Thymoquinone Plus Immunotherapy in Extra-Pulmonary Neuroendocrine Carcinoma: Case Series for a Novel Combination
Abstract
1. Introduction
2. Case Series
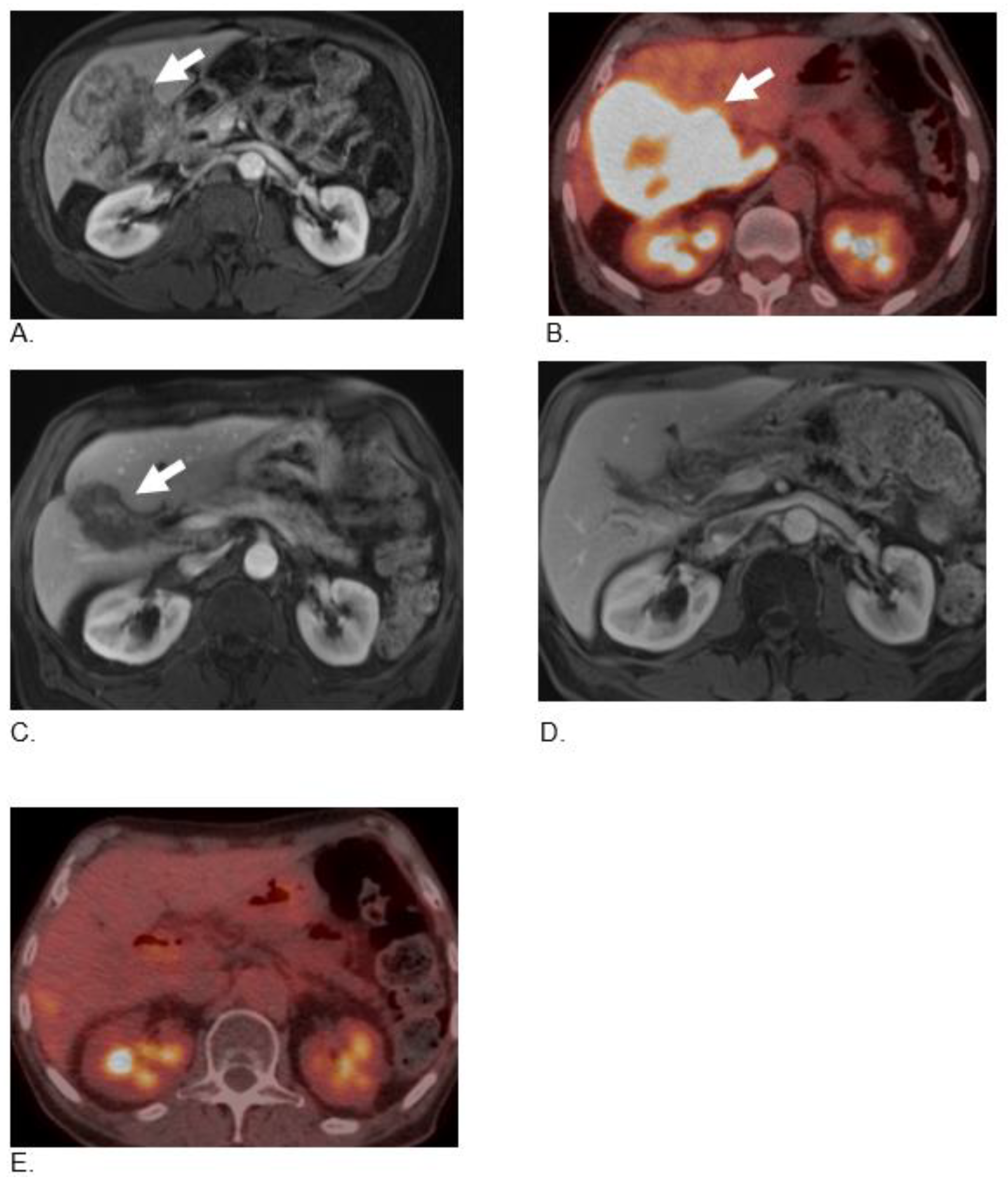
2.1. Case 1
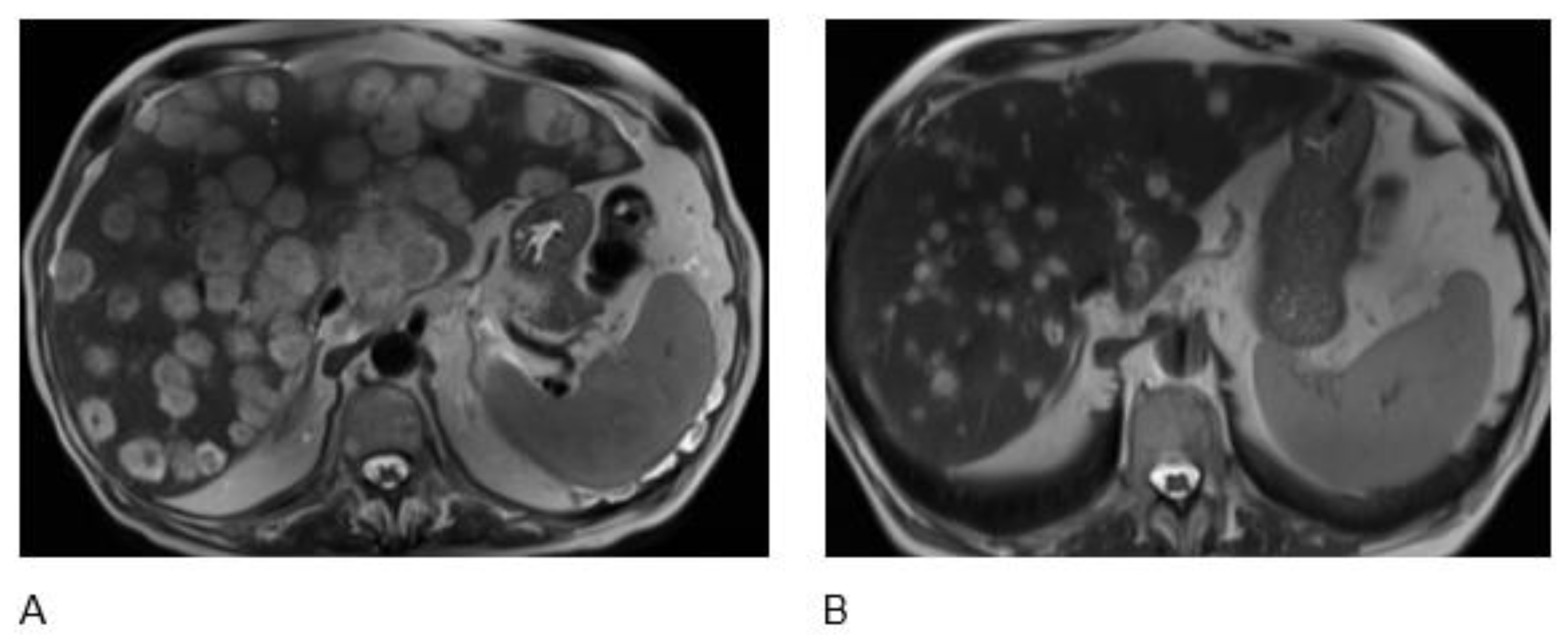
2.2. Case 2
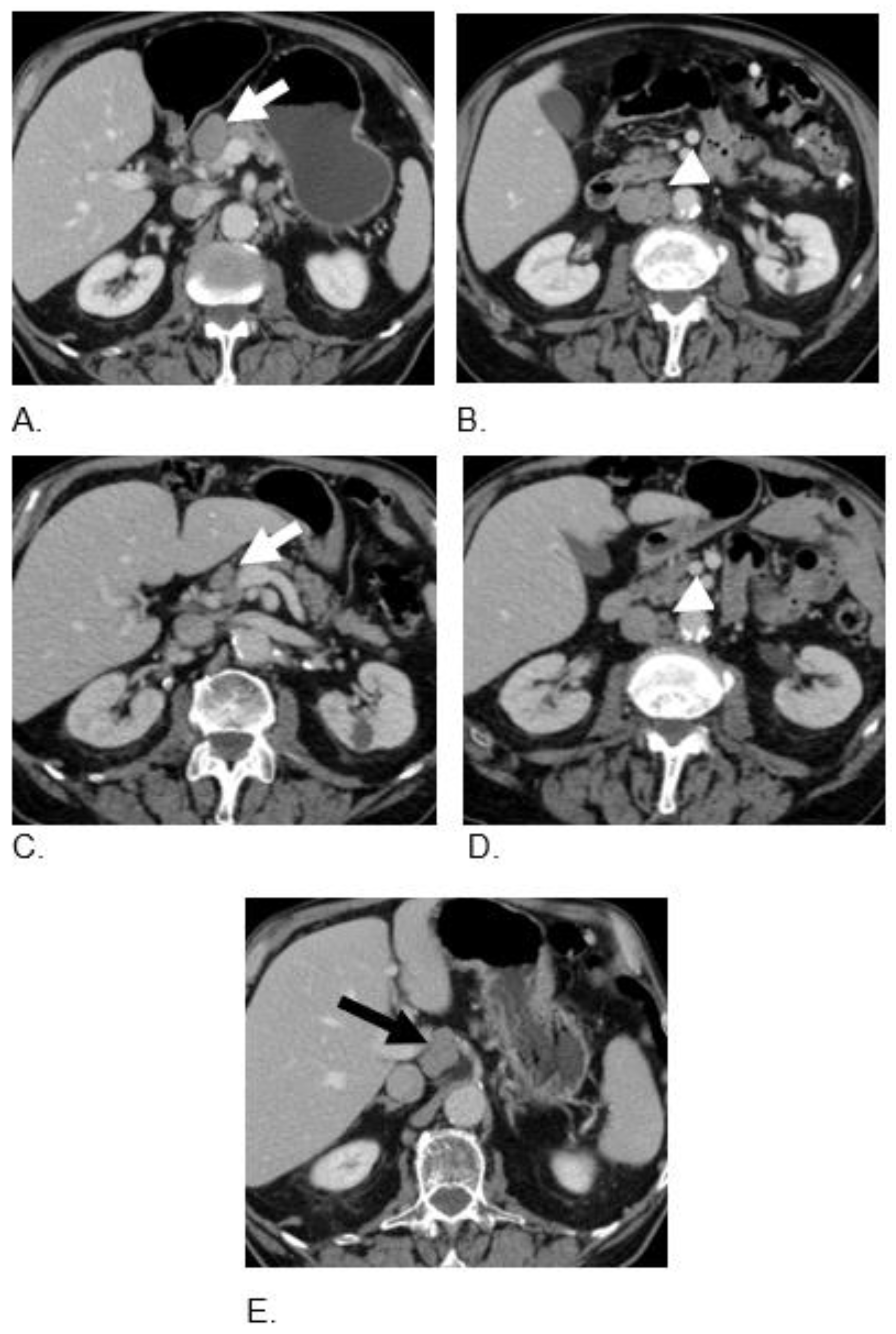
2.3. Case 3
2.4. Case 4
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TQ | Thymoquinone |
| NET | Neuroendocrine tumors |
| NEC | Neuroendocrine carcinoma |
| GEP | Gastroenteropancreatic |
| VEGF | Vascular endothelial growth factor |
| ERK | Extracellular-regulated kinase |
References
- Díez, M.; Teulé, A.; Salazar, R. Gastroenteropancreatic neuroendocrine tumors: Diagnosis and treatment. Ann. Gastroenterol. 2013, 26, 29–36. [Google Scholar] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.M.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendo-crine neoplasms: An international agency for research on cancer (iarc) and world health or-ganization (who) expert consensus. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Nasir, A.; Hodul, P.; Kvols, L. Biology and treatment of metastatic gastrointestinal neuroendocrine tumors. Gastrointest. Cancer Res. 2008, 2, 113–125. [Google Scholar]
- Patta, A.; Fakih, M. First-line cisplatin plus etoposide in high-grade metastatic neuroendocrine tumors of colon and rectum (MCRC NET): Review of 8 cases. Anticancer Res. 2011, 31, 975–978. [Google Scholar]
- Zhu, J.; Strosberg, J.R.; Dropkin, E.; Strickler, J. Treatment of High-Grade Metastatic Pancreatic Neuroendocrine Carcinoma with FOLFIRINOX. J. Gastrointest. Cancer 2015, 46, 166–169. [Google Scholar] [CrossRef]
- Oberg, K.; Casanovas, O.; Castaño, J.P.; Chung, D.; Delle Fave, G.; Denèfle, P.; Harris, P.; Khan, M.S.; Kulke, M.H.; Scarpa, A.; et al. Molecular pathogenesis of neuroendocrine tumors: Implications for current and fu-ture therapeutic approaches. Clin. Cancer Res. 2013, 19, 2842–2849. [Google Scholar] [CrossRef]
- Kuiper, P.; Hawinkels, L.J.; de Jonge-Muller, E.S.; Biemond, I.; Lamers, C.B.; Verspaget, H.W. Angiogenic markers endoglin and vascular endothelial growth factor in gastroen-teropancreatic neuroendocrine tumors. World. J. Gastroenterol. 2011, 17, 219–225. [Google Scholar] [CrossRef]
