Impact of Regulatory Approval Status on CADTH Reimbursement of Oncology Drugs and Role of Real-World Evidence on Conditional Approvals from 2019 to 2021
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence—What Is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Big Data and Pharmacovigilance: Data Mining for Adverse Drug Events and Interactions. Pharm. Ther. 2018, 43, 340–351. [Google Scholar]
- Franklin, J.M.; Patorno, E.; Desai, R.J.; Glynn, R.J.; Martin, D.; Quinto, K.; Pawar, A.; Bessette, L.G.; Lee, H.; Garry, E.M.; et al. Emulating Randomized Clinical Trials With Nonrandomized Real-World Evidence Studies: First Results From the RCT DUPLICATE Initiative. Circulation 2021, 143, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Health Canada Website on RWE. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/optimizing-real-world-evidence-regulatory-decisions.html (accessed on 23 July 2022).
- Health Canada Website on RWE. Available online: https://www.canada.ca/en/services/health/publications/drugs-health-products/real-world-data-evidence-drug-lifecycle-report.html (accessed on 23 July 2022).
- Health Canada Website on RWE Strategy. Available online: https://www.canada.ca/en/health-canada/corporate/transparency/regulatory-transparency-and-openness/improving-review-drugs-devices/real-world-evidence-medical-device-strategy.html (accessed on 23 July 2022).
- CADTH Website on RWE. Available online: https://www.cadth.ca/real-world-evidence-decision-making (accessed on 23 July 2022).
- Lau, C.; Jamali, F.; Loebenberg, R. Health Canada Usage of Real World Evidence (RWE) in Regulatory Decision Making compared with FDA/EMA usage based on publicly available information: Real-World Evidence used by Health Canada in Regulatory Decision Making. J. Pharm. Pharm. Sci. 2022, 25, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Institut National D’excellence en Santé et en Services Sociaux (INESSS) Website. Available online: https://www.inesss.qc.ca/en/index.html (accessed on 14 August 2022).
- CADTH Website. Available online: https://www.cadth.ca/sites/default/files/pdf/es0323-rwe-in-single-drug-appraisal.pdf (accessed on 23 July 2022).
- Canadian Cancer Society. Canadian Cancer Statistics Website. Available online: https://cancer.ca/en/research/cancer-statistics/canadian-cancer-statistics (accessed on 23 July 2022).
- Woo, A. Cancer Remains Leading Cause of Death in Canada but Overall Death Rate down, Five-Year Survival Rate up, Report Shows. The Globe and Mail 4 November 2021. Available online: https://www.theglobeandmail.com/canada/article-cancer-remains-leading-cause-of-death-in-canada-but-overall-death-rate/ (accessed on 14 August 2022).
- Beaver, J. Accelerated Approval for Oncology Drug Products: Regulatory Overview. Presented at U.S. FDA Oncologic Drugs Advisory Committee Meeting: Pembrolizumab Metastatic Cisplatin-ineligible Urothelial Carcinoma, 28 April 2021. Available online: https://www.fda.gov/media/147923/download (accessed on 23 July 2022).
- Health Canada Notice of Compliance with Conditions (NOC/c) Website. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/notice-compliance/conditions.html (accessed on 23 July 2022).
- Health Canada Notice of Compliance (NOC) Database Website. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/notice-compliance/database.html (accessed on 23 July 2022).
- CADTH. The pCODR Expert Review Committee (pERC) Website. Available online: https://www.cadth.ca/collaboration-and-outreach/advisory-bodies/pcodr-expert-review-committee-perc (accessed on 23 July 2022).
- CADTH Website. Available online: https://www.cadth.ca/pcodr-expert-review-committee-perc (accessed on 23 July 2022).
- Patel, D.; Grimson, F.; Mihaylova, E.; Wagner, P.; Warren, J.; van Engen, A.; Kim, J. Use of External Comparators for Health Technology Assessment Submissions Based on Single-Arm Trials. Value Health 2021, 24, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Liberti, L. The Relationship of Conditional Regulatory Approvals by HTA Recommendations–Outcome and Timing, CADTH Symposium 2019, Edmonton, Alberta, Concurrent Session B3. Available online: https://www.cadth.ca/sites/default/files/symp-2019/presentations/april15-2019/B3-presentation-lliberti.pdf (accessed on 1 September 2022).
