Nicotine-Free E-Cigarettes Might Promote Tobacco Smoking Reduction Better Than Nicotine Delivery Devices: Results of a Double-Blind Randomized Controlled Trial at 1 Year
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Design
2.2. Procedure
- -
- To be involved in the COSMOS II study;
- -
- To be chronic smokers (have smoked at least ten cigarettes a day for the past 10 years);
- -
- To have a motivational score higher than 10 and not be treated at a smoking center.
- -
- Symptomatic cardiovascular disease;
- -
- Symptomatic severe respiratory disease;
- -
- Regular psychotropic medication use;
- -
- Current or past history of alcohol abuse;
- -
- Use of smokeless tobacco or NRT.
2.3. Instruments
2.4. Statistical Analysis
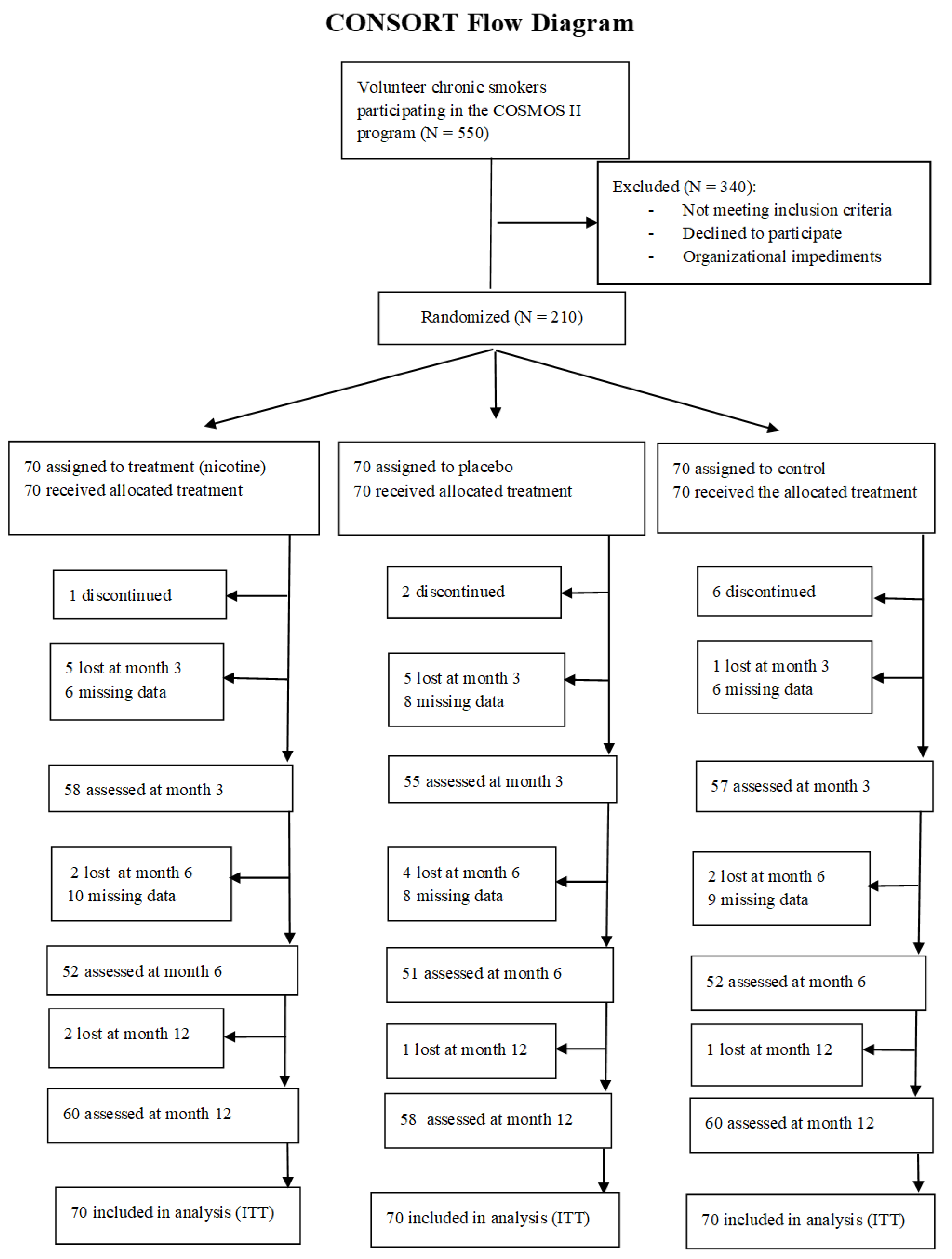
3. Results
3.1. Sample Characteristics
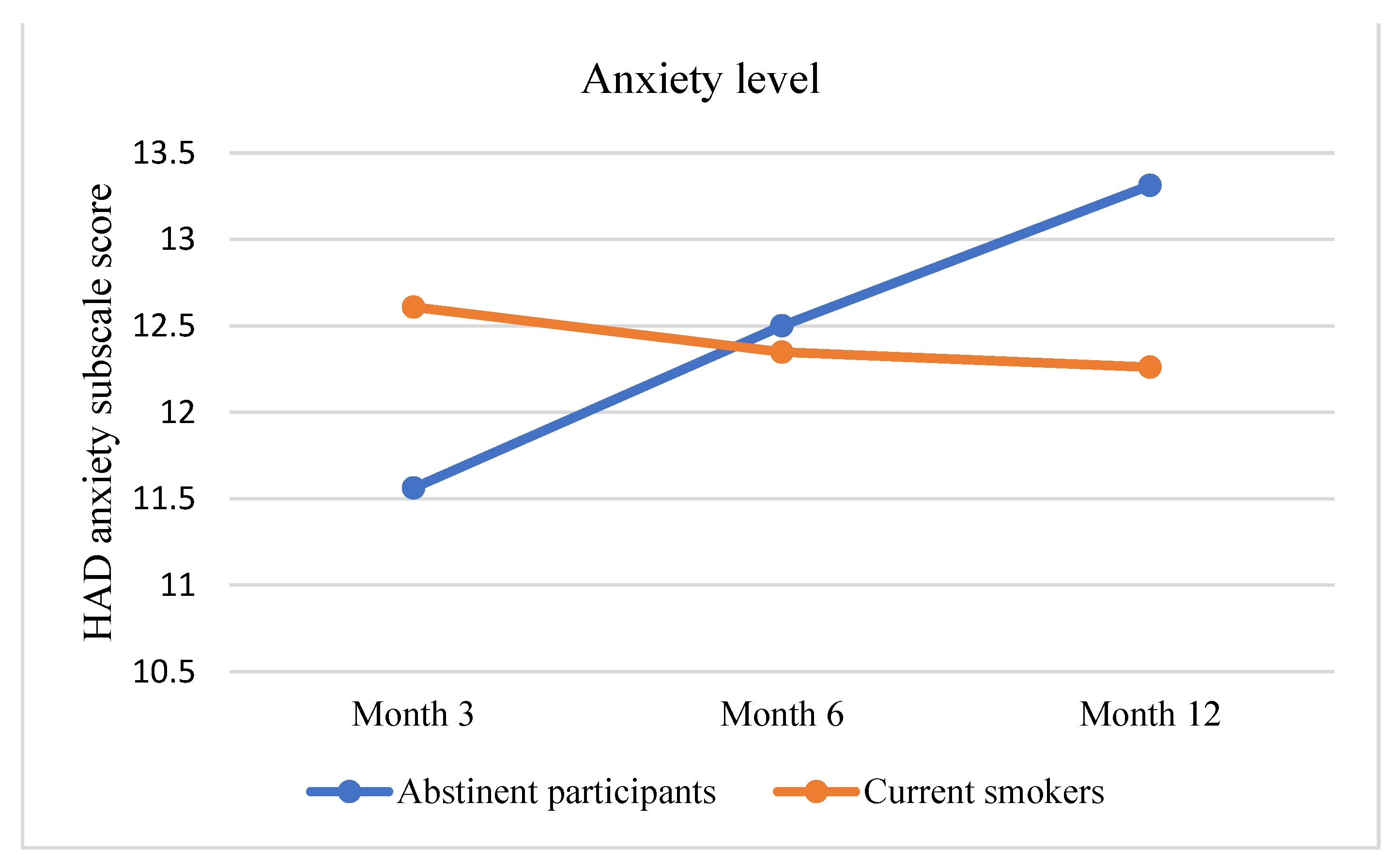
3.2. Primary Outcome Assessment: Improvement in Pulmonary Health
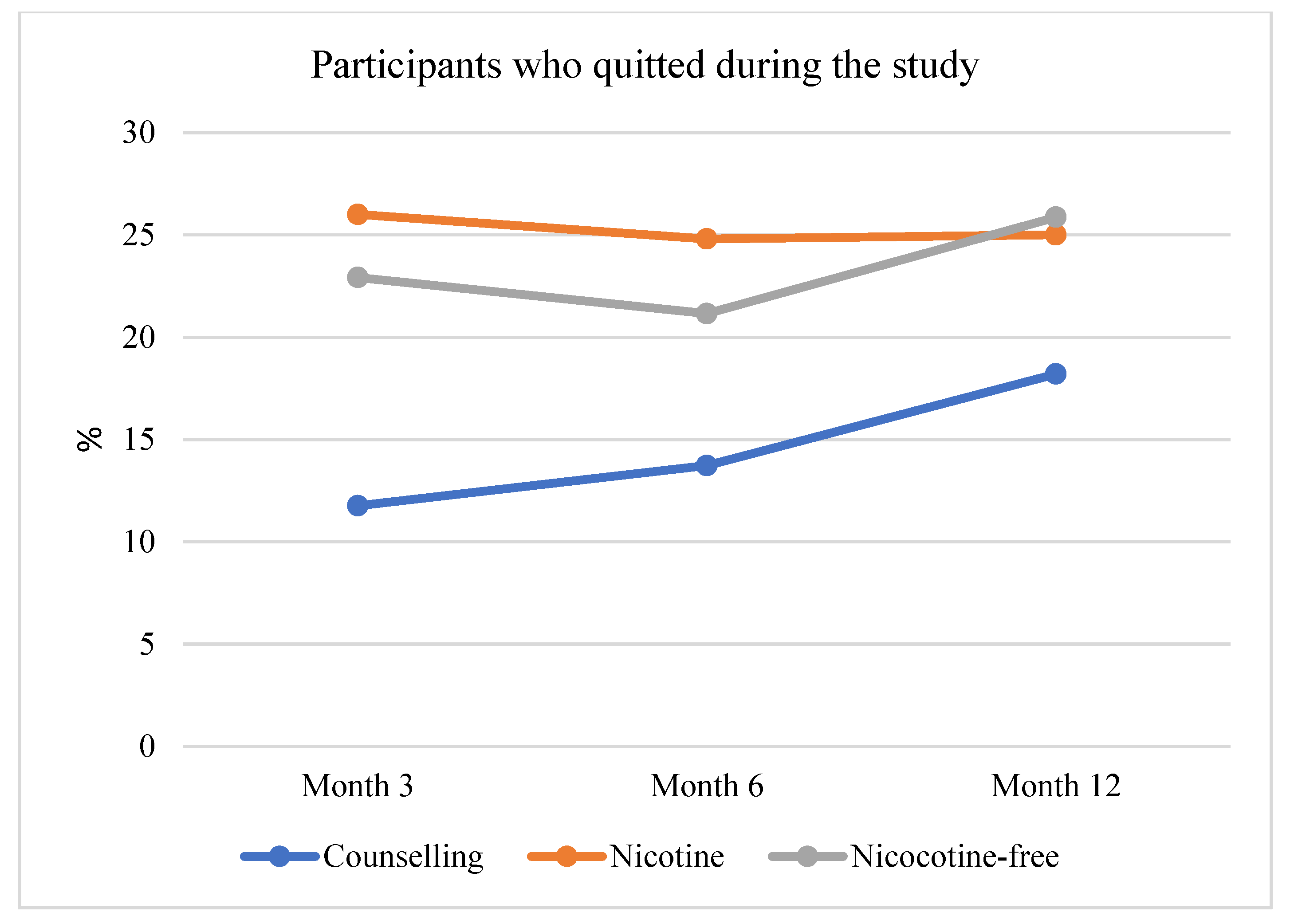
3.3. Secondary Outcome: Reduction of Tobacco Smoking
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farsalinos, K.E.; Niaura, R. E-cigarettes and Smoking Cessation in the United States According to Frequency of E-cigarette Use and Quitting Duration: Analysis of the 2016 and 2017 National Health Interview Surveys. Nicotine Tob. Res. 2020, 22, 655–662. [Google Scholar] [CrossRef]
