Reproductive Outcomes in Young Breast Cancer Survivors Treated (15–39) in Ontario, Canada
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
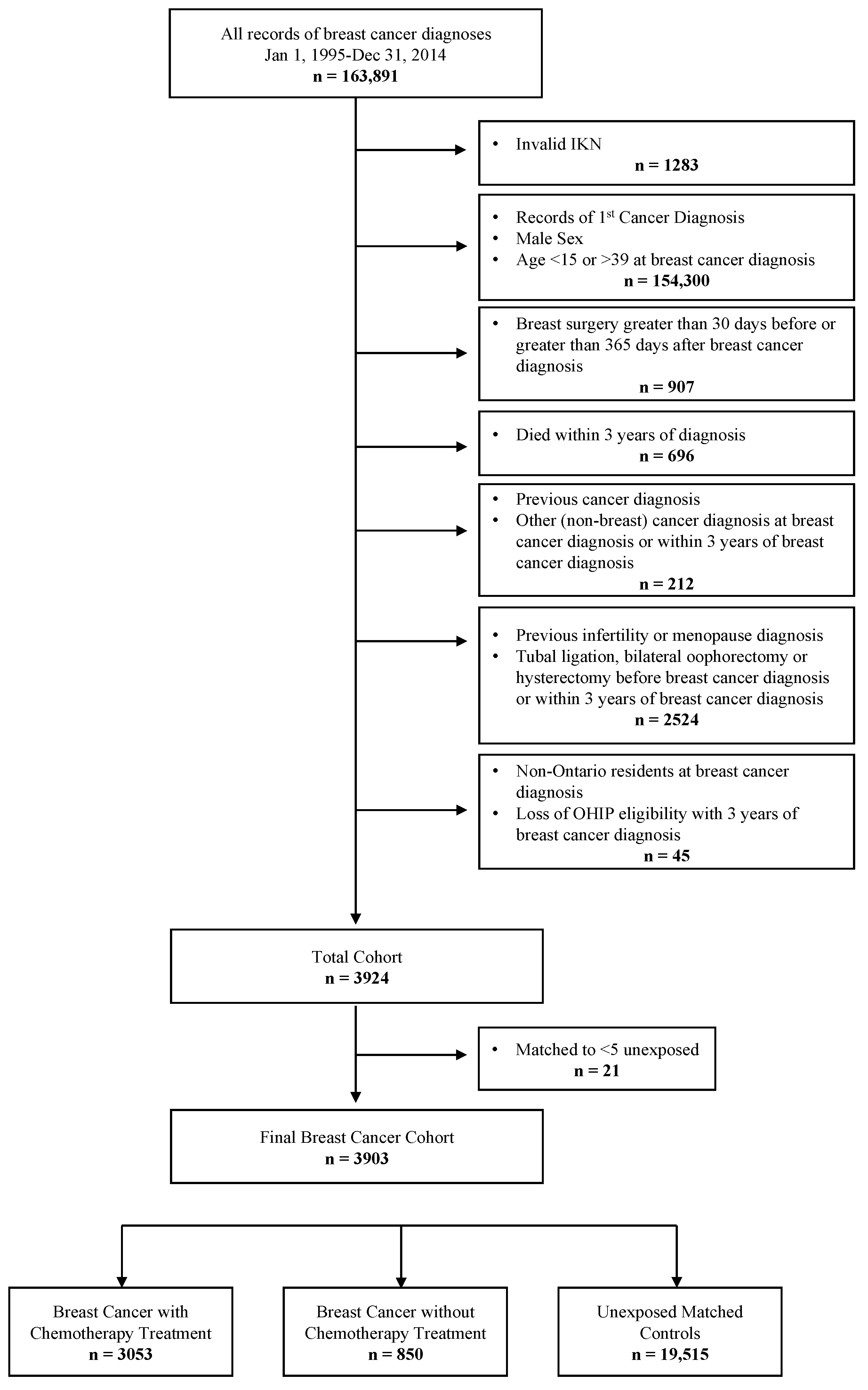
2.3. Cohort Creation
2.4. Covariates
2.5. Endpoints
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