- Health Canada Website on Summary Basis of Decision. Available online: https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?lang=en&linkID=SBD00524 (accessed on 23 July 2022).
- CADTH Reimbursement Review Reports Website. Available online: https://www.cadth.ca/reimbursement-review-reports (accessed on 23 July 2022).
- CADTH Recommendation for Idecabtagene Vicleucel. 2021. Available online: https://www.cadth.ca/sites/default/files/DRR/2021/PG0240%20Abecma%20-%20CADTH%20Final.pdf (accessed on 23 July 2022).
- Pan-Canadian Oncology Drug Review Final Clinical Guidance Report Brigatinib (Alunbrig) for Non-Small Cell Lung Cancer. 2019. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10167BrigatinibNSCLC_fnCGR_NOREDACT_POST01AUG2019_final.pdf (accessed on 23 July 2022).
- Pan-Canadian Oncology Drug Review Final Clinical Guidance Report Pralatrexate (Folotyn) for Peripheral T-Cell Lymphoma. 2019. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10138PralatrexatePTCL_fnCGR_NOREDACT_POST04Apr2019_final.pdf (accessed on 23 July 2022).
- Pan-Canadian Oncology Drug Review Final Clinical Guidance Report Enasidenib (Idhifa) for Acute Myeloid Leukemia. 2019. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10144EnasidenibAML_fnCGR_NOREDACT_Post_31Oct2019_final.pdf (accessed on 23 July 2022).
- Pan-Canadian Oncology Drug Review Final Clinical Guidance Report Cemiplimab (Libtayo) for Cutaneous Squamous Cell Carcinoma. 2020. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10187CemiplimabCSCC_fnCGR_REDACT_EarlyConv_22Jan2020_final.pdf (accessed on 23 July 2022).
- Pan-Canadian Oncology Drug Review Final Clinical Guidance Report Lorlatinib (Lorbrena) for Non-Small Cell Lung Cancer. 2020. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10183LorlatinibNSCLC_fnCGR_NOREDACT_Post_30Jan2020_final.pdf (accessed on 23 July 2022).
- CADTH pCODR Final Clinical Guidance Report: Polatuzumab Vedotin (Polivy). Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2021/10227PolatuzumabDLBCL_fnCGR_NOREDACT_EC21Apr2021_final.pdf (accessed on 23 July 2022).
- Pan-Canadian Oncology Drug Review Final Clinical Guidance Report Larotrectinib (Vitrakvi) for Neurotrophic Tyrosine Receptor Kinase (NTRK) Positive Solid Tumours. 2019. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2019/10159LarotrectinibNTRK%2BSolidTumours_fnCGR_REDACT_Post_31Oct2019_final.pdf (accessed on 23 July 2022).
- CADTH Reimbursement Review: CADTH Reimbursement Recommendation (Draft) Selpercatinib (Retevmo). 2022. Available online: https://www.cadth.ca/sites/default/files/DRR/2022/PC0261%20Retevmo%20-%20Draft%20CADTH%20Recommendation_for%20posting%20March%2031%2C%202022.pdf (accessed on 23 July 2022).
- CADTH Reimbursement Review: CADTH Reimbursement Recommendation (Draft) Tepotinib (Tepmetko). 2022. Available online: https://www.cadth.ca/sites/default/files/DRR/2022/PC0255%20Tepmetko%20-%20Draft%20CADTH%20Recommendation%20for%20Posting%20March%203%2C%202022.pdf (accessed on 23 July 2022).
- Health Canada Website on Guidance Document. Available online: https://www.canada.ca/en/health-canada/corporate/about-health-canada/legislation-guidelines/acts-regulations/service-standards-high-volume-regulatory-authorizations/service-standards-drug-submission-evaluations-pharmaceuticals-biologic-products-under-food-drug-regulations.html (accessed on 23 July 2022).
- Gotfrit, J.; Jackson, A.; Shin, J.J.W.; Stewart, D.J.; Mallick, R.; Wheatley-Price, P. Determinants of the Cancer Drug Funding Process in Canada Curr. Oncol. 2022, 29, 1997–2007. [Google Scholar]
- CADTH Procedures for Reimbursement Reviews Website. Available online: https://www.cadth.ca/cadth-procedures-reimbursement-reviews (accessed on 23 July 2022).