- Pierce, J.P.; Benmarhnia, T.; Chen, R.; White, M.; Abrams, D.B.; Ambrose, B.K.; Blanco, C.; Borek, N.; Choi, K.; Coleman, B.; et al. Role of e-cigarettes and pharmacotherapy during attempts to quit cigarette smoking: The PATH Study 2013–16. PLoS ONE 2020, 15, e0237938. [Google Scholar] [CrossRef]
- Masiero, M.; Lucchiari, C.; Mazzocco, K.; Veronesi, G.; Maisonneuve, P.; Jemos, C.; Salè, E.O.; Spina, S.; Bertolotti, R.; Pravettoni, G. E-cigarettes May Support Smokers with High Smoking-Related Risk Awareness to Stop Smoking in the Short Run: Preliminary Results by Randomized Controlled Trial. Nicotine Tob. Res. 2019, 21, 119–126. [Google Scholar] [CrossRef]
- Rigotti, N.A. Randomized Trials of e-Cigarettes for Smoking Cessation. JAMA—J. Am. Med. Assoc. 2020, 324, 1835–1837. [Google Scholar] [CrossRef]
- Wills, T.A.; Pagano, I.; Williams, R.J.; Tam, E.K. E-cigarette use and respiratory disorder in an adult sample. Drug Alcohol Depend. 2019, 194, 363–370. [Google Scholar] [CrossRef]
- Hartmann-Boyce, J.; McRobbie, H.; Lindson, N.; Bullen, C.; Begh, R.; Theodoulou, A.; Notley, C.; Rigotti, N.A.; Turner, T.; Butler, A.R.; et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2020, 10, CD010216. [Google Scholar] [CrossRef]
- Chun, L.F.; Moazed, F.; Calfee, C.S.; Matthay, M.A.; Gotts, J.E. Pulmonary toxicity of e-cigarettes. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2017, 313, L193–L206. [Google Scholar] [CrossRef]
- Grabovac, I.; Oberndorfer, M.; Fischer, J.; Wiesinger, W.; Haider, S.; Dorner, T.E. Effectiveness of Electronic Cigarettes in Smoking Cessation: A Systematic Review and Meta-analysis. Nicotine Tob. Res. 2020, 23, 625–634. [Google Scholar] [CrossRef]