- Pavel, M.E.; Hassler, G.; Baum, U.; Hahn, E.G.; Lohmann, T.; Schuppan, D. Circulating levels of angiogenic cytokines can predict tumour progression and prognosis in neuroendocrine carcinomas. Clin. Endocrinol. 2005, 62, 434–443. [Google Scholar] [CrossRef]
- Trisciuoglio, D.; Iervolino, A.; Zupi, G.; Del Bufalo, D. Involvement of PI3K and MAPK Signaling in bcl-2-induced Vascular Endothelial Growth Factor Expression in Melanoma Cells. Mol. Biol. Cell 2005, 16, 4153–4162. [Google Scholar] [CrossRef]
- Mafficini, A.; Scarpa, A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Rev. 2019, 40, 506–536. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Al Baghdadi, T.; et al. A Phase II Basket Trial of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.R.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.L.; Nagrial, A.; et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and Anti-Inflammatory Properties of Nigella sativa Oil in Human Pre-Adipocytes. Antioxidants 2019, 8, 51. [Google Scholar] [CrossRef]
- Shoieb, A.M.; Elgayyar, M.; Dudrick, P.S.; Bell, J.L.; Tithof, P.K. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int. J. Oncol. 2003, 22, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Diab-Assaf, M.; Boltze, C.; Al-Hmaira, J.; Hartig, R.; Roessner, A.; Schneider-Stock, R. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int. J. Oncol. 2004, 25, 857–866. [Google Scholar]
- Kundu, J.; Choi, B.Y.; Jeong, C.H.; Kundu, J.K.; Chun, K.S. Thymoquinone induces apoptosis in human co-lon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2- and Src mediated phosphorylation of EGF receptor tyrosine kinase. Oncol. Rep. 2014, 32, 821–828. [Google Scholar] [CrossRef]
- El-Baba, C.; Mahadevan, V.; Fahlbusch, F.B.; Mohan, S.S.; Rau, T.T.; Gali-Muhtasib, H.; Schneider-Stock, R. Thymoquinone-induced conformational changes of PAK1 interrupt prosurvival MEK-ERK signal-ing in colorectal cancer. Mol. Cancer 2014, 13, 201. [Google Scholar] [CrossRef]