- Bucher, H.E.; Guyatt, G.H.; Griffith, L.E.; Walter, S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 1997, 5, 683–691. [Google Scholar] [CrossRef]
- Anderson, S.K.; Penner, N.; Chambers, A.; Trudeau, M.E.; Chan, K.K.W.; Cheung, M.C. Conditional Approval of Cancer Drugs in Canada: Accountability and Impact on Public Funding. Curr. Oncol. 2019, 26, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Hunt, M.; Beaver, J.A.; Gustav, P.G.; Gravel, C.; Raven, A.; Regier, D.; Ho, C. Access to new therapies: FDA accelerated approvals and the corresponding Canadian regulatory and funding decisions. J. Clin. Oncol. 2020, 38, e19066. [Google Scholar] [CrossRef]
- Jansen, J.P.; Trikalinos, T.; Cappelleri, J.C.; Daw, J.; Andes, S.; Eldessouki, R.; Salanti, G. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: An ISPOR-AMCP-NPC Good Practice Task Force report. Value Health 2014, 17, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Putting Real-World Healthcare Data to Work. Available online: https://rwe-navigator.eu/use-real-world-evidence/adjusting-for-bias-in-non-randomised-and-observational-studies/ (accessed on 23 July 2022).
- CADTH Reimbursement Recommendation: CADTH Final Clinical Guidance Report on Brukinsa. Available online: https://www.cadth.ca/sites/default/files/DRR/2022/PC0267-Brukinsa.pdf (accessed on 13 October 2022).
- CADTH Reimbursement Recommendation: CADTH Final Clinical Guidance Report on Tukysa. Available online: https://www.cadth.ca/sites/default/files/DRR/2022/PC0243-Tukysa-combined.pdf (accessed on 13 October 2022).
- CADTH Reimbursement Recommendation: CADTH Final Clinical Report on Odomzo. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2021/10215SonidegibBCC_inCGR_NOREDACT_Post04Mar2021_final.pdf (accessed on 13 October 2022).
- Phillippo, D.M.; Ades, A.E.; Dias, S.; Palmer, S.; Abrams, K.R.; Welton, N.J. Methods for Population-Adjusted Indirect Comparisons in Health Technology Appraisal. Med. Decis. Mak. 2018, 38, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Facey, K.M.; Rannanheimo, P.; Batchelor, L.; Borchardt, M.; de Cock, J. Real-world evidence to support Payer/HTA decisions about highly innovative technologies in the EU—Actions for stakeholders. Int. J. Technol. Assess. Health Care 2020, 36, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Sammon, C.J.; Leahy, T.P.; Gsteiger, S.; Ramagopalan, S. Real-world evidence and nonrandomized data in health technology assessment: Using existing methods to address unmeasured confounding? J. Comp. Eff. Res. 2020, 9, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Arora, P. Using Quantitative Bias Analysis in Real World Data Strategy. 2022. Available online: https://www.cytel.com/blog/using-quantitative-bias-analysis-in-real-world-data-strategy (accessed on 23 July 2022).
- CADTH Guidance Working Group Website. Available online: https://www.cadth.ca/news/introducing-cadths-rwe-guidance-working-group (accessed on 14 August 2022).