- Walker, N.; Parag, V.; Verbiest, M.; Laking, G.; Laugesen, M.; Bullen, C. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: A pragmatic, randomised trial. Lancet Respir. Med. 2020, 8, 54–64. [Google Scholar] [CrossRef]
- Hajek, P.; Phillips-Waller, A.; Przulj, D.; Pesola, F.; Smith, K.M.; Bisal, N.; Li, J.; Parrott, S.; Sasieni, P.; Dawkins, L.; et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N. Engl. J. Med. 2019, 380, 629–637. [Google Scholar] [CrossRef]
- Benmarhnia, T.; Pierce, J.P.; Leas, E.; White, M.M.; Strong, D.R.; Noble, M.L.; Trinidad, D.R. Can E-Cigarettes and Pharmaceutical Aids Increase Smoking Cessation and Reduce Cigarette Consumption? Findings from a Nationally Representative Cohort of American Smokers. Am. J. Epidemiol. 2018, 187, 2397–2404. [Google Scholar] [CrossRef]
- Watkins, S.L.; Glantz, S.A.; Chaffee, B.W. Association of Noncigarette Tobacco Product Use With Future Cigarette Smoking Among Youth in the Population Assessment of Tobacco and Health (PATH) Study, 2013–2015. JAMA Pediatr. 2018, 172, 181–187. [Google Scholar] [CrossRef]
- Eisenberg, M.J.; Hébert-Losier, A.; Windle, S.B.; Greenspoon, T.; Brandys, T.; Fülöp, T.; Nguyen, T.; Elkouri, S.; Montigny, M.; Wilderman, I.; et al. Effect of e-Cigarettes Plus Counseling vs Counseling Alone on Smoking Cessation: A Randomized Clinical Trial. JAMA—J. Am. Med. Assoc. 2020, 324, 1844–1854. [Google Scholar] [CrossRef]
