Is Laparoscopic Hepatectomy Safe for Giant Liver Tumors? Proposal from a Single Institution for Totally Laparoscopic Hemihepatectomy Using an Anterior Approach for Giant Liver Tumors Larger Than 10 cm in Diameter
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
2.3. Surgical Procedure
2.3.1. Right Hemihepatectomy
2.3.2. Left Hemihepatectomy
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gagner, M.; Rheault, M.; Dubuc, J. Laparoscopic partial hepatectomy for liver tumor. Surg. Endosc. 1992, 6, 97–98. [Google Scholar]
- Buell, J.F.; Cherqui, D.; Geller, D.A.; O’Rourke, N.; Iannitti, D.; Dagher, I.; Koffron, A.J.; Thomas, M.; Gayet, B.; Han, H.S.; et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann. Surg. 2009, 250, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Makabe, K.; Nitta, H.; Takahara, T.; Hasegawa, Y.; Kanno, S.; Nishizuka, S.; Sasaki, A.; Wakabayashi, G. Efficacy of occlusion of hepatic artery and risk of carbon dioxide gas embolism during laparoscopic hepatectomy in a pig model. J. Hepatobiliary Pancreat. Sci. 2014, 21, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Honda, G.; Kurata, M.; Tadano, S.; Sakamoto, K.; Okuda, Y.; Abe, K. An experimental study on the relationship among airway pressure, pneumoperitoneum pressure, and central venous pressure in pure laparoscopic hepatectomy. Ann. Surg. 2016, 263, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’Rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2018, 268, 11–18. [Google Scholar]
- Abu Hilal, M.; Aldrighetti, L.; Dagher, I.; Edwin, B.; Troisi, R.I.; Alikhanov, R.; Aroori, S.; Belli, G.; Besselink, M.; Briceno, J.; et al. The Southampton Consensus Guidelines for laparoscopic liver surgery: From indication to implementation. Ann. Surg. 2018, 268, 11–18. [Google Scholar] [CrossRef]
- Ban, D.; Tanabe, M.; Ito, H.; Otsuka, Y.; Nitta, H.; Abe, Y.; Hasegawa, Y.; Katagiri, T.; Takagi, C.; Itano, O.; et al. A novel difficulty scoring system for laparoscopic liver resection. J. Hepatobiliary Pancreat. Sci. 2014, 21, 745–753. [Google Scholar] [CrossRef]
