Effects of Serum Lipids on the Long-Term Prognosis of Ampullary Adenocarcinoma Patients after Curative Pancreatoduodenectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Covariates
2.3. Statistical Analysis
3. Results
3.1. Determination of Cutoff Values for Serum Lipids
3.2. Basic Characteristics of Patients
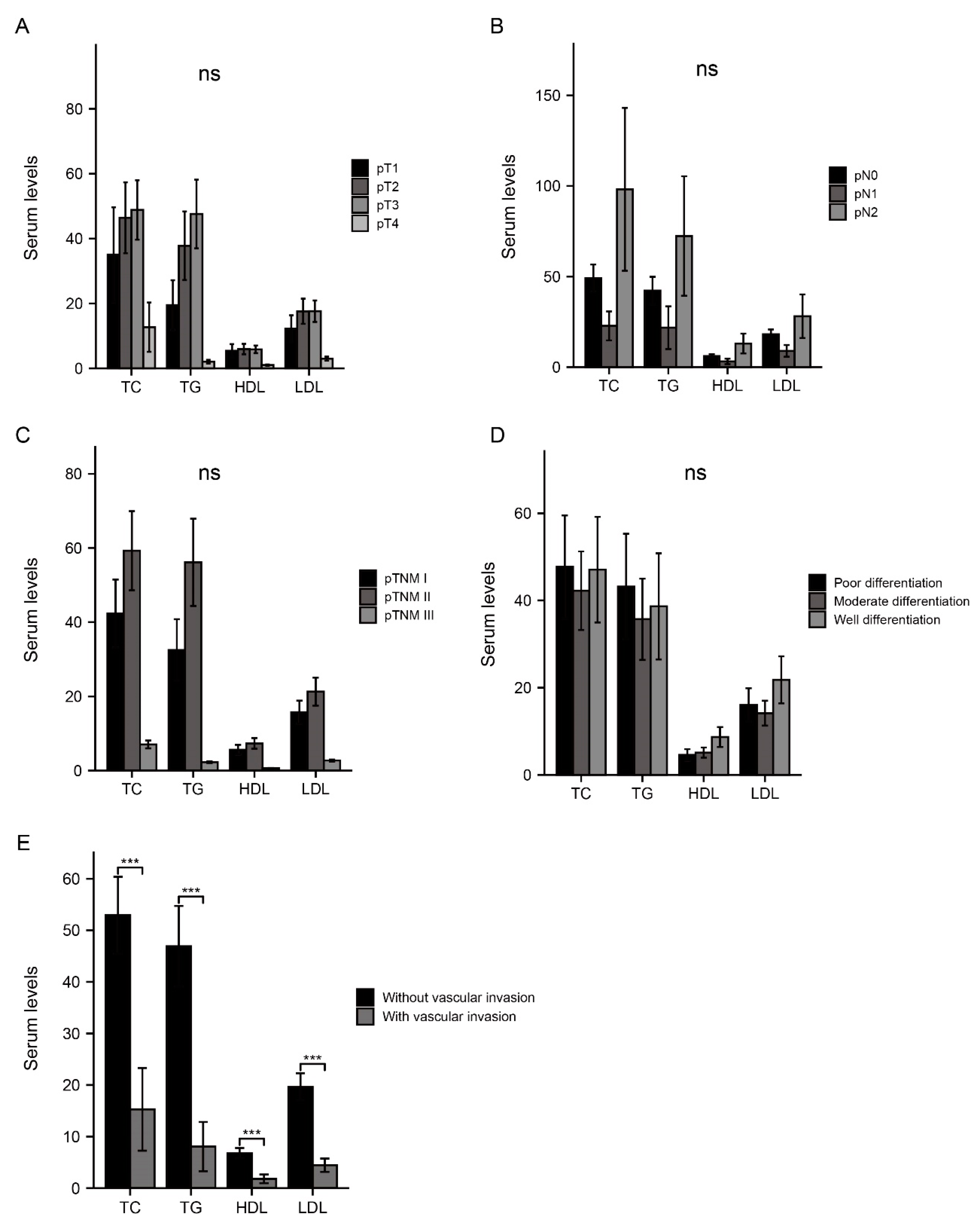
3.3. Association between Serum Lipids and Invasive Factors
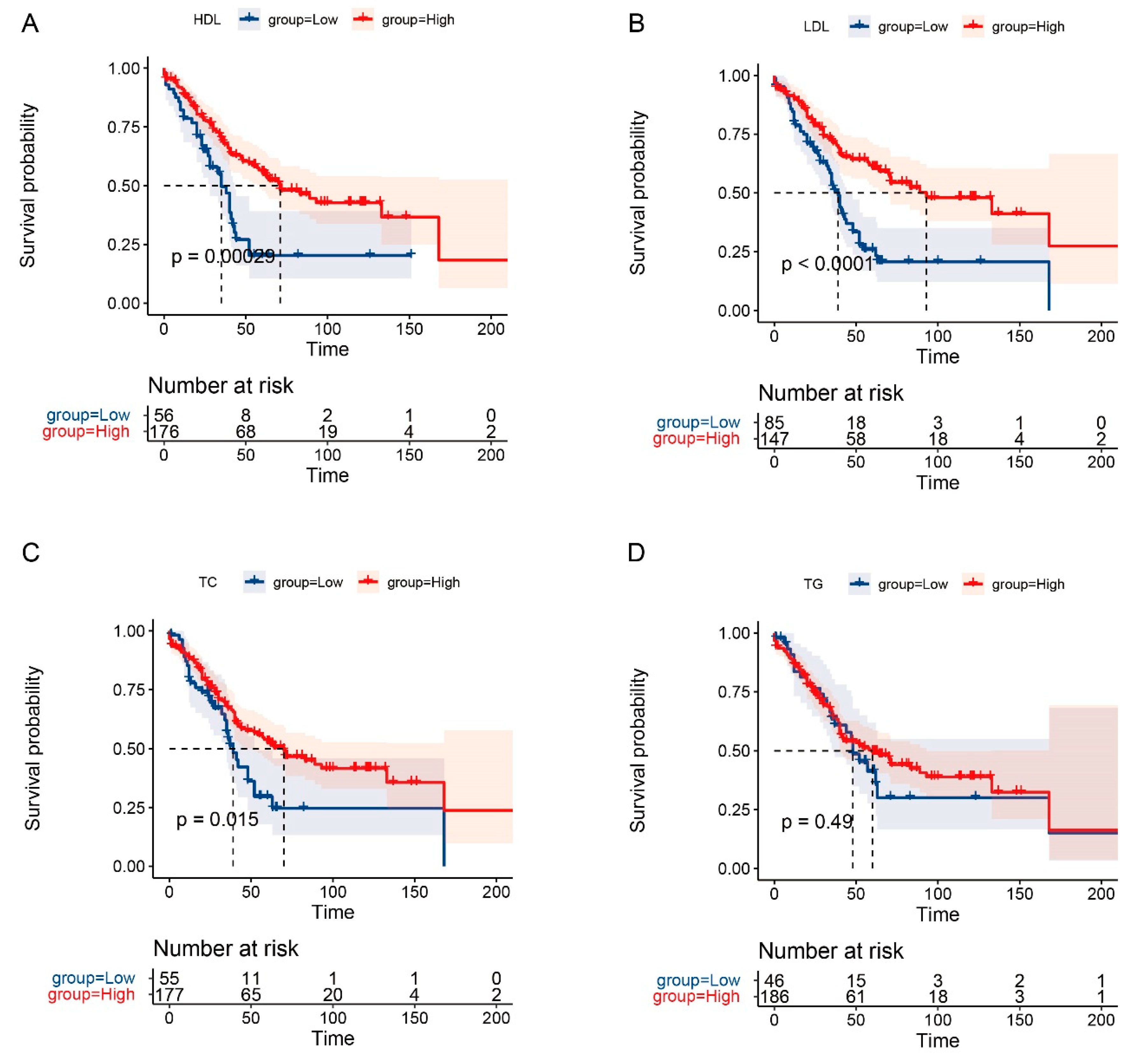
3.4. The Prognostic Role of Serum Lipids in OS and RFS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okano, K.; Oshima, M.; Yachida, S.; Kushida, Y.; Kato, K.; Kamada, H.; Wato, M.; Nishihira, T.; Fukuda, Y.; Maeba, T.; et al. Factors predicting survival and pathological subtype in patients with ampullary adenocarcinoma. J. Surg. Oncol. 2014, 110, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Dadduzio, V.; Lombardi, L.; Ricci, A.D.; Gadaleta-Caldarola, G. Ampullary Carcinoma: An Overview of a Rare Entity and Discussion of Current and Future Therapeutic Challenges. Curr. Oncol. 2021, 28, 3393–3402. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.H.; Bekaii-Saab, T. Ampullary cancer: An overview. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, 112–115. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Schwartz, A.M.; Batich, K.; Henson, D.E. Cancers of the ampulla of vater: Demographics, morphology, and survival based on 5625 cases from the SEER program. J. Surg. Oncol. 2009, 100, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. (Lond.) 2018, 38, 27. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2021, 218, e20201606. [Google Scholar] [CrossRef]
- Luo, X.; Cheng, C.; Tan, Z.; Li, N.; Tang, M.; Yang, L.; Cao, Y. Emerging roles of lipid metabolism in cancer metastasis. Mol. Cancer 2017, 16, 76. [Google Scholar] [CrossRef]
- Cao, Y. Adipocyte and lipid metabolism in cancer drug resistance. J. Clin. Investig. 2019, 129, 3006–3017. [Google Scholar] [CrossRef]
- Merino Salvador, M.; Gómez de Cedrón, M.; Moreno Rubio, J.; Falagán Martínez, S.; Sánchez Martínez, R.; Casado, E.; Ramírez de Molina, A.; Sereno, M. Lipid metabolism and lung cancer. Crit. Rev. Oncol. Hematol. 2017, 112, 31–40. [Google Scholar] [CrossRef]
- Alannan, M.; Fayyad-Kazan, H.; Trézéguet, V.; Merched, A. Targeting Lipid Metabolism in Liver Cancer. Biochemistry 2020, 59, 3951–3964. [Google Scholar] [CrossRef] [PubMed]
- Cedó, L.; Reddy, S.T.; Mato, E.; Blanco-Vaca, F.; Escolà-Gil, J.C. HDL and LDL: Potential New Players in Breast Cancer Development. J. Clin. Med. 2019, 8, 853. [Google Scholar] [CrossRef] [PubMed]