- Gordon, T.; Karey, E.; Rebuli, M.E.; Escobar, Y.-N.H.; Jaspers, I.; Chen, L.C. E-Cigarette Toxicology. Annu. Rev. Pharmacol. Toxicol. 2021, 62, 301–322. [Google Scholar] [CrossRef]
- Kenkel, D.; Mathios, A.; Wang, H. E-Cigarettes and Respiratory Disease: A Replication, Extension, and Future Directions. SSRN 2020, 27507. [Google Scholar] [CrossRef]
- Cobb, C.O.; Lester, R.C.; Rudy, A.K.; Hoetger, C.; Scott, M.; Austin, M.; Montpetit, A.; Lipato, T.; Graham, A.L.; Barnes, A.J.; et al. Tobacco-use behavior and toxicant exposure among current dual users of electronic cigarettes and tobacco cigarettes. Exp. Clin. Psychopharmacol. 2020, 29, 625–635. [Google Scholar] [CrossRef]
- McRobbie, H.; Phillips, A.; Goniewicz, M.L.; Smith, K.M.; Knight-West, O.; Przulj, D.; Hajek, P. Effects of Switching to Electronic Cigarettes with and without Concurrent Smoking on Exposure to Nicotine, Carbon Monoxide, and Acrolein. Cancer Prev. Res. 2015, 8, 873–878. [Google Scholar] [CrossRef]
- Czoli, C.D.; Fong, G.T.; Goniewicz, M.L.; Hammond, D. Biomarkers of Exposure Among “Dual Users” of Tobacco Cigarettes and Electronic Cigarettes in Canada. Nicotine Tob. Res. 2019, 21, 1259–1266. [Google Scholar] [CrossRef]
- Wang, J.B.; Olgin, J.E.; Nah, G.; Vittinghoff, E.; Cataldo, J.K.; Pletcher, M.J.; Marcus, G.M. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS ONE 2018, 13, e0198681. [Google Scholar] [CrossRef]
- Parekh, T.; Pemmasani, S.; Desai, R. Risk of Stroke With E-Cigarette and Combustible Cigarette Use in Young Adults. Am. J. Prev. Med. 2020, 58, 446–452. [Google Scholar] [CrossRef]
