Consolidation Systemic Therapy in Locally Advanced, Inoperable Nonsmall Cell Lung Cancer—How to Identify Patients Which Can Benefit from It?
Abstract
1. Introduction
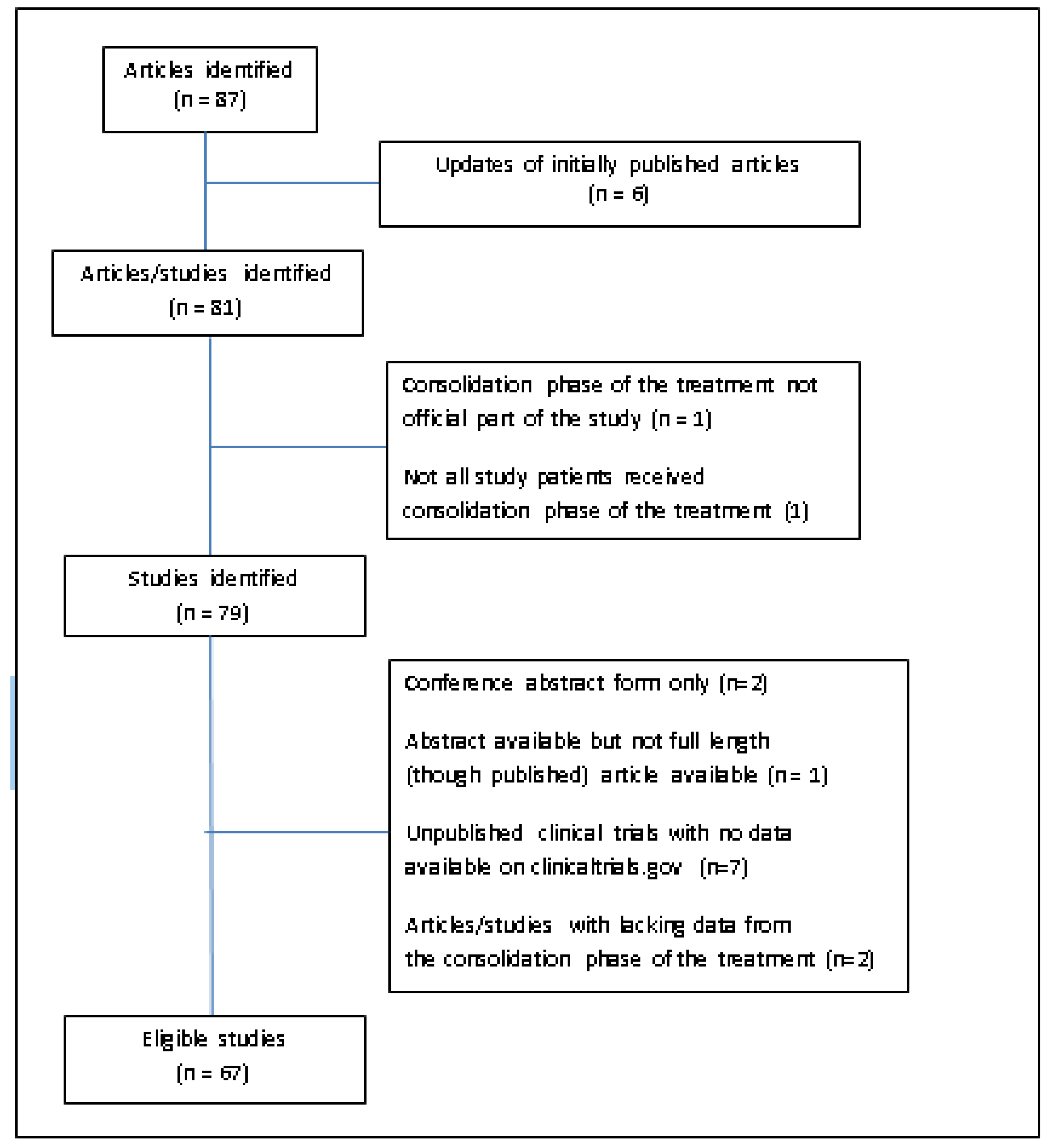
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Aupérin, A.; Le Péchoux, C.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, N.; Roquéi Figuls, M.; Farré Bernadó, N.; Macbeth, F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst. Rev. 2010, 6, CD002140. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-Y.; Zhou, H.; Li, H.-L.; Guan, P.; Yin, Z.H.; Guan, P.; Zhou, B.S. Chemo-radiotherapy for advanced non-small cell lung cancer: Concurrent or sequential? It’s no longer the question: A systematic review. Int. J. Cancer 2010, 127, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Vokes, E.E.; Herndon, J.E.; Crawford, J.; Leopold, K.A.; Perry, M.C.; Miller, A.A.; Green, M.R. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB nonsmall-cell lung cancer: Cancer and Leukemia Group B study 9431. J. Clin. Oncol. 2002, 20, 4191–4198. [Google Scholar] [CrossRef]
- Vokes, E.E.; Herndon, J.E.; Kelley, M.J.; Cicchetti, M.G.; Ramnath, N.; Neill, H.; Atkins, J.N.; Watson, D.M.; Akerley, W.; Green, M.R. Cancer and Leukemia Group BInduction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J. Clin. Oncol. 2007, 25, 1698–1704. [Google Scholar] [CrossRef]
- Le Péchoux, C.; Arriagada, R.; Le Chevalier, T.; Bretel, J.; Cosset, B.P.; Ruffié, P.; Baldeyrou, P.; Grunenwald, D. Concurrent cisplatin vindesine and hyperfractionated thoracic radiotherapy in locally advanced nonsmall cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 519–525. [Google Scholar] [CrossRef]
- Choy, H.; Akerley, W.; Safran, H.; Graziano, S.; Chung, C.; Williams, T.; Cole, B.; Kennedy, T. Multiinstitutional phase II trial of paclitaxel, carboplatin, and concurrent radiation therapy for locally advanced non-small-cell lung cancer. J. Clin. Oncol. 1998, 16, 3316–3322. [Google Scholar] [CrossRef]
- Lochrin, C.; Goss, G.; Stewart, D.J.; Cross, P.; Agboola, O.; Dahrouge, S.; Tomiak, E.; Evans, W.K. Concurrent chemotherapy with hyperfractionated accelerated thoracic irradiation in stage III non-small cell lung cancer. Lung Cancer 1999, 23, 19–30. [Google Scholar] [CrossRef]
- Choy, H.; Devore, R.F., 3rd; Hande, K.R.; Porter, L.L.; Rosenblatt, P.; Yunus, F.; Schlabach, L.; Smith, C.; Shyr, Y.; Johnson, D.H. A phase II study of paclitaxel, carboplatin, and hyperfractionated radiation therapy for locally advanced inoperable non-small-cell lung cancer (a Vanderbilt Cancer Center Affiliate Network Study). Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 931–937. [Google Scholar] [CrossRef]
- Lau, D.; Leigh, B.; Gandara, D.; Edelman, M.; Morgan, R.; Israel, V.; Lara, P.; Wilder, R.; Ryu, J.; Doroshow, J. Twice-weekly paclitaxel and weekly carboplatin with concurrent thoracic radiation followed by carboplatin/paclitaxel consolidation for stage III non-small-cell lung cancer: A California Cancer Consortium phase II trial. J. Clin. Oncol. 2001, 19, 442–447. [Google Scholar] [CrossRef]
- Ratanatharathorn, V.; Lorvidhaya, V.; Maoleekoonpairoj, S.; Phromratanapongse, P.; Sirilerttrakul, S.; Kraipiboon, P.; Cheirsilpa, A.; Tangkaratt, S.; Srimuninnimit, V.; Pattaranutaporn, P. Phase II trial of paclitaxel, carboplatin, and concurrent radiation therapy for locally advanced non-small-cell lung cancer. Lung Cancer 2001, 1, 257–265. [Google Scholar] [CrossRef]
- Albain, K.S.; Crowley, J.J.; Turrisi, A.T.; Gandara, D.R.; Farrar, W.B.; Clark, J.I.; Beasley, K.R.; Livingston, R.B. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: A Southwest Oncology Group phase II study, SWOG 9019. J. Clin. Oncol. 2002, 20, 3454–3460. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ahn, Y.C.; Kim, H.; Lee, S.-H.; Park, S.H.; Lee, K.-E.; Lim, D.H.; Park, J.; Kim, K.; Jung, C.W.; et al. A phase II trial of concurrent chemoradiation therapy followed by consolidation chemotherapy with oral etoposide and cisplatin for locally advanced inoperable non-small cell lung cancers. Lung Cancer 2003, 42, 227–235. [Google Scholar] [CrossRef]