- Ai, J.H.; Li, J.W.; Chen, J.; Bie, P.; Wang, S.G.; Zheng, S.G. Feasibility and safety of laparoscopic liver resection for hepatocellular carcinoma with a tumor size of 5–10 cm. PLoS ONE 2013, 8, e72328. [Google Scholar] [CrossRef]
- Shelat, V.G.; Cipriani, F.; Basseres, T.; Armstrong, T.H.; Takhar, A.S.; Pearce, N.W.; AbuHilal, M. Pure laparoscopic liver resection for large malignant tumors: Does size matter? Ann. Surg. Oncol. 2015, 22, 1288–1293. [Google Scholar] [CrossRef]
- Kabir, T.; Syn, N.L.; Guo, Y.; Lim, K.I.; Goh, B.K.P. Laparoscopic liver resection for huge (≥10 cm) hepatocellular carcinoma: A coarsened exact-matched single-surgeon study. Surg. Oncol. 2021, 37, 101569. [Google Scholar] [CrossRef]
- Ariizumi, S.I.; Nanashima, A.; Yamamoto, M. Anterior approach in right hepatectomy. J. Hepatobiliary Pancreat. Sci. 2018, 25, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Pringle, J.H. Notes on the arrest of hepatic hemorrhage due to trauma. Ann. Surg. 1908, 48, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.X.; Yu, Z.Y.; Bai, Y.N. Laparoscopic hepatectomy for liver metastases from colorectal cancer: A meta-analysis. J. Laparoendosc. Adv. Surg. Tech. 2014, 24, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Beppu, T.; Wakabayashi, G.; Hasegawa, K.; Gotohda, N.; Mizuguchi, T.; Takahashi, Y.; Hirokawa, F.; Taniai, N.; Watanabe, M.; Katou, M.; et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: A multi-institutional Japanese study. J. Hepatobiliary Pancreat. Sci. 2015, 22, 711–720. [Google Scholar] [CrossRef]
- Fretland, Å.A.; Dagenborg, V.J.; Bjørnelv, G.M.W.; Kazaryan, A.M.; Kristiansen, R.; Fagerland, M.W.; Hausken, J.; Tønnessen, T.I.; Abildgaard, A.; Barkhatov, L.; et al. Laparoscopic versus open resection for colorectal liver metastases: The OSLO-COMET randomized controlled trial. Ann. Surg. 2018, 267, 199–207. [Google Scholar] [CrossRef]
- Xiong, J.J.; Altaf, K.; Javed, M.A.; Huang, W.; Mukherjee, R.; Mai, G.; Sutton, R.; Liu, X.-B.; Hu, W.M. Meta-analysis of laparoscopic vs. open liver resection for hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 6657–6668. [Google Scholar] [CrossRef]