- Lucchiari, C.; Masiero, M.; Veronesi, G.; Maisonneuve, P.; Spina, S.; Jemos, C.; Salè, E.O.; Pravettoni, G.; Krebs, P.; Walker, N. Benefits of E-Cigarettes Among Heavy Smokers Undergoing a Lung Cancer Screening Program: Randomized Controlled Trial Protocol. JMIR Res. Protoc. 2016, 5, e21. [Google Scholar] [CrossRef]
- Lucchiari, C.; Masiero, M.; Mazzocco, K.; Veronesi, G.; Maisonneuve, P.; Jemos, C.; Salè, E.O.; Spina, S.; Bertolotti, R.; Pravettoni, G. Benefits of e-cigarettes in smoking reduction and in pulmonary health among chronic smokers undergoing a lung cancer screening program at 6 months. Addict. Behav. 2020, 103, 106222. [Google Scholar] [CrossRef]
- Veronesi, G.; Maisonneuve, P.; Bellomi, M.; Rampinelli, C.; Durli, I.; Bertolotti, R.; Spaggiari, L. Estimating Overdiagnosis in Low-Dose Computed Tomography Screening for Lung Cancer: A cohort study. Ann. Intern. Med. 2012, 157, 776–784. [Google Scholar] [CrossRef]
- Fekketich, A.K.; Fossati, R.; Apolone, G. An Evaluation of the Italian Version of the Fagerström Test for Nicotine Dependence. Psychol. Rep. 2009, 102, 687–694. [Google Scholar] [CrossRef]
- Marino, L. La disassuefazione dal fumo: L’ambulatorio. In L’epidemia Di Fumo in Italia; Nardini, S., Donner, C., Eds.; EDI-AIPO Scientifica: Pisa, Italy, 2002; Available online: https://www.cigaretteless.it/sites/default/files/Cigaretteless_Diario-del-fumatore.pdf (accessed on 19 March 2019).