- Gandara, D.R.; Chansky, K.; Albain, K.S.; Leigh, B.R.; Gaspar, L.E.; Lara, P.N., Jr.; Burris, H.; Gumerlock, P.; Kuebler, J.P.; Bearden, J.D., 3rd; et al. Southwest Oncology Group. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: Phase II Southwest Oncology Group Study S9504. J. Clin. Oncol. 2003, 21, 2004–2010. [Google Scholar] [CrossRef]
- Solomon, B.; Ball, D.L.; Richardson, G.; Smith, J.G.; Millward, M.; MacManus, M.; Michael, M.; Wirth, A.; O’Kane, C.; Muceniekas, L.; et al. Phase I/II study of concurrent twice-weekly paclitaxel and weekly cisplatin with radiation therapy for stage III non-small cell lung cancer. Lung Cancer 2003, 41, 353–361. [Google Scholar] [CrossRef]
- Sakai, H.; Yoneda, S.; Kobayashi, K.; Komagata, H.; Kosaihira, S.; Kazumoto, T.; Saito, Y. Phase II study of bi-weekly docetaxel and carboplatin with concurrent thoracic radiation therapy followed by consolidation chemotherapy with docetaxel plus carboplatin for stage III unresectable non-small cell lung cancer. Lung Cancer 2004, 43, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.; Altýnbas, M.; Eroglu, C.; Karahacioglu, E.; Er, O.; Ozkan, M.; Bilgin, M.; Canoz, O.; Gulmez, I.; Gulec, M. Preliminary results of a Phase II study of weekly Paclitaxel (PTX) and Carboplatin (CBDCA) administered concurrently with thoracic radiation therapy (TRT) followed by consolidation chemotherapy with PTX/CBDCA for stage III unresectable non-small-cell lung cancer (NSCLC). Am. J. Clin. Oncol. 2004, 27, 603–610. [Google Scholar]
- Fournel, P.; Robinet, G.; Thomas, P.; Souquet, P.-J.; Léna, L.; Vergnenégre, A.; Delhoume, J.Y.; Le Treut, J.; Silvani, J.A.; Dansin, E.; et al. Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J. Clin. Oncol. 2005, 23, 5910–5917. [Google Scholar]
- Belani, C.P.; Choy, H.; Bonomi, P.; Scott, C.; Travis, P.; Haluschak, J.; Curran, W.J., Jr. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: A randomized phase II locally advanced multi-modality protocol. J. Clin. Oncol. 2005, 23, 5883–5891. [Google Scholar] [CrossRef]
- Keene, K.S.; Harman, E.M.; Knauf, D.G.; McCarley, D.; Zlotecki, R.A. Five-year results of a Phase II trial of hyperfractionated radiotherapy and concurrent daily cisplatin chemotherapy for stage III non-small-cell lung cancer. Am. J. Clin. Oncol. 2005, 28, 217–222. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ohsugi, S.; Natsuhara, A.; Tsubokura, T.; Harada, H.; Ueda, M.; Arimoto, T.; Hara, H.; Yamada, T.; Takesako, T.; et al. Phase I/II trial of biweekly docetaxel and cisplatin with concurrent thoracic radiation for stage III nonsmall- cell lung cancer. Cancer Chemother. Pharmacol. 2006, 58, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.M.; Chansky, K.; Lau, D.H.; Leigh Gaspar, L.E.; Weiss, G.R.; Wozniak, A.J.; Crowley, J.J.; Gandara, D.R. SWOG S9712 Phase II study of consolidation paclitaxel after concurrent chemoradiation in poor-risk stage III non-small-cell lung cancer: SWOG S9712. J. Clin. Oncol. 2006, 24, 5242–5246. [Google Scholar] [CrossRef] [PubMed]
- Sekine, I.; Nokihara, H.; Sumi, M.; Saijo, N.; Nishiwaki, Y.; Ishikura, S.; Mori, K.; Tsukiyama, I.; Tamura, T. Docetaxel consolidation therapy following cisplatin, vinorelbine, and concurrent thoracic radiotherapy in patients with unresectable stage III nonsmall cell lung cancer. J. Thorac. Oncol. 2006, 1, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, E.; Khatab, A.; Al-Adwy, E.R.; Al-Assal, G.M. Limited Field Radiotherapy Concomitant with Cisplatin/Etoposide Followed by Consolidation Docetaxel for the Treatment of Inoperable Stage III Non- Small Cell Lung Cancer. J. Egypt Natl. Cancer Inst. 2007, 19, 28–38. [Google Scholar]
- Rusu, P.; Ciuleanu, T.E.; Cernea, D.; Pelau, D.; Gaal, V.; Cebotaru, C.; Guttman, T.; Todor, N.; Ghilezan, N. Concurrent chemoradiotherapy with vinorelbine and a platinum compound followed by consolidation chemotherapy for unresectable stage III non-small cell lung cancer: Preliminary results of a phase II study. J. BUON 2007, 12, 33–39. [Google Scholar]