- Stoykova, G.E.; Schlaepfer, I.R. Lipid Metabolism and Endocrine Resistance in Prostate Cancer, and New Opportunities for Therapy. Int. J. Mol. Sci. 2019, 20, 2626. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Park, B.J.; Nam, J.H.; Kook, M.C. Effect of Helicobacter pylori eradication and high-density lipoprotein on the risk of de novo gastric cancer development. Gastrointest. Endosc. 2019, 90, 448–456.e1. [Google Scholar] [CrossRef]
- Pakiet, A.; Kobiela, J.; Stepnowski, P.; Sledzinski, T.; Mika, A. Changes in lipids composition and metabolism in colorectal cancer: A review. Lipids Health Dis. 2019, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Lin, Y.; Zhang, H.; Liu, C.; Cheng, Z.; Yang, X.; Zhang, J.; Xiao, Y.; Sang, N.; Qian, X.; et al. Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death Dis. 2020, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liu, W.; Xu, S.; Sun, L. Associations of preoperative serum high-density lipoprotein cholesterol and low-density lipoprotein cholesterol levels with the prognosis of ovarian cancer. Arch. Gynecol. Obstet. 2022, 305, 683–691. [Google Scholar] [CrossRef] [PubMed]
- SPSS: IBM Corp. IBM SPSS Statistics for Windows, Version 25.5; IBM Corp: Armonk, NY, USA, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 22 April 2022).