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Costantini, M.; Musso, M.; Viterbori, P.; Bonci, F.; Del Mastro, L.; Garrone, O.; Venturini, M.; Morasso, G. Detecting psychological distress in cancer patients: Validity of the Italian version of the Hospital Anxiety and Depression Scale. Support. Care Cancer 1999, 7, 121–127. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Birring, S.S.; Prudon, B.; Carr, A.J.; Singh, S.J.; Morgan, M.D.L.; Pavord, I. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003, 58, 339–343. [Google Scholar] [CrossRef]
- Berkhof, F.F.; Boom, L.N.; Hertog, N.E.T.; Uil, S.M.; Kerstjens, H.A.M.; Berg, J.W.K.V.D. The validity and precision of the leicester cough questionnaire in COPD patients with chronic cough. Health Qual. Life Outcomes 2012, 10, 4. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Stylianou, M.; Agapiou, A. Main and side stream effects of electronic cigarettes. J. Environ. Manag. 2019, 238, 10–17. [Google Scholar] [CrossRef]
- Polosa, R.; O’Leary, R.; Tashkin, D.; Emma, R.; Caruso, M. The effect of e-cigarette aerosol emissions on respiratory health: A narrative review. Expert Rev. Respir. Med. 2019, 13, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, D.N.; Glantz, S.A. Association of E-Cigarette Use with Respiratory Disease Among Adults: A Longitudinal Analysis. Am. J. Prev. Med. 2020, 58, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Vansickel, A.R.; Cobb, C.O.; Weaver, M.F.; Eissenberg, T.E. A Clinical Laboratory Model for Evaluating the Acute Effects of Electronic “Cigarettes”: Nicotine Delivery Profile and Cardiovascular and Subjective Effects. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Ebbert, J.O.; Agunwamba, A.A.; Rutten, L.J. Counseling Patients on the Use of Electronic Cigarettes. Mayo Clin. Proc. 2015, 90, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.J.; Benowitz, N.L. The Past, Present, and Future of Nicotine Addiction Therapy. Annu. Rev. Med. 2016, 67, 467–486. [Google Scholar] [CrossRef]
- Fiore, M.C.; Jaén, C.R.; Baker, T.B.; Bailey, W.C.; Benowitz, N.L.; Curry, S.J.; Dorfman, S.F.; Froelicher, E.S.; Goldstein, M.G.; Healton, C.G.; et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline; U.S. Department of Health & Human Services: Rockville, MD, USA, 2008. [CrossRef]
- Minnix, J.A.; Karam-Hage, M.; Blalock, J.A.; Cinciripini, P.M. The importance of incorporating smoking cessation into lung cancer screening. Transl. Lung Cancer Res. 2018, 7, 272–280. [Google Scholar] [CrossRef]