- Hanna, N.; Neubauer, M.; Yiannoutsos, C.; McGarry, R.; Arseneau, J.; Ansari, R.; Reynolds, C.; Govindan, R.; Melnyk, A.; Fisher, W.; et al. Hoosier Oncology Group; US Oncology. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J. Clin. Oncol. 2008, 26, 5755–5760. [Google Scholar]
- Kelly, K.; Chansky, K.; Gaspar, L.E.; Albain, K.S.; Jett, J.; Ung, Y.C.; Lau, D.H.M.; Crowley, J.J.; Gandara, D.R. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J. Clin. Oncol. 2008, 6, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Hughes, R.S.; Sandler, A.B.; Dowlati, A.; Schwartzberg, L.S.; Dobbs, T.; Schlabach, L.; Wu, J.; Muldowney, N.J.; Choy, H. A phase II study of concurrent chemoradiation with weekly docetaxel, carboplatin, and radiation therapy followed by consolidation chemotherapy with docetaxel and carboplatin for locally advanced inoperable non-small cell lung cancer (NSCLC). J. Thorac. Oncol. 2009, 4, 722–727. [Google Scholar] [CrossRef]
- Mutter, R.; Lu, B.; Carbone, D.; Csiki, I.; Moretti, L.; Johnson, D.H.; Morrow, J.D.; Sandler, A.B.; Shyr, Y.; Ye, F.; et al. A phase II study of celecoxib in combination with paclitaxel, carboplatin and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin. Cancer Res. 2009, 15, 2158–2165. [Google Scholar] [CrossRef]
- Seung, S.K.; Ross, H.J. Phase II trial of combined modality therapy with concurrent topotecan plus radiotherapy followed by consolidation chemotherapy for unresectable Stage III and selected Stage IV non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 802–809. [Google Scholar] [CrossRef]
- Ohyanagi, F.; Yamamoto, N.; Horiike, A.; Harada, H.; Kozuka, T.; Murakami, H.; Gomi, K.; Takahashi, T.; Morota, M.; Nishimura, T.; et al. Phase II trial of S-1 and cisplatin with concurrent radiotherapy for locally advanced non-small-cell lung cancer. Br. J. Cancer 2009, 101, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, T.; Van Houtte, P.; Paesmans, M.; Giner, V.; Lecomte, J.; Koumakis, G.; Richez, M.; Holbrechts, S.; Roelandts, M.; Meert, A.P.; et al. A phase III randomised study comparing concomitant radiochemotherapy as induction versus consolidation treatment in patients with locally advanced unresectable non-small cell lung cancer. Lung Cancer 2009, 64, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Oshita, F.; Ohe, M.; Honda, T.; Murakami, S.; Kondo, T.; Saito, H.; Noda, K.; Yamashita, K.; Nakayama, Y.; Yamada, K. Phase II study of nedaplatin and irinotecan with concurrent thoracic radiotherapy in patients with locally advanced non-small-cell lung cancer. Br. J. Cancer 2010, 103, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Nakagawa, K.; Nishimura, Y.; Tsujino, K.; Satouchi, M.; Kudo, S.; Hida, T.; Kawahara, M.; Takeda, K.; Katakami, N.; et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J. Clin. Oncol. 2010, 28, 3739–3745. [Google Scholar] [CrossRef]
- Movsas, B.; Langer, C.J.; Ross, H.J.; Wang, L.; Jotte, R.M.; Feigenberg, S.; Xu, F.; Huang, C.H.; Monberg, M.J.; Obasaju, C.K. Randomized phase II trial of cisplatin, etoposide, and radiation followed by gemcitabine alone or by combined gemcitabine and docetaxel in stage III A/B unresectable non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 673–679. [Google Scholar] [CrossRef]
- Bastos, B.R.; Hatoum, G.F.; Walker, G.R.; Tolba, K.; Takita, C.; Gomez, J.; Santos, E.S.; Lopes, G.; Raez, L.E. Efficacy and toxicity of chemoradiotherapy with carboplatin and irinotecan followed by consolidation docetaxel for unresectable stage III non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 533–539. [Google Scholar] [CrossRef]