- Torres, M.P.; Ponnusamy, M.P.; Chakraborty, S.; Smith, L.M.; Das, S.; Arafat, H.A.; Batra, S.K. Effects of thymo-quinone in the expression of mucin 4 in pancreatic cancer cells: Implications for the development of novel cancer therapies. Mol. Cancer Ther. 2010, 9, 1419–1431. [Google Scholar] [CrossRef]
- Relles, D.; Chipitsyna, G.I.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Thymoquinone Promotes Pancreatic Cancer Cell Death and Reduction of Tumor Size through Combined Inhibition of Histone Deacetylation and Induction of Histone Acetylation. Adv. Prev. Med. 2016, 2016, 1407840. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Zhao, B.; Li, S.; Zhang, Y.; Pan, S.; Wu, Y.; Wang, J.; Wang, D.; Pan, H.; et al. Thymo-quinone induces G2/M arrest, inactivates PI3K/Akt and nuclear factor-κB pathways in human cholangiocarcinomas both in vitro and in vivo. Oncol. Rep. 2014, 31, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.Q.; Wang, J.; Guo, X.F.; Liu, Z.; Dong, W.G. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J. Gastroenterol. 2016, 22, 4149–4159. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.E.; Abd-Allah, A.R.; Korashy, H.M.; Attia, S.M.; Alzahrani, A.Z.; Saquib, Q.; Bakheet, S.A.; Abdel-Hamied, H.E.; Jamal, S.; Rishi, A.K. Thymoquinone suppression of the human hepatocellular carci-noma cell growth involves inhibition of IL-8 expression, elevated levels of TRAIL receptors, oxi-dative stress and apoptosis. Mol. Cell Biochem. 2014, 389, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Jehan, S.; Zhong, C.; Li, G.; Zulqarnain Bakhtiar, S.; Li, D.; Sui, G. Thymoquinone Selectively Induces Hepatocellular Carcinoma Cell Apoptosis in Synergism With Clinical Therapeutics and Depend-ence of p53 Status. Front. Pharmacol. 2020, 11, 555283. [Google Scholar] [CrossRef]
- Lang, M.; Borgmann, M.; Oberhuber, G.; Evstatiev, R.; Jimenez, K.; Dammann, K.W.; Jambrich, M.; Khare, V.; Campregher, C.; Ristl, R.; et al. Thymoquinone attenuates tumor growth in ApcMin mice by interference with Wnt-signaling. Mol. Cancer 2013, 12, 41. [Google Scholar] [CrossRef]
- Iwasa, S.; Morizane, C.; Okusaka, T.; Ueno, H.; Ikeda, M.; Kondo, S.; Tanaka, T.; Nakachi, K.; Mitsunaga, S.; Kojima, Y.; et al. Cisplatin and Etoposide as First-line Chemotherapy for Poorly Differentiated Neuroendocrine Carcinoma of the Hepatobiliary Tract and Pancreas. Jpn. J. Clin. Oncol. 2010, 40, 313–318. [Google Scholar] [CrossRef]