- Chang, J.T.; Anic, G.M.; Rostron, B.L.; Tanwar, M.; Chang, C.M. Cigarette Smoking Reduction and Health Risks: A Systematic Review and Meta-analysis. Nicotine Tob. Res. 2021, 23, 635–642. [Google Scholar] [CrossRef]
- Moldovanu, D.; de Koning, H.J.; van der Aalst, C.M. Lung cancer screening and smoking cessation efforts. Transl. Lung Cancer Res. 2021, 10, 1099–1109. [Google Scholar] [CrossRef]
- Bracken-Clarke, D.; Kapoor, D.; Baird, A.M.; Buchanan, P.J.; Gately, K.; Cuffe, S.; Finn, S.P. Vaping and lung cancer – A review of current data and recommendations. Lung Cancer 2021, 153, 11–20. [Google Scholar] [CrossRef]
- Arnold, M.J.; Nollen, N.L.; Mayo, M.S.; Ahluwalia, J.S.; Leavens, E.L.; Zhang, G.; Rice, M.; Pulvers, K. Harm Reduction Associated with Dual Use of Cigarettes and e-Cigarettes in Black and Latino Smokers: Secondary Analyses from a Randomized Controlled e-Cigarette Switching Trial. Nicotine Tob. Res. 2021, 23, 1972–1976. [Google Scholar] [CrossRef]
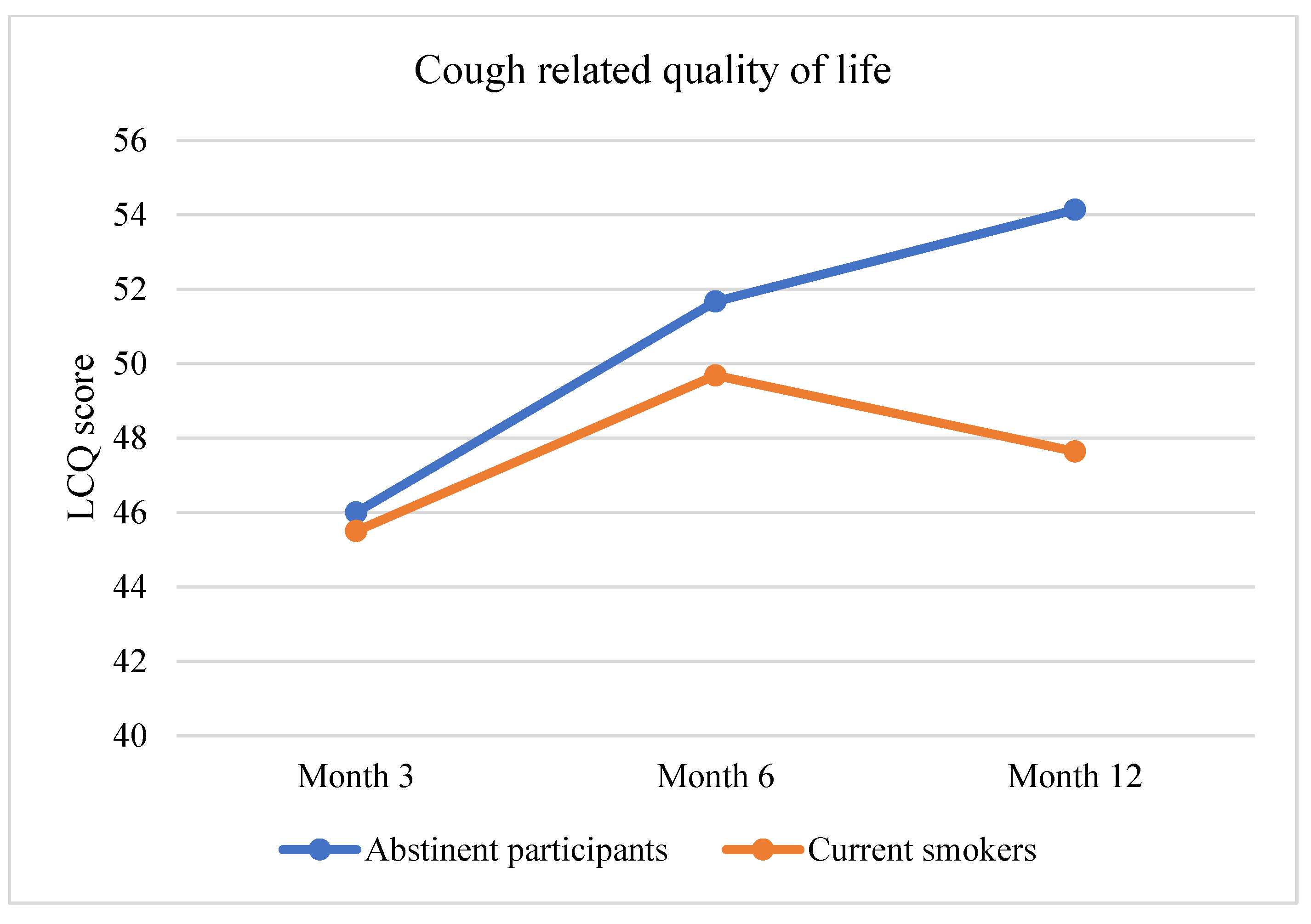
| Variable | Arm 1 | Arm 2 | Arm 3 | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Smoking starting age | 17.55 | 3.77 | 16.90 | 3.58 | 17.76 | 3.68 |
| Daily cigarettes | 16.18 | 7.23 | 13.71 | 7.22 | 13.93 | 7.20 |
| e-CO (ppm value) | 13.11 | 7.01 | 12.21 | 7.17 | 14.63 | 6.99 |
| Dependence Level | 4.48 | 1.69 | 4.20 | 1.80 | 4.67 | 1.72 |
| Motivational Level | 12.00 | 3.30 | 13.16 | 3.09 | 12.42 | 4.11 |
| Anxiety (HADs) | 12.17 | 2.20 | 12.45 | 2.37 | 12.12 | 2.24 |
| Depression (HADs) | 9.13 | 1.57 | 8.90 | 1.81 | 8.32 | 1.37 |
| LCQ | 48.83 | 7.16 | 46.14 | 6.12 | 50.30 | 5.47 |
| Cough % | Catarrh % | Breathlessness % | Bronchitis % | ||
|---|---|---|---|---|---|
| Nicotine e-cigarette | |||||
| Abstinent participants | 13.3 | 10.3 | 61.1 | 9.1 | |
| Current smokers | 51.1 | 47.1 | 80.0 | 15.2 | |
| Nicotine-free e-cigarette | |||||
| Abstinent participants | 13.3 | 13.0 | 63.0 | 9.6 | |
| Current smokers | 37.2 | 40.0 | 72.1 | 10.0 | |
| Control | |||||
| Abstinent participants | 10.0 | 13.0 | 59.5 | 13.1 | |
| Current smokers | 40.0 | 42.6 | 82.0 | 8.1 | |
| All participants | |||||
| Abstinent participants | 12.5 | 11.8 | 61.2 | 10.2 | |
| Current smokers | 48.0 | 43.9 | 80.1 | 10.5 | |
| Burning Throat | Cough | Nausea | Headache | Insomnia | Stomachache | Confusion | |