- Han, D.; Tiruneh, M.; Chambers, A.; Haynes, A. VP02 Real-World Evidence (RWE) And CADTH Pan-Canadian Oncology Drug Review. Int. J. Technol. Assess. Health Care 2018, 34, 159–160. [Google Scholar] [CrossRef]
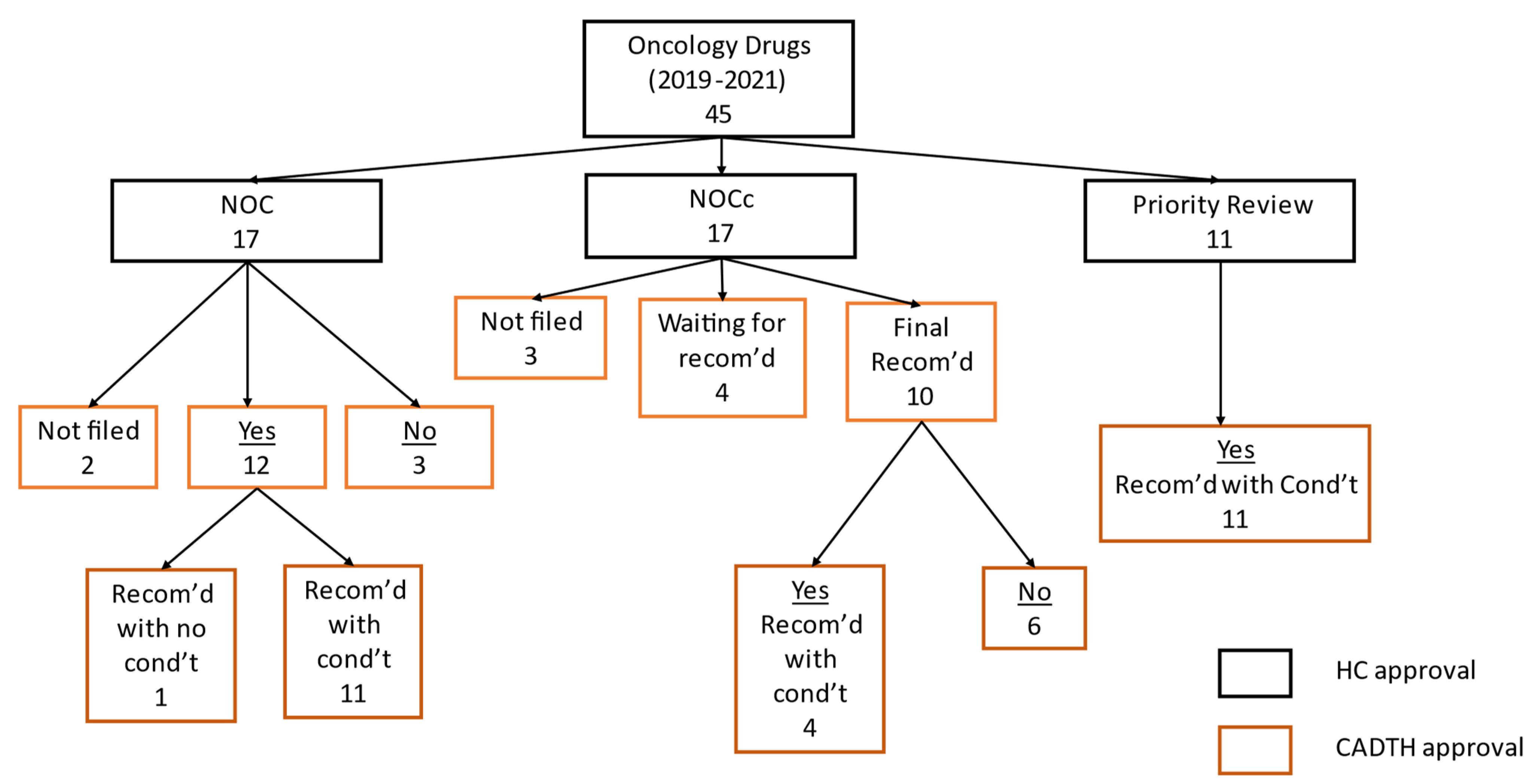
| Health Canada Review of Drugs with SBD Published between 2019 and 2021 | Number of Submissions n = 45 % (n) | Days in Review: Mean (95% CI) | Conditional and Priority Review Times as Compared to Standard Review Times | Comparing Priority Review Time as to Conditional Review Time |
|---|---|---|---|---|
| Standard review (NOC) % (n) | 37.78 (17) | 368 (346, 391) | ||
| Conditional Review (NOCc) % (n) | 37.78 (17) | 285 (206, 309) | p < 0.001 | |
| Priority Review (PR) % (n) | 24.44 (11) | 224 (174, 274) | p < 0.001 | p = 0.025 |
| CADTH Review of Oncology Drugs (with Final Recommendations Only) | Standard Approvals (n = 15) a | Conditional Approvals NOCc (n = 10) b | Priority Reviews PR (n = 11) | Conditional Review Times and Priority Review Times Compared to Standard Review Times |
|---|---|---|---|---|
| Review time (days) Mean (95% CI) | 238 (216, 260) | 240 (258, 188) | 252 (223, 308) | Conditional to Standard: p = 0.98 Priority to standard: p = 0.57 |
| Reimburse without conditions % (n) | 6.7 (1) | 0 | 0 | NA |
| Reimburse with conditions % (n) | 73.3 (11) | 40 (4) | 100 (11) | NA |
| Do not reimburse % (n) | 20 (3) | 60 (6) | 0 | NA |
| Parameters with Impact on RWE Validity | Examples Extracted from CADTH Review Reports | Decisions on Reimbursement [Ref] |
|---|---|---|
| Bias | ||
| High risk of selection bias owing to the retrospective nature of the historical comparator. | +ve [24] |
| The bias resulting from missing covariates is very difficult to quantify, and as a result, it is unclear what impact the missing covariates have on the results of the MAICs. | −ve [23] |
| There is also a potential measurement bias due to differences in the frequency and conduct of disease assessments in clinical practice versus the trial setting. | +ve [30] |
| Heterogenicity | ||
| These ITCs have a number of limitations that impact their internal and external validity, such as not being able to comprehensively assess the clinical heterogeneities across the included individual studies and their influence on the study results due to the lack of certain patient characteristics, uncertainty still exists on the treatment effect of selpercatinib despite of various adjustments, and generalizability of the study findings to patients with RET fusion-positive could be limited. | +ve [30] |
| There was limited assessment and reporting of clinically important heterogeneity, and the statistical analyses completed are unlikely to have accounted for all major differences. | −ve [31] | |
| Unresolved Confounders | ||
| The major concerns with the submitted report are related to the quality of the analysis, limited control of prognostic factors and effect modifiers, and the heterogeneity of the evidence used. | +ve [29] |