- Blumenschein, G.R., Jr.; Paulus, R.; Curran, W.J.; Robert, F.; Fossella, F.; Werner-Wasik, M.; Herbst, R.S.; Doescher, P.O.; Choy, H.; Komaki, R. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J. Clin. Oncol. 2011, 29, 2312–2318. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.; Ma, S.; Ji, Y.; Sun, X.; Jiang, H.; Chen, J.; Du, X.; Zheng, Y.; Qiu, G. Concomitant chemoradiotherapy using pemetrexed and carboplatin for unresectable stage III non-small cell lung cancer (NSCLC): Preliminary results of a phase II study. Lung Cancer 2011, 72, 327–332. [Google Scholar] [CrossRef]
- Govindan, R.; Bogart, J.; Stinchcombe, T.; Wang, X.; Hodgson, L.; Kratzke, R.; Garst, G.; Brotherton, T.; Vokes, E.E. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J. Clin. Oncol. 2011, 29, 3120–3125. [Google Scholar] [CrossRef]
- Brunsvig, P.F.; Kyte, J.A.; Kersten, C.; Sundstrøm, S.; Møller, M.; Nyakas, M.; Hansen, G.L.; Gaudernack, G.; Aamdal, S. Telomerase peptide vaccination in NSCLC: A Phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a Phase I/II trial. Clin. Cancer Res. 2011, 17, 6847–6857. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Ruckdeschel, J.C.; Patel, B.B.; Wozniak, W.; Konski, A.; Valdivieso, M.; Hackstock, D.; Chen, W.; Belzer, K.; Burger, A.M.; et al. Phase II study of pemetrexed and cisplatin, with chest radiotherapy followed by docetaxel in patients with stage III non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, Y.; Seto, T.; Sasaki, T.; Yamanaka, T.; Okamoto, I.; Takeda, K.; Tanaka, M.; Katakami, N.; Sawa, T.; Kudoh, S.; et al. S-1 plus cisplatin with concurrent radiotherapy for locally advanced non-small cell lung cancer. A multi-institutional Phase II trial (West Japan Thoracic Oncology Group 3706). J. Thorac. Oncol. 2011, 6, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Takada, M.; Ando, M.; Okishio, K.; Atagi, S.; Fujita, Y.; Tomizawa, Y.; Hayashihara, K.; Okano, Y.; Takahashi, F.; et al. A multi-institutional phase II trial of consolidation of S-1 after concurrent chemoradiotherapy with cisplatin and vinorelbine for locally advanced non-small cell lung cancer. Eur. J. Cancer 2012, 48, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Schuette, W.; Krzakowski, M.J.; Massuti, B.; Otterson, G.A.; Lizambri, R.; Wei, H.; Berger, D.P.; Chen, Y. Randomized Phase II study of palifermin for reducing dysphagia in patients receiving concurrent chemoradiotherapy for locally advanced unresectable non-small cell lung cancer. J. Thorac. Oncol. 2012, 7, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Choy, H.; Schwartzberg, L.S.; Dakhil, S.R.; Garon, E.B.; Gerber, D.E.; Choksi, J.K.; Govindan, R.; Peng, G.; Koustenis, A.; Treat, J.; et al. Phase 2 study of pemetrexed plus carboplatin, or pemetrexed plus cisplatin with concurrent radiation therapy followed by pemetrexed consolidation in patients with favorable-prognosis inoperable stage IIIA/B non-small-cell lung cancer. J. Thorac. Oncol. 2013, 8, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Kotsakis, A.; Tarhini, A.A.; Heron, D.E.; Smith, R.; Friedland, D.; Petro, D.P.; Raez, L.E.; Brahmer, J.R.; Greenberger, J.S.; et al. A multicenter phase II study of cetuximab in combination with chest radiotherapy and consolidation chemotherapy in patients with stage III non-small cell lung cancer. Lung Cancer 2013, 81, 416–421. [Google Scholar] [CrossRef]
- Garrido, P.; Rosell, R.; Arellano, A.; Andreu, F.; Dómine, M.; Perez-Casas, A.; Cardenal, F.; Arnaiz, M.D.; Morán, T.; Morera, R.; et al. Randomized phase II trial of non-platinum induction or consolidation chemotherapy plus concomitant chemoradiation in stage III NSCLC patients: Mature results of the Spanish Lung Cancer Group 0008 study. Lung Cancer 2013, 81, 84–90. [Google Scholar] [CrossRef]
- Kaira, K.; Tomizawa, Y.; Yoshino, R.; Yoshii, A.; Matsuura, M.; Iwasaki, Y.; Koga, Y.; Ono, A.; Nishioka, M.; Kamide, Y.; et al. Phase II study of oral S-1 and cisplatin with concurrent radiotherapy for locally advanced non-small-cell lung cancer. Lung Cancer 2013, 82, 449–454. [Google Scholar] [CrossRef]
- Takayama, K.; Inoue, K.; Tokunaga, S.; Matsumoto, T.; Oshima, T.; Kawasaki, M.; Imanaga, T.; Kuba, M.; Takeshita, M.; Harada, T.; et al. Phase II study of concurrent thoracic radiotherapy in combination with weekly paclitaxel plus carboplatin in locally advanced non-small cell lung cancer: LOGIK0401. Cancer Chemother. Pharmacol. 2013, 72, 1353–1359. [Google Scholar] [CrossRef]