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Radišauskas, R.; Kuzmickienė, I.; Milinavičienė, E.; Everatt, R. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina (Kaunas Lithuania) 2016, 52, 89–98. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Berrington de González, A.; Freedman, N.D.; Huxley, R.; Mok, Y.; Jee, S.H.; Samet, J.M. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. 2011, 29, 1592–1598. [Google Scholar] [CrossRef]
- Van Duijnhoven, F.J.; Bueno-De-Mesquita, H.B.; Calligaro, M.; Jenab, M.; Pischon, T.; Jansen, E.H.; Frohlich, J.; Ayyobi, A.; Overvad, K.; Toft-Petersen, A.P.; et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut 2011, 60, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Törnberg, S.A.; Holm, L.E.; Carstensen, J.M. Breast cancer risk in relation to serum cholesterol, serum beta-lipoprotein, height, weight, and blood pressure. Acta Oncol. (Stockholm Sweden) 1988, 27, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Onwuka, J.U.; Okekunle, A.P.; Olutola, O.M.; Akpa, O.M.; Feng, R. Lipid profile and risk of ovarian tumours: A meta-analysis. BMC Cancer 2020, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Li, N.; Wang, G.; Feng, X.; Chen, S.; Su, K.; Li, F.; Wei, L.; Li, X.; Xie, S.; et al. Independent and joint associations of blood lipids and lipoproteins with lung cancer risk in Chinese males: A prospective cohort study. Int. J. Cancer 2019, 144, 2972–2984. [Google Scholar] [CrossRef]
- Lim, U.; Gayles, T.; Katki, H.A.; Stolzenberg-Solomon, R.; Weinstein, S.J.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Serum high-density lipoprotein cholesterol and risk of non-hodgkin lymphoma. Cancer Res. 2007, 67, 5569–5574. [Google Scholar] [CrossRef]
- Zhou, P.; Li, B.; Liu, B.; Chen, T.; Xiao, J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: A systematic review and meta-analysis. Clin. Chim. Acta 2018, 477, 94–104. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, T.; Luo, F.; Yang, Y.; Zhao, S.; Huang, Y.; Zhao, H.; Zhang, L.; Zhao, Y. Clinical Significance of Kinetics of Low-Density Lipoprotein Cholesterol and Its Prognostic Value in Limited Stage Small Cell Lung Cancer Patients. Cancer Control 2021, 28, 10732748211028257. [Google Scholar] [CrossRef]
- Zhou, T.; Zhan, J.; Fang, W.; Zhao, Y.; Yang, Y.; Hou, X.; Zhang, Z.; He, X.; Zhang, Y.; Huang, Y.; et al. Serum low-density lipoprotein and low-density lipoprotein expression level at diagnosis are favorable prognostic factors in patients with small-cell lung cancer (SCLC). BMC Cancer 2017, 17, 269. [Google Scholar] [CrossRef]
- Li, A.J.; Elmore, R.G.; Chen, I.Y.; Karlan, B.Y. Serum low-density lipoprotein levels correlate with survival in advanced stage epithelial ovarian cancers. Gynecol. Oncol. 2010, 116, 78–81. [Google Scholar] [CrossRef]