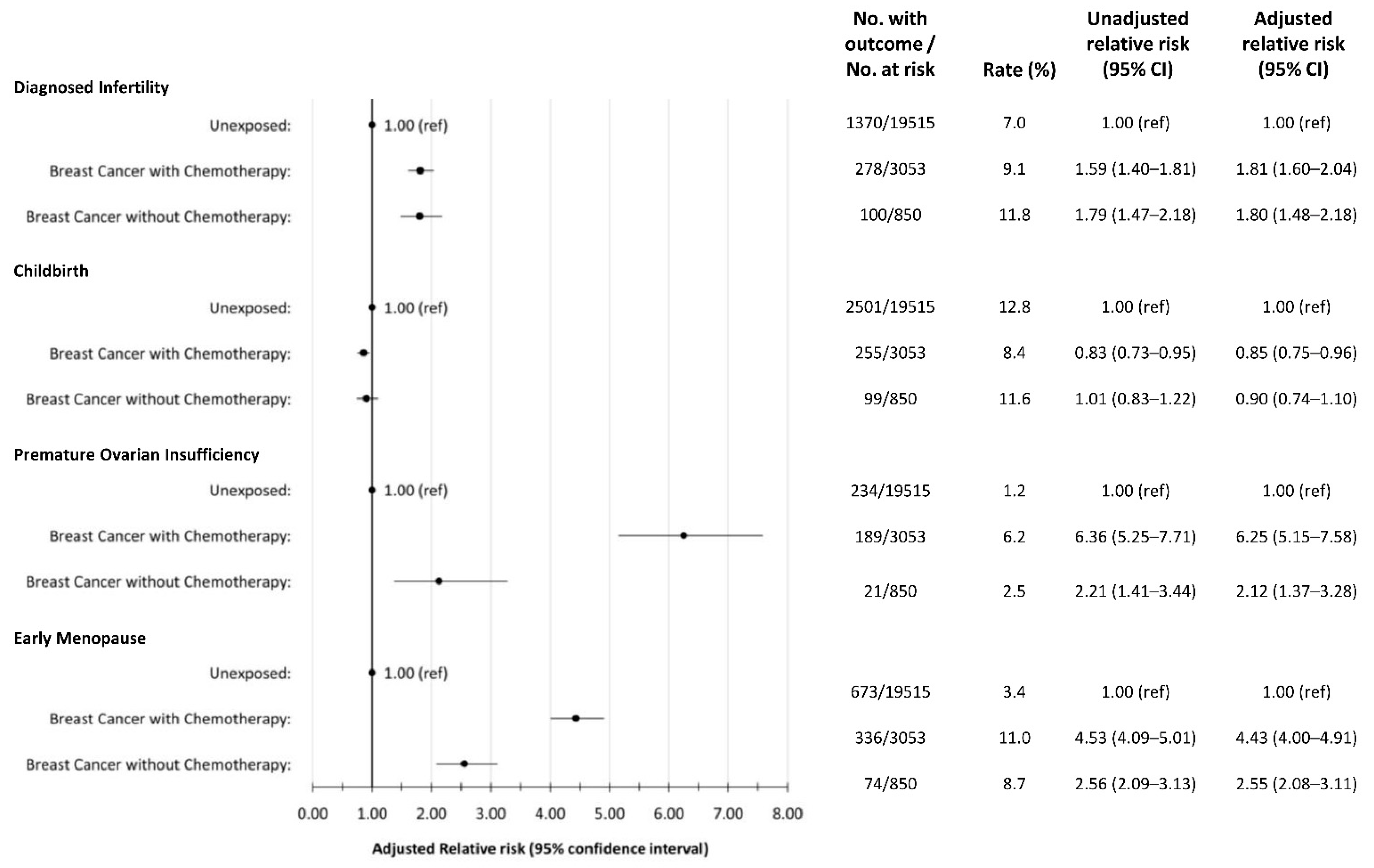
3.2. Infertility
3.3. Childbirth
3.4. Premature Ovarian Insufficiency and Early Menopause
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15–39). Available online: https://seer.cancer.gov/statfacts/html/aya.html (accessed on 2 March 2022).