- Casal Rubio, J.; Firvida-Perez, J.L.; Lazaro-Quintela, M.; Barón-Duarte, F.J.; Alonso-Jáudenes, G.; Santomé, L.; Afonso-Afonso, F.J.; Amenedo, M.; Huidobro, G.; Campos-Balea, B.; et al. A phase II trial of erlotinib as maintenance treatment after concurrent chemoradiotherapy in stage III non-small-cell lung cancer: A Galician Lung Cancer Group study. Cancer Chemother. Pharmacol. 2014, 73, 451–457. [Google Scholar] [CrossRef]
- Butts, C.; Socinski, M.A.; Mitchell, P.L.; Thatcher, N.; Havel, L.; Krzakowski, M.; Nawrocki, S.; Ciuleanu, T.-E.; Bosquée, L.; Trigo, J.M.; et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III nonsmall- cell lung cancer (start): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 59–68. [Google Scholar] [CrossRef]
- Zhang, J.; Gay, H.A.; Russo, S.; Parent, T.; Aljumaily, R.; Walker, P.R. Phase II study of low-dose paclitaxel with timed thoracic radiotherapy followed by adjuvant gemcitabine and carboplatin in unresectable stage III non-small cell lung cancer. Lung Cancer 2014, 83, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Dilling, T.J.; Extermann, M.; Kim, J.; Thompson, L.M.; Yue, B.; Stevens, C.W.; Antonia, S.; Gray, J.; Williams, C.; Haura, E.; et al. Phase 2 study of concurrent cetuximab plus definitive thoracic radiation therapy followed by consolidation docetaxel plus cetuximab in poor prognosis or elderly patients with locally advanced non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Ahn, Y.C.; Kim, J.-H.; Lee, C.G.; Cho, E.K.; Lee, K.C.; Chen, M.; Kim, D.-W.; Kim, H.-K.; Min, Y.J.; et al. Multinational randomized Phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J. Clin. Oncol. 2015, 33, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar]
- Komaki, R.; Allen, P.K.; Wei, X.; Liao, Z.; Milas, L.; Cox, J.D.; O’Reilly, M.S.; Chang, J.Y.; McAleer, M.F.; Jeter, M.; et al. Adding erlotinib to chemoradiation improves overall survival but not progression-free survival in stage III non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 317–324. [Google Scholar] [CrossRef]
- Wozniak, A.J.; Moon, J.; Thomas Jr, C.R.; Kelly, K.; Mack, P.C.; Gaspar, L.E.; Raben, D.; Fitzgerald, T.J.; Pandya, K.J.; Gandara, D.R. A Pilot trial of cisplatin/etoposide/radiotherapy followed by consolidation docetaxel and the combination of bevacizumab (NSC-704865) in patients with inoperable locally advanced stage III non-small-cell lung cancer: SWOG S0533. Clin. Lung Cancer 2015, 16, 340–347. [Google Scholar] [CrossRef]
- Flentje, M.; Huber, R.M.; Engel-Riedel, W.; Andreas, S.; Kollmeier, J.; Staar, S.; Dickgreber, N.; Vaissiere, N.; De Almeida, C.; Edlich, B.; et al. GILT-A randomised phase III study of oral vinorelbine and cisplatin with concomitant radiotherapy followed by either consolidation therapy with oral vinorelbine and cisplatin or best supportive care alone in stage III non-small cell lung cancer. Strahlenther. Onkol. 2016, 192, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Senan, S.; Brade, A.; Wang, L.H.; Vansteenkiste, J.; Dakhil, S.; Biesma, B.; Aguillo, M.M.; Aerts, J.; Govindan, R.; Rubio-Viqueira, B.; et al. PROCLAIM: Randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2016, 34, 953–962. [Google Scholar] [CrossRef]
- Fournel, P.; Vergnenegre, A.; Robinet, G.; Léna, H.; Gervais, R.; Le Caer, H.; Souquet, P.-J.; Chavaillon, J.-M.; Bozonnat, M.-C.; Daurès, J.-P.; et al. GFPC and IFCT Team. Induction or consolidation chemotherapy for unresectable stage III non-small-cell lung cancer patients treated with concurrent chemoradiation: A randomized phase II trial GFPC—IFCT 02-01. Eur. J. Cancer. 2016, 52, 181–187. [Google Scholar] [CrossRef]