- Mitry, E.; Baudin, E.; Ducreux, M.; Sabourin, J.C.; Rufie, P.; Aparicio, T.; Lasser, P.; Elias, D.; Duvillard, P.; Schlumberger, M.; et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br. J. Cancer 1999, 81, 1351–1355. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Halfdanarson, T.; Gile, J.; Morse, B.; Sommerer, K.; Strosberg, J. Efficacy of ipilimumab and nivolumab in patients with high-grade neuroendocrine neoplasms. In Proceedings of the ASCO-Gastrointestinal Cancers Symposium, Virtual Meeting, 15–17 January 2021. [Google Scholar]
- Hauser, H.; Le, T.; Chou, J.F.; Heffernan, O.; DeMore, A.; Gonen, S.; Capanu, M.; Reidy, D.L.; Raj, N.P. Treatment response and clinical outcomes of neuro-endocrine neoplasms (NENs) treated with immune checkpoint inhibitors (ICIs): A single institu-tion experience. In Proceedings of the NANETs Annual Symposium, Virtual, 18 May 2021. [Google Scholar]
- Yi, T.; Cho, S.G.; Yi, Z.; Pang, X.; Rodriguez, M.; Wang, Y.; Sethi, G.; Aggarwal, B.B.; Liu, M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther. 2008, 7, 1789–1796. [Google Scholar] [CrossRef]
- Erdurmus, M.; Yagci, R.; Yilmaz, B.; Hepsen, I.F.; Turkmen, C.; Aydin, B.; Karadag, R. Inhibitory effects of topical thymoquinone on corneal neovascularization. Cornea 2007, 26, 715–719. [Google Scholar] [CrossRef]
- Xu, N.; Lao, Y.; Zhang, Y.; Gillespie, D.A. Akt: A double-edged sword in cell proliferation and genome sta-bility. J. Oncol. 2012, 2012, 951724. [Google Scholar] [CrossRef]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellu-lar localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Finley, S.D. ERK and Akt exhibit distinct signaling responses following stimulation by pro-angiogenic factors. Cell Commun. Signal. 2020, 18, 114. [Google Scholar] [CrossRef]