- National Cancer Institute. Cancer Stat Facts: Female Breast Cancer. Available online: http://seer.cancer.gov/statfacts/html/breast.html (accessed on 2 March 2022).
- Canadian Partnership Against Cancer. Adolescents & Young Adults with Cancer: A System Performance Report. Toronto (ON): Canadian Partnership Against Cancer. 15 April 2017. Available online: https://s22457.pcdn.co/wp-content/uploads/2019/01/Adolescents-and-young-adults-with-cancer-EN.pdf (accessed on 14 April 2021).
- Hickey, M.; Peate, M.; Saunders, C.M.; Friedlander, M. Breast cancer in young women and its im-pact on reproductive function. Human reproduction update. Hum. Reprod. Update 2009, 15, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Breast.PDF [Internet]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 24 October 2021).
- Henry, N.L.; Somerfield, M.R.; Abramson, V.G.; Allison, K.H.; Anders, C.K.; Chingos, D.T.; Hurria, A.; Openshaw, T.H.; Krop, I.E. Role of patient and disease factors in adjuvant systemic therapy decision making for early-stage, operable breast cancer: American Society of Clinical Oncology endorsement of Cancer Care Ontario guide-line recommendations. Am. J. Clin. Oncol. 2016, 34, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.A.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clini-cal practice guideline focused update. J. Clin. Oncol. 2019, 37, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Ciruelos, E.; Burstein, H.J.; et al. Tai-loring adjuvant endocrine therapy for premenopausal breast cancer. NEJM 2018, 379, 122–137. [Google Scholar] [CrossRef]
- Velez, M.P.; Richardson, H.; Baxter, N.N.; McClintock, C.; Greenblatt, E.; Barr, R.; Green, M. Risk of infertility in female adolescents and young adults with cancer: A population-based cohort study. Hum. Reprod. 2021, 36, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, A.; Pudwell, J.; McClintock, C.; Velez, M.P. Premature ovarian insufficiency in female adolescent and young adult survivors of cancer: A population-based cohort study. J. Obstet. Gynaecol. Can. 2021, 43, 658. [Google Scholar] [CrossRef]
- Imai, A.; Furui, T. Chemotherapy-induced female infertility and protective action of gonadotro-pin-releasing hormone analogues. JOG 2007, 27, 20–24. [Google Scholar]
- Levine, J.M.; Kelvin, J.F.; Quinn, G.; Gracia, C.R. Infertility in reproductive-age female cancer survivors. Cancer 2015, 121, 1532–1539. [Google Scholar] [CrossRef]
- Partridge, A.H.; Ruddy, K.J. Fertility and adjuvant treatment in young women with breast cancer. Breast 2007, 16, 175–181. [Google Scholar] [CrossRef]
- Morarji, K.; McArdle, O.; Hui, K.; Gingras-Hill, G.; Ahmed, S.; Greenblatt, E.M.; Warner, E.; Sridhar, S.; Ali, A.M.F.; Azad, A.; et al. Ovarian Function after Chemotherapy in Young Breast Cancer Survivors. Curr. Oncol. 2017, 24, 494–502. [Google Scholar] [CrossRef]