- Hasegawa, T.; Futamura, Y.; Horiba, A.; Yoshida, T.; Suzuki, T.; Kato, T.; Kaito, D.; Ohno, Y.; Iida, T.; Hayashi, S.; et al. A phase II study of nab-paclitaxel plus carboplatin in combination with thoracic radiation in patients with locally advanced non-small-cell lung cancer. J. Radiat. Res. 2016, 57, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-J.; Deng, Q.-H.; Yu, X.-M.; Ji, Y.-L.; Zheng, Y.-D.; Jiang, H.; Xu, Y.-P.; Ma, S.-L. A phase II study of Endostatin in combination with paclitaxel, carboplatin, and radiotherapy in patients with unresectable locally advanced non-small cell lung cancer. BMC Cancer 2016, 16, 266. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after chemoradiotherapy in Stage III non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Hughes, B.G.M.; Ahern, E.; Lehman, M.; Pratt, G.; Dauth, M.; Pritchard, W.; Wockner, L.; Horwood, K. A phase II trial of concurrent chemotherapy with intravenous cisplatin and vinorelbine and radiotherapy followed by consolidation chemotherapy with oral vinorelbine in locally advanced non–small cell lung cancer (NSCLC): The CONCAVE study. Asia Pac. J. Clin. Oncol. 2017, 13, 137–144. [Google Scholar] [CrossRef]
- Levy, A.; Bardet, E.; Lacas, B.; Pignon, J.-P.; Adam, J.; Lacroix, L.; Artignan, X.; Verrellem, P.; Le Péchoux, C. A phase II open-label multicenter study of gefitinib in combination with irradiation followed by chemotherapy in patients with inoperable stage III non-small cell lung cancer. Oncotarget 2017, 8, 15924–15933. [Google Scholar] [CrossRef] [PubMed]
- Takhar, H.; Singhal, N.; Mislang, A.; Kumar, R.; Kim, L.; Selva-Nayagam, S.; Pittman, K.; Karapetis, C.; Borg, M.; Olver, I.N.; et al. Phase II study of celecoxib with docetaxel chemoradiotherapy followed by consolidation chemotherapy docetaxel pluscisplatin with maintenance celecoxib in inoperable stage II Inonsmall cell lung cancer. Asia Pac. J. Clin. Oncol. 2018, 14, 91–100. [Google Scholar] [CrossRef]
- Kawano, Y.; Sasaki, T.; Yamaguchi, H.; Hirano, K.; Horiike, A.; Satouchi, M.; Hosokawa, S.; Morinaga, R.; Komiya, K.; Inoue, K.; et al. Phase I/II study of carboplatin plus nab-paclitaxel and concurrent radiotherapy for patients with locally advanced non–small cell lung cancer. Lung Cancer 2018, 125, 136–141. [Google Scholar] [CrossRef]
- Sasaki, T.; Seto, T.; Yamanaka, T.; Kunitake, N.; Shimizu, J.; Kodaira, T.; Nishio, M.; Kozuka, T.; Takahashi, T.; Harada, H.; et al. A randomised phase II trial of S-1 plus cisplatin versus vinorelbine plus cisplatin with concurrent thoracic radiotherapy for unresectable, locally advanced non-small cell lung cancer: WJOG5008L. Br. J. Cancer 2018, 119, 675–682. [Google Scholar] [CrossRef]
- Durm, G.A.; Jabbour, S.K.; Althouse, S.K.; Liu, Z.; Sadiq, A.A.; Zon, R.T.; Jalal, S.I.; Kloecker, G.H.; Williamson, M.J.; Reckamp, K.L.; et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN14-179. Cancer 2020, 126, 4353–4361. [Google Scholar] [CrossRef]
- Patel, J.D.; Lee, J.-W.; Carbone, D.P.; Wagner, H.; Shanker, A.; de Aquino, M.T.P.; Horn, L.; Johnson, M.L.; Gerber, D.E. Phase II Study of immunotherapy with Tecemotide and Bevacizumab after chemoradiation in patients with unresectable stage III non-squamous non-small cell lung Ccncer (NS-NSCLC): A trial of the ECOG-ACRIN Cancer Research Group (E6508). Clin. Lung Cancer 2020, 21, 520–526. [Google Scholar] [CrossRef]
- Lin, S.H.; Lin, Y.; Yao, L.; Kalhor, N.; Carter, B.W.; Altan, M.; Blumenschein, G.; Byers, L.A.; Fossella, F.; Gibbons, D.L.; et al. Phase II Trial of Concurrent Atezolizumab with Chemoradiation for Unresectable NSCLC. J. Thorac. Oncol. 2020, 15, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Skinner, H.; Hu, C.; Tsakiridis, T.; Santana-Davila, R.; Lu, B.; Erasmus, J.J.; Doemer, A.J.; Videtic, G.M.M.; Coster, J.; Yang, A.X.; et al. Addition of metformin to concurrent chemoradiation in patients with locally advanced non-small cell lung cancer. The NRG-LU001 phase 2 randomized clinical trial. JAMA Oncol. 2021, 7, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Jeremić, B. Standard treatment option in Stage III non-small cell lung cancer: Case against trimodal therapy and consolidation therapy. Clin. Lung Cancer 2015, 16, 80–85. [Google Scholar] [CrossRef]