- Yuan, B.; Fu, J.; Yu, W.L.; Fu, X.H.; Qiu, Y.H.; Yin, L.; Zhu, B.; Zhang, Y.J. Prognostic value of serum high-density lipoprotein cholesterol in patients with gallbladder cancer. Rev. Esp. De Enferm. Dig. Organo Off. De La Soc. Esp. De Patol. Dig. 2019, 111, 839–845. [Google Scholar] [CrossRef]
- Zamanian-Daryoush, M.; Lindner, D.; Tallant, T.C.; Wang, Z.; Buffa, J.; Klipfell, E.; Parker, Y.; Hatala, D.; Parsons-Wingerter, P.; Rayman, P.; et al. The cardioprotective protein apolipoprotein A1 promotes potent anti-tumorigenic effects. J. Biol. Chem. 2013, 288, 21237–21252. [Google Scholar] [CrossRef] [PubMed]
- Smythies, L.E.; White, C.R.; Maheshwari, A.; Palgunachari, M.N.; Anantharamaiah, G.M.; Chaddha, M.; Kurundkar, A.R.; Datta, G. Apolipoprotein A-I mimetic 4F alters the function of human monocyte-derived macrophages. Am. J. Physiol. Cell Physiol. 2010, 298, C1538–C1548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.J.; Zhu, N.; Shi, Y.N.; Wang, Y.X.; Zhang, C.J.; Deng, C.F.; Liao, D.F.; Qin, L. Targeting HDL in tumor microenvironment: New hope for cancer therapy. J. Cell. Physiol. 2021, 236, 7853–7873. [Google Scholar] [CrossRef] [PubMed]
- Bursill, C.A.; Castro, M.L.; Beattie, D.T.; Nakhla, S.; van der Vorst, E.; Heather, A.K.; Barter, P.J.; Rye, K.A. High-density lipoproteins suppress chemokines and chemokine receptors in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1773–1778. [Google Scholar] [CrossRef]
- Pagès, G.; Berra, E.; Milanini, J.; Levy, A.P.; Pouysségur, J. Stress-activated protein kinases (JNK and p38/HOG) are essential for vascular endothelial growth factor mRNA stability. J. Biol. Chem. 2000, 275, 26484–26491. [Google Scholar] [CrossRef]
- Mazzuferi, G.; Bacchetti, T.; Islam, M.O.; Ferretti, G. High density lipoproteins and oxidative stress in breast cancer. Lipids Health Dis. 2021, 20, 143. [Google Scholar] [CrossRef]
- Pekkanen, J.; Nissinen, A.; Vartiainen, E.; Salonen, J.T.; Punsar, S.; Karvonen, M.J. Changes in serum cholesterol level and mortality: A 30-year follow-up. The Finnish cohorts of the seven countries study. Am. J. Epidemiol. 1994, 139, 155–165. [Google Scholar] [CrossRef]
- Jacobs, D.; Blackburn, H.; Higgins, M.; Reed, D.; Iso, H.; McMillan, G.; Neaton, J.; Nelson, J.; Potter, J.; Rifkind, B.; et al. Report of the Conference on Low Blood Cholesterol: Mortality Associations. Circulation 1992, 86, 1046–1060. [Google Scholar] [CrossRef]
- Stein, E.A.; Raal, F.J. Targeting LDL: Is lower better and is it safe. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 309–324. [Google Scholar] [CrossRef]
- Alsheikh-Ali, A.A.; Maddukuri, P.V.; Han, H.; Karas, R.H. Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: Insights from large randomized statin trials. J. Am. Coll. Cardiol. 2007, 50, 409–418. [Google Scholar] [CrossRef]

| Characteristics | Overall (N = 232) |
|---|---|
| Gender, n (%) | |
| Female | 95 (40.9%) |
| Male | 137 (59.1%) |
| Age, year | |
| Media (interquartile range) | 58 (49.75–65) |
| Tumor diameter, cm | |