- Murphy, D.A.; Makonnen, S.; Lassoued, W.; Feldman, M.D.; Carter, C.; Lee, W.M. Inhibition of tumor endo-thelial ERK activation, angiogenesis, and tumor growth by sorafenib (BAY43-9006). Am. J. Pathol. 2006, 169, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 5, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Suzuki, H.; Fuchino, R.; Yamasaki, A.; Nagai, S.; Yanai, K.; Koga, K.; Nakamura, M.; Tanaka, M.; Morisaki, T.; et al. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 2009, 29, 881. [Google Scholar] [PubMed]
- Taylor, M.H.; Lee, C.H.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Re-nal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163. [Google Scholar] [CrossRef]
- Taylor, M.H.; Rasco, D.W.; Brose, M.S.; Vogelzang, N.J.; Richey, S.L.; Cohn, A.L.; Richards, D.A.; Stepan, D.E.; Dutcus, C.E.; Guo, M.; et al. A phase 1b/2 trial of lenvatinib plus pembrolizumab in patients with squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2018, 36, 6016. [Google Scholar] [CrossRef]
- Halperin, D.M.; Liu, S.; Dasari, A.; Fogelman, D.R.; Bhosale, P.; Mahvash, A.; Dervin, S.; Estrella, J.; Cortazar, P.; Maru, D.M.; et al. A phase II trial of atezolizumab and bevacizumab in patients with advanced, progressive neuroendocrine tumors (NETs). J. Clin. Oncol. 2020, 38, 619. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Fayyad, M.W. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: A comprehensive review. Int. Immunopharmacol. 2015, 28, 295–304. [Google Scholar] [CrossRef]
- Shabsoug, B.; Khalil, R.; Abuharfeil, N. Enhancement of natural killer cell activity in vitro against hu-man tumor cells by some plants from Jordan. J. Immunotoxicol. 2008, 5, 279–285. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Hmaidan, R.; Carr, R.I. Nigella sativa modulates splenocyte proliferation, Th1/Th2 cytokine profile, macrophage function and NK anti-tumor activity. J. Ethnopharmacol. 2010, 131, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L.; Alenzi, F.Q.; Attia, W.Y. Thymoquinone, the active ingredient of Nigella sativa seeds, en-hances survival and activity of antigen-specific CD8-positive T cells in vitro. Br. J. Biomed. Sci. 2011, 68, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Namazi, N.; Alizadeh, M.; Farajnia, S. Nigella sativa oil with a calorie-restricted diet can improve biomarkers of systemic inflammation in obese women: A randomized double-blind, pla-cebo-controlled clinical trial. J. Clin. Lipidol. 2016, 10, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Bamosa, A.O.; Kaatabi, H.; Lebdaa, F.M.; Elq, A.M.; Al-Sultanb, A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J. Physiol. Pharmacol. 2010, 54, 344–354. [Google Scholar]