| However, it is not clear if the underlying assumption of the unanchored MAIC that all effect modifiers and prognostic factors have been accounted for was accomplished. | −ve [23] | |
| None of the articles retrieved through the literature search spoke directly about the prognostic relevance of the specific gene fusion. All primary studies retrieved through the literature search were retrospective in design. In cases where presence of the specific gene fusion was verified, sample sizes were small, making the generalizability of findings difficult to determine. | −ve [30] | |
| Methodology issues | ||
| No statistical analyses were performed (e.g., multivariate regression model analyses) to identify a subset of variables most predictive of outcome to include for matching. Further, it is unknown how missing data on variables used for matching were handled in the analysis. | +ve [25] |
| These limitations, combined with the flaw in the presentation of the methodological quality of the included studies, limits the overall confidence in the results of the methodological quality of the included studies, limits the overall confidence in the results of this review. | −ve [28] | |
| Data issues | ||
| Fewer than 25% of the remaining adherent patients continued the assessment after week 29. When the data was presented in linear plots, there appears to be higher scores in the [treated] groups and scores remained flat in the BR group, however the significant amount of missing data limits confidence in this analysis. | +ve [29] |
| which implies potential bias due to the need to rely on multiple imputation methods, increasing the uncertainty in effect estimates. | −ve [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, C.; Dranitsaris, G. Impact of Regulatory Approval Status on CADTH Reimbursement of Oncology Drugs and Role of Real-World Evidence on Conditional Approvals from 2019 to 2021. Curr. Oncol. 2022, 29, 8031-8042. https://doi.org/10.3390/curroncol29110635
Lau C, Dranitsaris G. Impact of Regulatory Approval Status on CADTH Reimbursement of Oncology Drugs and Role of Real-World Evidence on Conditional Approvals from 2019 to 2021. Current Oncology. 2022; 29(11):8031-8042. https://doi.org/10.3390/curroncol29110635
Chicago/Turabian StyleLau, Catherine, and George Dranitsaris. 2022. "Impact of Regulatory Approval Status on CADTH Reimbursement of Oncology Drugs and Role of Real-World Evidence on Conditional Approvals from 2019 to 2021" Current Oncology 29, no. 11: 8031-8042. https://doi.org/10.3390/curroncol29110635
APA StyleLau, C., & Dranitsaris, G. (2022). Impact of Regulatory Approval Status on CADTH Reimbursement of Oncology Drugs and Role of Real-World Evidence on Conditional Approvals from 2019 to 2021. Current Oncology, 29(11), 8031-8042. https://doi.org/10.3390/curroncol29110635