- Takahara, T.; Wakabayashi, G.; Beppu, T.; Aihara, A.; Hasegawa, K.; Gotohda, N.; Hatano, E.; Tanahashi, Y.; Mizuguchi, T.; Kamiyama, T.; et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: A multi-institutional Japanese study. J. Hepatobiliary Pancreat. Sci. 2015, 22, 721–727. [Google Scholar] [CrossRef]
- Chen, K.; Pan, Y.; Wang, Y.F.; Zheng, X.Y.; Liang, X.; Yu, H.; Cai, X.J. Laparoscopic right hepatectomy for hepatocellular carcinoma: A propensity score matching analysis of outcomes compared with conventional open surgery. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 503–512. [Google Scholar] [CrossRef]
- Liu, C.L.; Fan, S.T.; Cheung, S.T.; Lo, C.M.; Ng, I.O.; Wong, J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: A prospective randomized controlled study. Ann. Surg. 2006, 244, 194–203. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.Q.; Wang, Q.; Yang, J.; Yang, J.Y. Anterior vs. conventional approach hepatectomy for large liver cancer: A meta-analysis. World J. Gastroenterol. 2014, 20, 17235–17243. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nitta, H.; Takahara, T.; Katagiri, H.; Kanno, S.; Umemura, A.; Sasaki, A. Anterior approach for pure laparoscopic donor right hepatectomy. Surg. Endosc. 2020, 34, 4677–4678. [Google Scholar] [CrossRef] [PubMed]
- Belghiti, J.; Guevara, O.A.; Noun, R.; Saldinger, P.F.; Kianmanesh, R. Liver hanging maneuver: A safe approach to right hepatectomy without liver mobilization. J. Am. Coll. Surg. 2001, 193, 109–111. [Google Scholar] [CrossRef]
- Kim, J.H. Pure Laparoscopic Right Hepatectomy Using Modified Liver Hanging Maneuver: Technical Evolution from Caudal Approach toward Ventral Approach. J. Gastrointest. Surg. 2018, 22, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
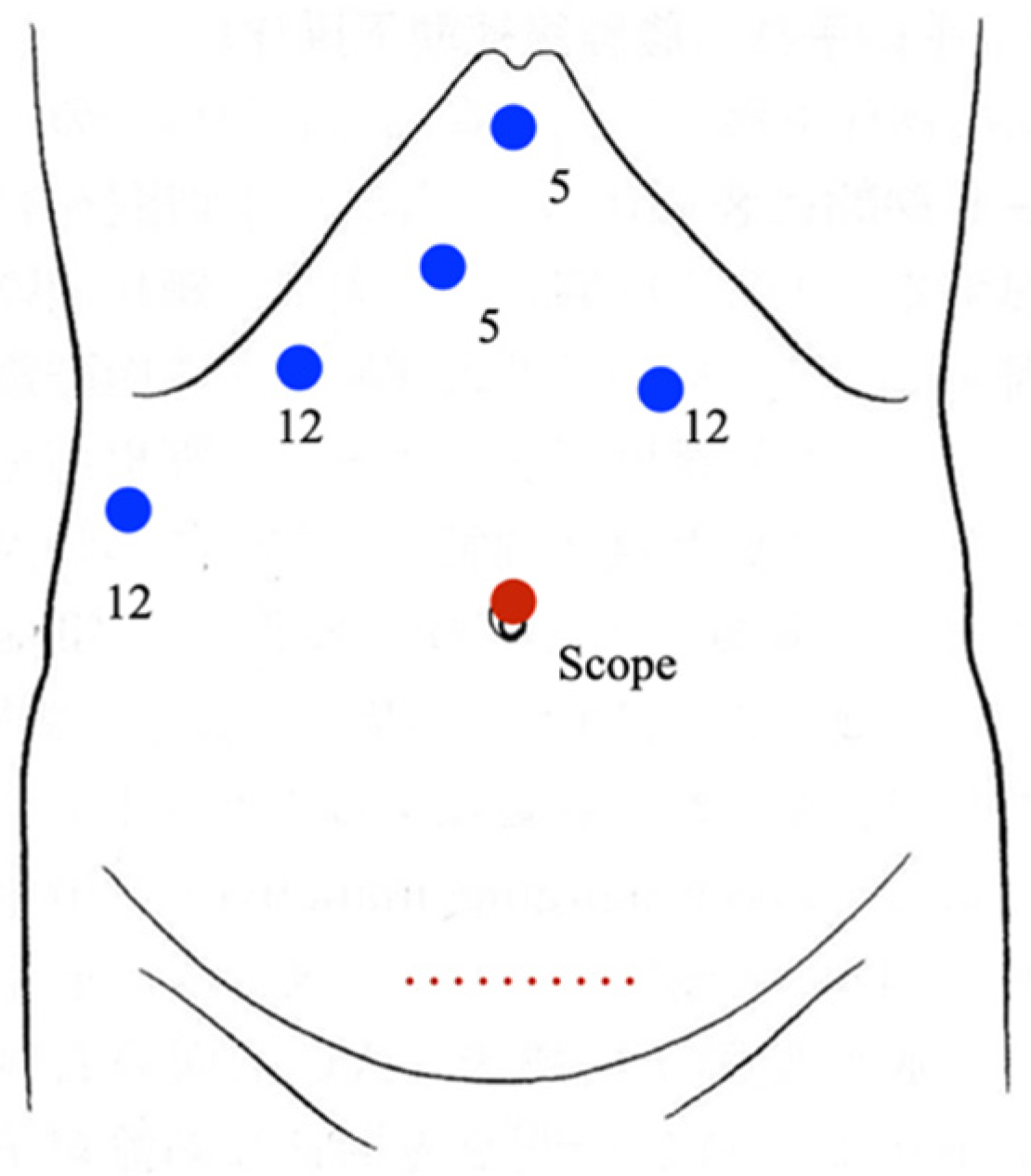
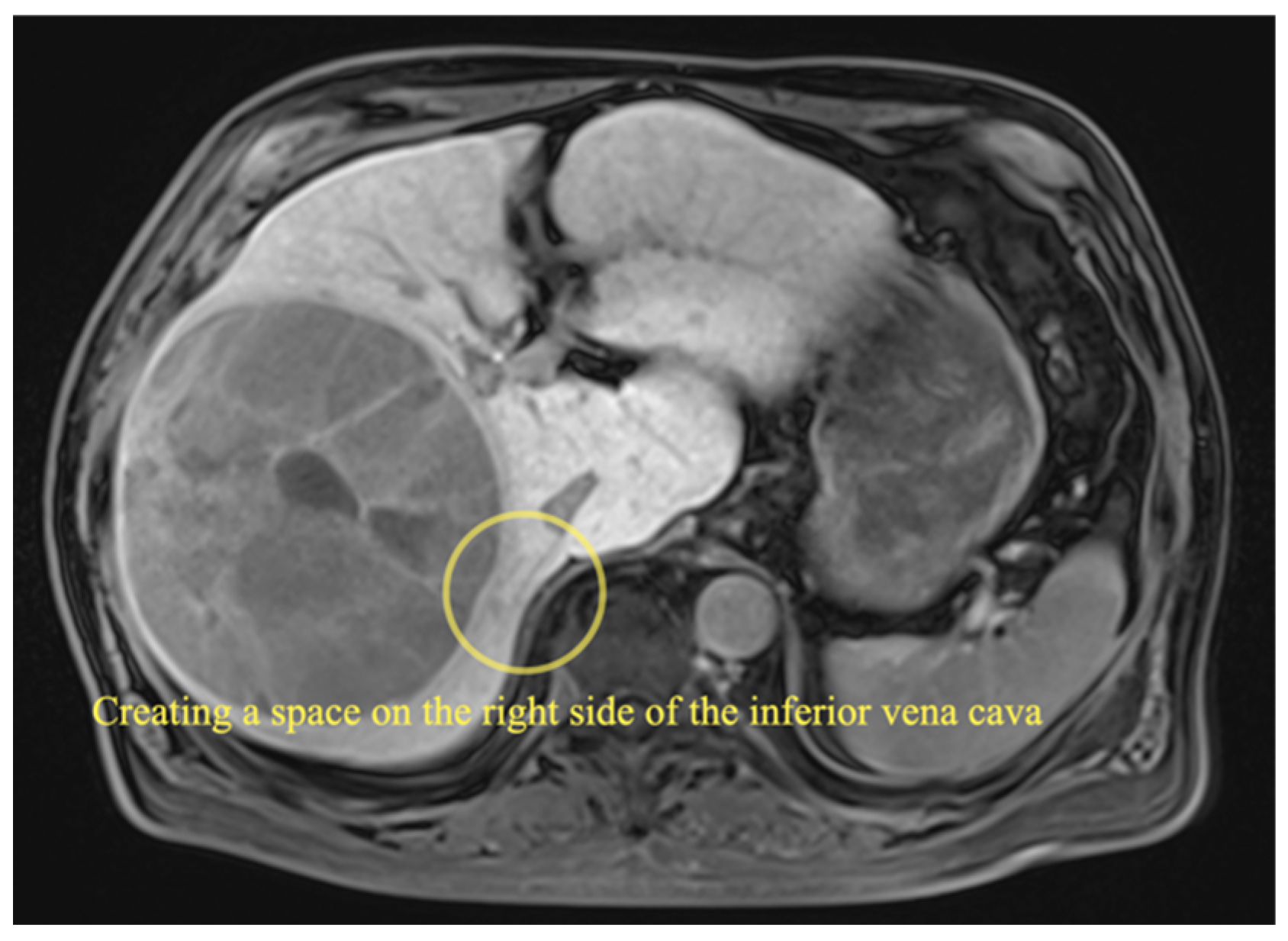