|---|---|---|---|---|---|---|---|
| Month 6 | |||||||
| Nicotine e-cigarette group | 15.9% | 5.8% | 5.8% | - | 1.4% | 4.3% | 1.4% |
| Nicotine-free e-cigarette group | 5.6% | 2.8% | 7.0% | 1.4% | - | 4.2% | - |
| Month 12 | |||||||
| Nicotine e-cigarette group | 7.8% | 3.8% | 4.1% | - | - | 4.3% | - |
| Nicotine-free e-cigarette group | 1.2% | - | 5.0% | - | - | 4.5% | - |
| Abstinent Participants | Current Smokers | Total | |
|---|---|---|---|
| Arm 1 (Nicotine e-cigarette) | 15 (25.0%) | 45 (75.0%) | 60 |
| Arm 2 (Nicotine-free e-cigarette) | 15 (25.9%) | 43 (74.1%) | 58 |
| Arm 3 (Control) | 10 (16.7%) | 50 (83.3%) | 60 |
| Tot | 40 (22.5%) | 138 (77.5%) | 178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucchiari, C.; Masiero, M.; Mazzocco, K.; Veronesi, G.; Maisonneuve, P.; Jemos, C.; Salè, E.O.; Spina, S.; Bertolotti, R.; Busacchio, D.; et al. Nicotine-Free E-Cigarettes Might Promote Tobacco Smoking Reduction Better Than Nicotine Delivery Devices: Results of a Double-Blind Randomized Controlled Trial at 1 Year. Curr. Oncol. 2022, 29, 8579-8590. https://doi.org/10.3390/curroncol29110676
Lucchiari C, Masiero M, Mazzocco K, Veronesi G, Maisonneuve P, Jemos C, Salè EO, Spina S, Bertolotti R, Busacchio D, et al. Nicotine-Free E-Cigarettes Might Promote Tobacco Smoking Reduction Better Than Nicotine Delivery Devices: Results of a Double-Blind Randomized Controlled Trial at 1 Year. Current Oncology. 2022; 29(11):8579-8590. https://doi.org/10.3390/curroncol29110676
Chicago/Turabian StyleLucchiari, Claudio, Marianna Masiero, Ketti Mazzocco, Giulia Veronesi, Patrick Maisonneuve, Costantino Jemos, Emanuela Omodeo Salè, Stefania Spina, Raffaella Bertolotti, Derna Busacchio, and et al. 2022. "Nicotine-Free E-Cigarettes Might Promote Tobacco Smoking Reduction Better Than Nicotine Delivery Devices: Results of a Double-Blind Randomized Controlled Trial at 1 Year" Current Oncology 29, no. 11: 8579-8590. https://doi.org/10.3390/curroncol29110676
APA StyleLucchiari, C., Masiero, M., Mazzocco, K., Veronesi, G., Maisonneuve, P., Jemos, C., Salè, E. O., Spina, S., Bertolotti, R., Busacchio, D., & Pravettoni, G. (2022). Nicotine-Free E-Cigarettes Might Promote Tobacco Smoking Reduction Better Than Nicotine Delivery Devices: Results of a Double-Blind Randomized Controlled Trial at 1 Year. Current Oncology, 29(11), 8579-8590. https://doi.org/10.3390/curroncol29110676