- Bencheqroun, H.; Ahmed, Y.; Kocak, M.; Villa, E.; Barrera, C.; Mohiuddin, M.; Fortunet, R.; Iyoha, E.; Bates, D.; Okpalor, C.; et al. A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of ThymoQuinone Formula (TQF) for Treating Outpatient SARS-CoV. Pathogens 2022, 11, 551. [Google Scholar] [CrossRef]

| Study | Cancer Type | Model (Cell Lines/Mice) | Mechanism of Action of TQ |
|---|---|---|---|
| Kundu, J. [17] | Colorectal Cancer | HCT116 Cell lines |
|
| Gali-Muhtasib, H. [16] | Colorectal Cancer | HCT-116 Cell lines |
|
| El-Baba [18] | Colorectal Cancer | HCT116w, DLD-1, HT29 Cell lines |
|
| Torres, M.P. [19] | Pancreatic | FG/COLO357, CD18/HPAF Cell lines |
|
| Relles, D. [20] | Pancreatic | AsPC-1 and MiaPaCa-2 Cell lines and Xenografts |
|
| Xu, D. [21] | Cholangiocarcinoma | TFK-1, HuCCT1 Cell lines |
|
| Zhu, W.Q. [22] | Gastric Cancer | HGC27, BGC823, SGC7901 Cell lines |
|
| Ashour, A.E. [23] | Hepatocellular Carcinoma (HCC) | HepG2 Cell lines |
|
| Shah, J. [24] | Hepatocellular Carcinoma (HCC) | HepG2 and SMMC-7721 cell lines |
|
| Lang, M. [25] | Small Intestine | APCMin mice |
|
| Primary Tumor | Histological Differentiation | Patient Age at Diagnosis (Years) | Gender | First Line Treatment | Second Line Treatment | Maintenance Therapy | Outcome |
|---|---|---|---|---|---|---|---|
| Unknown Primary | EP Poorly differentiated neuroendocrine carcinoma (Small cell) | 77 | M | Carboplatin and Etoposide | Nivolumab 240 mg intravenous (i.v) every 2 weeks and Ipilimumab 1 mg/kg i.v. every 6 weeks for 4 cycles plus TQ-BSO (3 tablets 500 mg daily) | Nivolumab 240 mg i.v. every 2 weeks, plus BSO (TQ) tablets 1500 mg daily | Alive with PR and PFS of 24 mos |
| Gall Bladder | Poorly differentiated neuroendocrine carcinoma (Small cell) | 75 | M | Carboplatin and Etoposide | Nivolumab 3 mg/kg i.v and Ipilimumab 1 mg/kg i.v. every 3 weeks for 4 cycles plus TQ-BSO (3 tablets 500 mg daily) | Nivolumab 240 mg i.v. every 2 weeks, plus BSO (TQ) tablets 1500 mg daily | Alive with CR and PFS of 22 mos |