| Case | Age/Sex | Disease | Types of Liver Resection | Conversion | Operating Time (min) | Blood Loss (mL) | Resected Tumor Size (cm) | Resected Liver Volume (g) | Complications (Clavien-Dindo ≥ III) | Postoperative Hospital Stay (Days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78/M | HCC | Right | 319 | 329 | 12.5 | 1250 | 38 | ||
| 2 | 59/M | HCC | Right | 410 | 249 | 11.0 | 1200 | 8 | ||
| 3 | 48/M | HCC | Left | 410 | 483 | 13.0 | 1250 | 12 | ||
| 4 | 60/F | Hemangioma | Right | 390 | 155 | 11.5 | 1380 | 7 | ||
| 5 | 43/M | Hemangioma | Extended left | 261 | 536 | 10.5 | 1050 | 14 | ||
| 6 | 74/M | HCC | Right | 266 | 20 | 12.0 | 1100 | 14 | ||
| 7 | 75/M | HCC | Right | 364 | 166 | 13.5 | 1592 | 7 | ||
| 8 | 61/F | Cyst | Right | 175 | 101 | 15.0 | 1150 | 10 | ||
| 9 | 82/F | HCC | Right | 357 | 122 | 13.6 | 1350 | Bile leakage | 117 | |
| 10 | 63/M | HCC | Left | 384 | 303 | 10.3 | 1000 | 13 | ||
| 11 | 9/M | HCC | Right | HALS | 441 | 356 | 13.0 | 1100 | 9 | |
| 12 | 69/M | HCC | Right | 259 | 30 | 10.5 | 1180 | 9 | ||
| 13 | 64/M | HCC | Right | 263 | 50 | 13.0 | 1340 | 12 | ||
| 14 | 73/M | HCC | Right | 291 | 450 | 11.7 | 1260 | 15 | ||
| 15 | 57/F | Cystadeno-carcinoma | Right | 336 | 148 | 12.0 | 1331 | 13 |
| Nongiant Tumors (n = 65) | Giant Tumors (n = 15) | p Value † | |
|---|---|---|---|
| Age * | 69 (26–85) | 63 (9–82) | 0.134 |
| Disease | HCC 28, ICC 11, Meta 21, Benign 5 | HCC 11, Cystadenocarcinoma 1, Benign 3 | 0.003 ‡ |
| Liver cirrhosis | 6 | 1 | 0.266 ‡ |
| Types of hemihepatectomy Right/Left | 23/42 | 12/3 | 0.004 |
| Operating time (min) * | 271 (165–559) | 336 (175–441) | 0.080 |
| Blood loss (ml) * | 72 (1–2473) | 166 (20–536) | 0.497 |
| Resected tumor size (cm) * | 4.0 (1.0–9.8) | 12.0 (10.5–15.0) | <0.001 |
| Resected liver volume (g) * | 410 (150–800) | 1250 (1000–1592) | <0.001 |
| Postoperative hospital stay (days) * | 12 (5–67) | 12 (7–117) | 0.496 |
| Complications (Clavien-Dindo ≧ III) | 9 | 1 | 0.664 ‡ |
| Nongiant Tumors (n = 23) | Giant Tumors (n = 12) | p Value † | |
|---|---|---|---|
| Age * | 69 (53–85) | 67 (9–82) | 0.320 |