- Del Mastro, L.; Catzeddu, T.; Venturini, M. Infertility and pregnancy after breast cancer: Current knowledge and future perspectives. Cancer. Treat. Rev. 2006, 32, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor–Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J. Clin. Oncol. 2016, 34, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Marton, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K. Births: Final report from 2019. CDC Natl. Rep. Vital Stat. 2021, 70, 4. [Google Scholar]
- Austin, P.C. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Be-tween Two Groups in Observational Research, Commun. Stat. 2009, 38, 1228–1234. [Google Scholar]
- Murphy, B.L.; Day, C.N.; Hoskin, T.L.; Habermann, E.B.; Boughey, J.C. Adolescents and Young Adults with Breast Cancer have More Aggressive Disease and Treatment Than Patients in Their Forties. Ann. Surg. Oncol. 2019, 26, 3920–3930. [Google Scholar] [CrossRef]
- Trivers, K.F.; Fink, A.K.; Partridge, A.H.; Oktay, K.; Ginsburg, E.S.; Li, C.; Pollack, L.A. Estimates of Young Breast Cancer Survivors at Risk for Infertility in the U.S. Oncol. 2014, 19, 814–822. [Google Scholar] [CrossRef]
- Oktay, K.; Harvey, B.; Partridge, A.H.; Quinn, G.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Korkidakis, A.; Lajkosz, K.; Green, M.; Strobino, D.; Velez, M.P. Patterns of Referral for Fertility Preservation Among Female Adolescents and Young Adults with Breast Cancer: A Population-Based Study. J. Adolesc. Young- Adult. Oncol. 2019, 8, 197–204. [Google Scholar] [CrossRef]
- Stensheim, H.; Cvancarova, M.; Møller, B.; Fosså, S.D. Pregnancy after adolescent and adult cancer: A population-based matched cohort study. Int. J. Cancer 2011, 129, 1225–1236. [Google Scholar] [CrossRef]
- Lambertini, M.; Blondeaux, E.; Bruzzone, M.; Perachino, M.; Anderson, R.A.; de Azambuja, E.; Poorvu, P.D.; Kim, H.J.; Villarreal-Garza, C.; Pistilli, B.; et al. Pregnancy After Breast Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2021, 39, 3293–3305. [Google Scholar] [CrossRef]
- Statistics Canada: Crude Birth Rate, Age-Specific Fertility Rates and Total Fertility Rates (Live Births). Available online: https://www150.statcan.gc.ca/n1/en/catalogue/13100418 (accessed on 14 April 2021).
- Gargus, E.; Deans, R.; Anazodo, A.; Woodruff, T.K. Management of Primary Ovarian Insufficiency Symptoms in Survivors of Childhood and Adolescent Cancer. J. Natl. Compr. Cancer. Netw. 2018, 16, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- den Brok, W.D.; Schrader, K.A.; Sun, S.; Tinker, A.V.; Zhao, E.Y.; Aparicio, S.; Gelmon, K.A. Ho-mologous recombination deficiency in breast cancer: A clinical review. JCO Precis. Oncol. 2017, 1, 1–13. [Google Scholar] [PubMed]
- Miao, Y.; Wang, P.; Xie, B.; Yang, M.; Li, S.; Cui, Z.; Fan, Y.; Li, M.; Xiong, B. BRCA2 deficiency is a potential driver for human primary ovarian insufficiency. Cell. Death. Dis. 2019, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.K. Oncofertility: A grand collaboration between reproductive medicine and oncolo-gy. Reproduction 2015, 150, S1–S10. [Google Scholar] [CrossRef]
| Variable | Value | Chemo No N = 850 | Chemo Yes N = 3053 | All BC combined N = 3903 | Non-Cancer Group N = 19,515 | Std Difference * |