| Mean (SD) | 2.40 (1.10) |
| Tumor differentiation, n (%) | |
| Poor differentiation | 78 (33.6%) |
| Moderate differentiation | 103 (44.4%) |
| Well differentiation | 51 (22.0%) |
| Lymph nodes resection, n (%) | |
| <17 | 163 (70.3%) |
| ≥17 | 69 (29.7%) |
| Vascular invasion, n (%) | |
| No | 184 (79.3%) |
| Yes | 48 (20.7%) |
| pT, n (%) | |
| T1 | 38 (16.4%) |
| T2 | 79 (34.1%) |
| T3 | 111 (47.8%) |
| T4 | 4 (1.7%) |
| pN, n (%) | |
| N0 | 169 (72.8%) |
| N1 | 53 (22.8%) |
| N2 | 10 (4.3%) |
| Stage, n (%) | |
| I | 101 (43.5%) |
| II | 101 (43.5%) |
| III | 30 (12.9%) |
| Postoperative complication, n (%) | |
| No | 141 (60.8%) |
| Yes | 91 (39.2%) |
| Postoperative adjuvant therapy, n (%) | |
| No | 173 (74.6%) |
| Yes | 59 (25.4%) |
| TC, n (%) | |
| <4.28 mmol/L | 55 (23.7%) |
| ≥4.28 mmol/L | 177 (76.3%) |
| TG, n (%) | |
| <1.11 mmol/L | 46 (19.8%) |
| ≥1.11 mmol/L | 186 (80.2%) |
| HDL-C, n (%) | |
| <0.39 mmol/L | 56 (24.1%) |
| ≥0.39 mmol/L | 176 (75.9%) |
| LDL-C, n (%) | |
| <2.56 mmol/L | 85 (36.6%) |
| ≥2.56 mmol/L | 147 (63.4%) |
| Complication | Overall (N = 91) |
|---|---|
| Abdominal infection | 11 (12.1%) |
| Abdominal infection and pancreatic fistula | 2 (2.2%) |
| Anastomotic leakage | 1 (1.1%) |
| Biliary/pancreatic fistula | 29 (31.9%) |
| Gastric emptying disorder | 20 (22.0%) |
| Hemorrhage | 17 (18.7%) |
| Hemorrhage and abdominal infection | 1 (1.1%) |
| Hemorrhage and pancreatic fistula | 1 (1.1%) |
| Hemorrhage, gastric emptying disorder, and abdominal infection | 1 (1.1%) |
| Intestinal fistula and abdominal infection | 1 (1.1%) |
| Lung infection | 5 (5.5%) |
| pancreatic fistula and incision fat liquefaction | 1 (1.1%) |
| Urinary system infection | 1 (1.1%) |
| Adjuvant Therapy Method | Overall (N = 59) |
|---|---|
| Chemotherapy | 48 (81.4%) |
| Combination of chemotherapy and radiation | 2 (3.4%) |
| Combination of chemotherapy and targeted therapy | 1 (1.7%) |
| Continuous hyperthermic peritoneal perfusion | 1 (1.7%) |
| Radiation | 4 (6.8%) |
| Unknown | 3 (5.1%) |
| Clavien–Dindo System for Comlication | Overall (N = 91) |
|---|---|
| Grade I | 1 (1.1%) |
| Grade II | 55 (60.4%) |
| Grade III | 29 (31.9%) |
| Grade IV | 6 (6.6%) |
| Variate | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | |
| Gender (male vs. female) | 1.08 | 0.74–1.58 | 0.683 | |||
| Age | 1.02 | 1–1.04 | 0.067 | |||
| Year of inclusion (after 2010 vs. before 2010) | 1.16 | 0.79–1.7 | 0.440 | |||
| Tumor diameter | 1.15 | 0.96–1.38 | 0.118 | |||
| Differentiation (well vs. non-well) | 0.61 | 0.38–0.99 | 0.044 | 1.02 | 0.46–2.24 | 0.965 |
| Lymph nodes resection (≥17 vs. <17) | 1.07 | 0.72–1.61 | 0.73 | |||
| Vascular invasion (yes vs. no) | 1.64 | 1.05–2.54 | 0.029 | 0.77 | 0.32–1.83 | 0.555 |
| pT (T3, T4 vs. T1, T2) | 2.19 | 1.5–3.19 | <0.001 | 1.71 | 0.88–3.34 | 0.116 |
| pN (N1, N2 vs. N0) | 2.28 | 1.54–3.39 | <0.001 | 1.6 | 0.55–2.5 | 0.682 |