| Disease | HCC 13, ICC 4, Meta 6 | HCC 9, Cystadenocarcinoma 1, Benign 2 | 0.022 ‡ |
| Liver cirrhosis | 2 | 0 | 0.443 ‡ |
| Operating time (min) * | 333 (181–559) | 327 (175–441) | 0.472 |
| Blood loss (ml) * | 153 (53–1232) | 151 (20–450) | 0.465 |
| Resected tumor size (cm) * | 4.5 (1.0–9.0) | 12.3 (10.5–15.0) | <0.001 |
| Resected liver volume (g) * | 720 (390–800) | 1255 (1100–1592) | <0.001 |
| Postoperative hospital stay (days) * | 13 (9–67) | 11 (7–117) | 0.976 |
| Complications (Clavien-Dindo ≧ III) | 6 | 1 | 0.373 ‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitta, H.; Sasaki, A.; Katagiri, H.; Kanno, S.; Umemura, A. Is Laparoscopic Hepatectomy Safe for Giant Liver Tumors? Proposal from a Single Institution for Totally Laparoscopic Hemihepatectomy Using an Anterior Approach for Giant Liver Tumors Larger Than 10 cm in Diameter. Curr. Oncol. 2022, 29, 8261-8268. https://doi.org/10.3390/curroncol29110652
Nitta H, Sasaki A, Katagiri H, Kanno S, Umemura A. Is Laparoscopic Hepatectomy Safe for Giant Liver Tumors? Proposal from a Single Institution for Totally Laparoscopic Hemihepatectomy Using an Anterior Approach for Giant Liver Tumors Larger Than 10 cm in Diameter. Current Oncology. 2022; 29(11):8261-8268. https://doi.org/10.3390/curroncol29110652
Chicago/Turabian StyleNitta, Hiroyuki, Akira Sasaki, Hirokatsu Katagiri, Shoji Kanno, and Akira Umemura. 2022. "Is Laparoscopic Hepatectomy Safe for Giant Liver Tumors? Proposal from a Single Institution for Totally Laparoscopic Hemihepatectomy Using an Anterior Approach for Giant Liver Tumors Larger Than 10 cm in Diameter" Current Oncology 29, no. 11: 8261-8268. https://doi.org/10.3390/curroncol29110652
APA StyleNitta, H., Sasaki, A., Katagiri, H., Kanno, S., & Umemura, A. (2022). Is Laparoscopic Hepatectomy Safe for Giant Liver Tumors? Proposal from a Single Institution for Totally Laparoscopic Hemihepatectomy Using an Anterior Approach for Giant Liver Tumors Larger Than 10 cm in Diameter. Current Oncology, 29(11), 8261-8268. https://doi.org/10.3390/curroncol29110652