|---|---|---|---|---|---|---|
| Age at surgery | Mean (SD) | 35.0 (4.1) | 35.0 (3.7) | 35.0 (3.8) | 35.0 (3.9) | 0 |
| Median (Q1–Q3) | 36 (33–38) | 36 (33–38) | 36 (33–38) | 36 (33–38) | 0 | |
| Min–Max | 15–40 | 18–40 | 15–40 | 15–41 | ||
| Age group | 15–24 - n (%) | 18 (2.1%) | 38 (1.2%) | 56 (1.4%) | 302 (1.5%) | 0.01 |
| 25–29 - n (%) | 78 (9.2%) | 265 (8.7%) | 343 (8.8%) | 1705 (8.7%) | 0 | |
| 30–34 - n (%) | 195 (22.9%) | 829 (27.2%) | 1024 (26.2%) | 5151 (26.4%) | 0 | |
| 35–41 - n (%) | 559 (65.8%) | 1921 (62.9%) | 2480 (63.5%) | 12,357 (63.3%) | 0 | |
| Parity | Nulliparous - n (%) | 500 (58.8%) | 1444 (47.3%) | 1944 (49.8%) | 11,777 (60.3%) | 0.21 |
| Parous - n (%) | 350 (41.2%) | 1609 (52.7%) | 1959 (50.2%) | 7738 (39.7%) | 0.21 | |
| Neighbourhood income quintile | 1 - Lowest quintile - n (%) | 147 (17.3%) | 520 (17.0%) | 667 (17.1%) | 3944 (20.2%) | 0.08 |
| 2 - n (%) | 154 (18.1%) | 593 (19.4%) | 747 (19.1%) | 3780 (19.4%) | 0.01 | |
| 3 - n (%) | 195 (22.9%) | 657 (21.5%) | 852 (21.8%) | 3807 (19.5%) | 0.06 | |
| 4 - n (%) | 173 (20.4%) | 652 (21.4%) | 825 (21.1%) | 4134 (21.2%) | 0 | |
| 5 - Highest quintile - n (%) | 181 (21.3%) | 631 (20.7%) | 812 (20.8%) | 3850 (19.7%) | 0.03 | |
| Immigrant | No - n (%) | 663 (78.0%) | 2391 (78.3%) | 3054 (78.2%) | 14,319 (73.4%) | 0.11 |
| Yes - n (%) | 187 (22.0%) | 662 (21.7%) | 849 (21.8%) | 5196 (26.6%) | 0.11 | |
| Rurality | Rural - n (%) | 811 (95.4%) | 2903 (95.1%) | 3714 (95.2%) | 18583 (95.2%) | 0 |
| Urban - n (%) | 39 (4.6%) | 150 (4.9%) | 189 (4.8%) | 932 (4.8%) | 0 | |
| Prior Endometriosis | No - n (%) | 835 (98.2%) | 2993 (98.0%) | 3828 (98.1%) | 19,261 (98.7%) | 0.05 |
| Yes - n (%) | 15 (1.8%) | 60 (2.0%) | 75 (1.9%) | 254 (1.3%) | 0.05 | |
| Prior PCOS | No - n (%) | 829 (97.5%) | 3010 (98.6%) | 3839 (98.4%) | 19,282 (98.8%) | 0.04 |
| Yes - n (%) | 21 (2.5%) | 43 (1.4%) | 64 (1.6%) | 233 (1.2%) | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rushton, M.; Pudwell, J.; Wei, X.; Powell, M.; Richardson, H.; Velez, M.P. Reproductive Outcomes in Young Breast Cancer Survivors Treated (15–39) in Ontario, Canada. Curr. Oncol. 2022, 29, 8591-8599. https://doi.org/10.3390/curroncol29110677
Rushton M, Pudwell J, Wei X, Powell M, Richardson H, Velez MP. Reproductive Outcomes in Young Breast Cancer Survivors Treated (15–39) in Ontario, Canada. Current Oncology. 2022; 29(11):8591-8599. https://doi.org/10.3390/curroncol29110677
Chicago/Turabian StyleRushton, Moira, Jessica Pudwell, Xuejiao Wei, Madeleine Powell, Harriet Richardson, and Maria P. Velez. 2022. "Reproductive Outcomes in Young Breast Cancer Survivors Treated (15–39) in Ontario, Canada" Current Oncology 29, no. 11: 8591-8599. https://doi.org/10.3390/curroncol29110677
APA StyleRushton, M., Pudwell, J., Wei, X., Powell, M., Richardson, H., & Velez, M. P. (2022). Reproductive Outcomes in Young Breast Cancer Survivors Treated (15–39) in Ontario, Canada. Current Oncology, 29(11), 8591-8599. https://doi.org/10.3390/curroncol29110677