| Postoperative complication (yes vs. no) | 1.31 | 0.9–1.92 | 0.157 | |||
| Clavien–Dindo system (grade III/IV vs. I/II) | 2.18 | 1.21–3.92 | 0.009 | 2.16 | 1.16–4.02 | 0.015 |
| Postoperative adjuvant therapy (yes vs. no) | 1.21 | 0.79–1.84 | 0.384 | |||
| TC (≥4.28 vs. <4.28) | 0.69 | 0.46–1.05 | 0.082 | |||
| TG (≥1.11 vs. <1.11) | 0.9 | 0.58–1.39 | 0.624 | |||
| HDL-C (≥0.39 vs. <0.39) | 0.52 | 0.35–0.78 | 0.002 | 1.2 | 0.59–2.45 | 0.620 |
| LDL-C (≥2.56 vs. <2.56) | 0.49 | 0.33–0.71 | <0.001 | 0.43 | 0.21–0.85 | 0.015 |
| Variate | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | |
| Gender (male vs. female) | 1.14 | 0.78–1.66 | 0.507 | |||
| Age | 1.02 | 1–1.04 | 0.031 | 1.03 | 1–1.06 | 0.084 |
| Year of inclusion (after 2010 vs. before 2010) | 1.22 | 0.84–1.79 | 0.296 | |||
| Tumor diameter | 1.1 | 0.92–1.31 | 0.288 | |||
| Differentiation (well vs. non-well) | 0.64 | 0.39–1.03 | 0.064 | |||
| Lymph nodes resection (≥17 vs. <17) | 1.15 | 0.77–1.72 | 0.508 | |||
| Vascular invasion (yes vs. no) | 1.53 | 0.99–2.38 | 0.057 | |||
| pT (T3, T4 vs. T1, T2) | 2.15 | 1.47–3.14 | <0.001 | 2.04 | 1.04–4.01 | 0.037 |
| pN (N1, N2 vs. N0) | 2.2 | 1.48–3.26 | <0.001 | 0.87 | 0.43–1.77 | 0.704 |
| Postoperative complication (yes vs. no) | 1.32 | 0.9–1.93 | 0.15 | |||
| Clavien–Dindo system (III/IV vs. I/II) | 2.13 | 1.19–3.81 | 0.011 | 2.14 | 1.16–3.95 | 0.015 |
| Postoperative adjuvant therapy (yes vs. no) | 1.16 | 0.76–1.76 | 0.502 | |||
| TC (≥4.28 vs. <4.28) | 0.6 | 0.39–0.91 | 0.016 | 0.54 | 0.25–1.17 | 0.116 |
| TG (≥1.11 vs. <1.11) | 0.86 | 0.55–1.33 | 0.495 | |||
| HDL-C (≥0.39 vs. <0.39) | 0.48 | 0.32–0.72 | <0.001 | 1.03 | 0.5–2.15 | 0.930 |
| LDL-C (≥2.56 vs. <2.56) | 0.44 | 0.3–0.65 | <0.001 | 0.54 | 0.24–1.2 | 0.129 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, X.; Sun, C.; Fei, H.; Li, Z.; Zhao, D. Effects of Serum Lipids on the Long-Term Prognosis of Ampullary Adenocarcinoma Patients after Curative Pancreatoduodenectomy. Curr. Oncol. 2022, 29, 9006-9017. https://doi.org/10.3390/curroncol29110706
Li Z, Zhang X, Sun C, Fei H, Li Z, Zhao D. Effects of Serum Lipids on the Long-Term Prognosis of Ampullary Adenocarcinoma Patients after Curative Pancreatoduodenectomy. Current Oncology. 2022; 29(11):9006-9017. https://doi.org/10.3390/curroncol29110706
Chicago/Turabian StyleLi, Zheng, Xiaojie Zhang, Chongyuan Sun, He Fei, Zefeng Li, and Dongbing Zhao. 2022. "Effects of Serum Lipids on the Long-Term Prognosis of Ampullary Adenocarcinoma Patients after Curative Pancreatoduodenectomy" Current Oncology 29, no. 11: 9006-9017. https://doi.org/10.3390/curroncol29110706
APA StyleLi, Z., Zhang, X., Sun, C., Fei, H., Li, Z., & Zhao, D. (2022). Effects of Serum Lipids on the Long-Term Prognosis of Ampullary Adenocarcinoma Patients after Curative Pancreatoduodenectomy. Current Oncology, 29(11), 9006-9017. https://doi.org/10.3390/curroncol29110706





