, 29(12), 9135-9149. https://doi.org/10.3390/curroncol29120715