| Colon MiNEN | Mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN). | 67 | M | Carboplatin and Etoposide | Nivolumab 3 mg/kg i.v and Ipilimumab 1 mg/kg i.v. every 3 weeks for 4 cycles plus TQ-BSO (1 teaspoon oil formula daily) | Nivolumab 240 mg i.v. every 2 weeks plus BSO (TQ) tablets 1500 mg daily | Alive with CR and PFS of 10 mos |
| Colon MiNEN | Mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN). 70% small cell and 30% adenocarcinoma | 85 | M | Adjuvant mFOLFOX | Nivolumab 3 mg/kg i.v and Ipilimumab 1 mg/kg i.v. every 3 weeks for 3 cycles plus TQ-BSO (3 tablets 500 mg daily) | BSO (TQ) tablets 1500 mg daily | Alive with PR and PFS of 12 month |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.; Azmi, A.S.; Asa, S.L.; Tirumani, S.H.; Mahipal, A.; Cjakrabarti, S.; Bajor, D.; Selfridge, J.E.; Kaseb, A.O. Thymoquinone Plus Immunotherapy in Extra-Pulmonary Neuroendocrine Carcinoma: Case Series for a Novel Combination. Curr. Oncol. 2022, 29, 9018-9030. https://doi.org/10.3390/curroncol29110707
Mohamed A, Azmi AS, Asa SL, Tirumani SH, Mahipal A, Cjakrabarti S, Bajor D, Selfridge JE, Kaseb AO. Thymoquinone Plus Immunotherapy in Extra-Pulmonary Neuroendocrine Carcinoma: Case Series for a Novel Combination. Current Oncology. 2022; 29(11):9018-9030. https://doi.org/10.3390/curroncol29110707
Chicago/Turabian StyleMohamed, Amr, Asfar S. Azmi, Sylvia L. Asa, Sree Harsha Tirumani, Amit Mahipal, Sakti Cjakrabarti, David Bajor, J. Eva Selfridge, and Ahmed O. Kaseb. 2022. "Thymoquinone Plus Immunotherapy in Extra-Pulmonary Neuroendocrine Carcinoma: Case Series for a Novel Combination" Current Oncology 29, no. 11: 9018-9030. https://doi.org/10.3390/curroncol29110707
APA StyleMohamed, A., Azmi, A. S., Asa, S. L., Tirumani, S. H., Mahipal, A., Cjakrabarti, S., Bajor, D., Selfridge, J. E., & Kaseb, A. O. (2022). Thymoquinone Plus Immunotherapy in Extra-Pulmonary Neuroendocrine Carcinoma: Case Series for a Novel Combination. Current Oncology, 29(11), 9018-9030. https://doi.org/10.3390/curroncol29110707