- Jeremic, B.; Cihoric, N.; Casas, F.; Gomez-Caamano Dubinsky, P. No role for trimodality therapy and consolidation chemotherapy compared with concurrent radiochemotherapy alone in stage III non-small-cell lung cancer. J. Clin. Oncol. 2016, 34, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, B.; Langenhoven, L. Consolidation therapy after concurrent radiochemotherapy? Still unclear who may potentially benefit! Lung Cancer 2013, 82, 509. [Google Scholar] [CrossRef]
- Colin, P.; Jovenin, N.; Ganem, G.; Duhamel, J.; Oster, J.; Guichard, F.; Cretin, J.; Terrioux, P.; Brechot, J.; Morere, J. Effect of paclitaxel-carboplatin (PC) consolidation chemotherapy after weekly PC concurrent chemo-radiotherapy (CCR) for patients with locally advanced non-small cell lung cancer (LA-NSCLC): 3-year definitive results of the B001-phase III GERCOR-study. J. Clin. Oncol. 2006, 24, 18. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, E.K.; Lee, J.S.; Park, I.I.S.; Do, K.-H.; Song, S.Y.; Park, J.W. Consolidation chemotherapy with monthly Paclitaxel and Cisplatin (PC) or observation after concurrent chemoradiotherapy for locally advanced non-small cell lung cancer (NSCLC): Randomized phase II study. J. Thorac. Oncol. 2007, 2, 449s. [Google Scholar] [CrossRef]
- Oshita, F.; Murakami, S.; Kondo, T.; Saito, H.; Yamada, K.; Nakayama, Y. Nedaplatin and irinotecan with concurrent thoracic radiotherapy followed by docetaxel consolidation in patients with locally advanced non-small cell lung cancer. J. Exp. Ther. Oncol. 2017, 12, 17–23. [Google Scholar]
- Rigas, J.R.; Carey, M.A.; Rubin, M.S. Efficiency of mainte nance erlotinib versus placebo in patients with unresectable stage III non-small lung cancer (NSCLC) following concurrent chemo radiation (D0410, NCT00153803). J. Thoracic. Oncol. 2009, 4, S371. [Google Scholar]
- Ph II Study of Concurrent Chemoradiotherapy with Weekly Docetaxel, Carboplatin and Radiation Therapy followed by consolidation Chemotherapy with Docetaxel and Carboplatin for Locally Advanced Inoperable Non Small Cell Lung Cancer (NSCLC). Available online: https://clinicaltrials.gov/ct2/show/NCT00664105?term=NCT00664105&draw=2&rank=1;ClinicalTrials.govIdentifier:NCT00664105 (accessed on 1 July 2022).
- Phase 1/2 Study of Pemetrexed (Alimta) Plus Carboplatin, or Pemetrexed Plus Cisplatin with Concurrent Radiation Therapy Followed by Every-21-Day Pemetrexed Consolidation in Patients with Favorable-Prognosis Inoperable Stage IIIA/B Non Small Cell Lung Cancer (NSCLC). Available online: https://https://clinicaltrials.gov/ct2/show/NCT00482014?term=NCT00482014&draw=2&rank=1.ClinicalTrials.govIdentifier:NCT00482014 (accessed on 1 July 2022).
- Carboplatin and Irinotecan Concomitantly with Radiation Therapy Followed by Consolidation Chemotherapy with Docetaxel for Locally Advanced Non Small cell lung cancer (NSCLC) (GIA 12177). Available online: https://clinicaltrials.gov/ct2/show/NCT00449020?term=NCT00449020&draw=2&rank=1.ClinicalTrials.govIdentifier:NCT00449020 (accessed on 1 July 2022).
- A Phase II Trial of Concurrent Cisplatin/Etoposide/Radiotherapy Followed by Consolidation Sorafenib in Patients with Inoperable Stage III Non-Small cell lung cancer (NSCLC). Available online: https://clinicaltrials.gov/ct2/show/NCT00417248?term=NCT00417248&draw=2&rank=1.ClinicalTrials.govIdentifier:NCT00417248 (accessed on 1 July 2022).
- A Phase II Study to Investigate a Combination of Metformin with Chemo-Radiotherapy in Patients with Locally Advanced Non-small cell lung cancer (NSCLC). Available online: https://clinicaltrials.gov/ct2/show/NCT02115464?term=NCT02115464&draw=2&rank=1.ClinicalTrials.govIdentifier:NCT02115464 (accessed on 1 July 2022).
- A Phase II Study of Radiation Therapy, Paclitaxel Poliglumex, and Carboplatin in Stage III Non-Small Cell Lung Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00352690?term=NCT00352690&draw=2&rank=1.ClinicalTrials.govIdentifier:NCT00352690 (accessed on 1 July 2022).
- Ichinose, Y.; Nakai, Y.; Kudoh, S.; Semba, H.; Yoshida, S.; Nukiwa, T.; Yamamoto, H.; Yamane, Y.; Niitani, H. Uracil/Tegafur plus cisplatin with concurrent radiotherapy for locally advanced non-small-cell lung cancer. A multi-institutional Phase II trial. Clin. Cancer Res. 2004, 10, 4369–4373. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Wu, S.; Ou, G.; Bi, N.; Li, W.; Ren, H.; Cao, J.; Liang, J.; Li, J.; Zhou, Z.; et al. Randomized phase II study of concurrent cisplatin/etoposide or paclitaxel/carboplatin and thoracic radiotherapy in patients with stage III non-small cell lung cancer. Lung Cancer 2012, 77, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, K.; Kurata, T.; Yamamoto, S.; Kawaguchi, T.; Kubo, A.; Isa, S.; Hasegawa, Y.; Ou, S.H.; Takada, M.; Ando, M. Is consolidation chemotherapy after concurrent chemo-radiotherapy beneficial for patients with locally advanced non-small-cell lung cancer? A pooled analysis of the literature. J. Thorac. Oncol. 2013, 8, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Liu, J.; Zhou, W.; Weng, K.; Long, B.; Wang, Y. The role of additional chemotherapy in combination with concurrent chemoradiotherapy for locally advanced inoperable non-small cell lung cancer: A systematic review and meta-analysis of 12 randomized trials. Cancer Investig. 2019, 37, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, X.; Kong, D.; Zhang, L.; Guo, Y.; Ren, J.; Hu, X.; Yang, J.; Gao, S. The effect of consolidation chemotherapy after concurrent chemoradiotherapy on the survival of patients with locally advanced non-small cell lung cancer: A meta-analysis. Int. J. Clin. Oncol. 2017, 22, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zang, J.; Xu, J.; Bai, C.; Qin, Y.; Liu, K.; Wu, C.; Wu, M.; He, Q.; Zhang, S.; et al. Maintenance therapy with continuous or switch strategy in advanced non-small cell lung cancer: A systematic review and meta-analysis. Chest 2011, 140, 117–126. [Google Scholar] [CrossRef] [PubMed]
| Item | N | % | |
|---|---|---|---|
| Study type | Pilot study | 2 | 3 |
| Phase I-II | 4 | 6 | |
| Phase II (single arm) | 42 | 63 | |
| Phase II (randomized) | 5 | 7 | |
| Phase III | 9 | 13 | |
| One arm from phase II | 3 | 5 | |
| One arm from phase III | 2 | 3 | |
| Institution type | Single institution | 18 | 27 |
| Multi-institutional | 49 | 73 | |
| Stage of the disease | IIA-IIIB | 1 | 1 |
| IIB-IIIB | 1 | 1 | |
| IIB-IIIC | 1 | 1 | |
| IIIA-IIIB | 57 | 85 | |
| IIIB | 4 | 6 | |
| III-IV | 3 | 5 | |
| Type of RT fractionation * | Conventional (1.8–2.0 Gy/fx) | 60 | 89 |
| Hfx (1.2–1.5 Gy/fx) | 4 | 6 | |
| Split course (1.8–3.0 Gy/fx) | 2 | 3 | |
| Not specified | 1 | 2 | |
| RT total dose range per fractionation type * | Conventional: 40–74 Gy | 61 | 91 |
| Hfx: 60–69.6 Gy | 4 | 6 | |
| Split course: 60–61 Gy | 2 | 3 | |
| Not specified: not specified | 1 | 1 | |
| No. drugs given concurrently with RT * | 1 drug | 8 | 11 |
| 2 drugs | 53 | 74 | |
| ≥3 drugs | 11 | 15 | |
| Type of drugs given concurrently with RT * | CHT alone | 58 | 82 |
| Targeted alone or in combination | 7 | 10 | |
| Immunotherapy alone or in combination | 2 | 3 | |
| CHT with non-anti-cancer drugs | 4 | 6 | |
| No. drugs given in consolidation phase * | 1 drug | 21 | 30 |
| 2 drugs | 40 | 56 | |
| ≥3 drugs | 11 | 15 | |
| Type of drugs given in consolidation phase * | CHT alone | 54 | 78 |
| Targeted alone or in combination | 6 | 9 | |
| Immunotherapy alone or in combination | 6 | 9 | |
| CHT with non-anti-cancer drugs | 3 | 4 | |
| Drugs given in consolidation phase * | Same as in concurrent phase | 31 | 46 |
| Different (switch) | 20 | 29 | |
| >1 drug remains the same | 17 | 25 | |
| Duration of the consolidation phase | 2 cycles | 31 | 46 |
| 3 cycles | 18 | 27 | |
| 2–4 | 1 | 2 | |
| 3–5 cycles | 1 | 2 | |
| 4 cycles | 8 | 12 | |
| Prolonged administration | 8 | 12 |
| Characteristic | N | % | |
|---|---|---|---|
| Time gap between concurrent and consolidation phase | No gap | 8 | 12 |
| 2 weeks | 3 | 4 | |
| 3 weeks | 9 | 13 | |
| 4 weeks | 15 | 23 | |
| Various | 24 | 36 | |
| unknown | 8 | 12 | |
| Evaluation conducted after concurrent part | Yes | 36 | 54 |
| No | 31 | 46 | |
| Response after concurrent part provided | Yes | 14 | 21 |
| No | 53 | 79 | |
| Response provided after the whole course of the treatment | Yes | 32 | 48 |
| No | 35 | 52 | |
| Type of patients continuing with consolidation treatment | All | 34 | 51 |
| Non-PD | 28 | 42 | |
| Responders | 2 | 3 | |
| Not specified | 3 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeremić, B.; Mariamidze, E.; Shoshiashvili, I.; Kiladze, I. Consolidation Systemic Therapy in Locally Advanced, Inoperable Nonsmall Cell Lung Cancer—How to Identify Patients Which Can Benefit from It? Curr. Oncol. 2022, 29, 8316-8329. https://doi.org/10.3390/curroncol29110656
Jeremić B, Mariamidze E, Shoshiashvili I, Kiladze I. Consolidation Systemic Therapy in Locally Advanced, Inoperable Nonsmall Cell Lung Cancer—How to Identify Patients Which Can Benefit from It? Current Oncology. 2022; 29(11):8316-8329. https://doi.org/10.3390/curroncol29110656
Chicago/Turabian StyleJeremić, Branislav, Elene Mariamidze, Inga Shoshiashvili, and Ivane Kiladze. 2022. "Consolidation Systemic Therapy in Locally Advanced, Inoperable Nonsmall Cell Lung Cancer—How to Identify Patients Which Can Benefit from It?" Current Oncology 29, no. 11: 8316-8329. https://doi.org/10.3390/curroncol29110656
APA StyleJeremić, B., Mariamidze, E., Shoshiashvili, I., & Kiladze, I. (2022). Consolidation Systemic Therapy in Locally Advanced, Inoperable Nonsmall Cell Lung Cancer—How to Identify Patients Which Can Benefit from It? Current Oncology, 29(11), 8316-8329. https://doi.org/10.3390/curroncol29110656







