Abstract
EGFR tyrosine kinase inhibitors (EGFR-TKIs) are breakthrough palliative treatments for advanced lung cancer patients with tumors harboring mutations in the EGFR gene. Using healthcare administrative data, three cohorts were created to describe the use of three EGFR-TKIs that are publicly funded in Quebec for specific indications (i.e., 1st-line gefitinib, 1st-line afatinib, and post-EGFR-TKI osimertinib). The main objective was to compare overall survival (OS) among patients receiving these treatments to those in previous experimental and real-world studies. The patients who received EGFR-TKIs for indications of interest between 1 April 2001, and 31 March 2019 (or 31 March 2020, for post-EGFR-TKI osimertinib) were included to estimate the Kaplan-Meier-based median OS for each cohort. An extensive literature search was conducted to include comparable studies. For the gefitinib 1st-line (n = 457), the afatinib 1st-line (n = 80), and the post-EGFR-TKI osimertinib (n = 119) cohorts, we found a median OS (in months) of 18.9 (95%CI: 16.3–21.9), 26.6 (95%CI: 13.7-NE) and 19.9 (95%CI: 17.4-NE), respectively. Out of the 20 studies that we retained from the literature review and where comparisons were feasible, 17 (85%) had similar OS results, which further confirms the value of these breakthrough therapies in real-world clinical practice.
1. Introduction
With about half of all cases being diagnosed at stage IV [1], lung cancer is the deadliest cancer in Canada, including Quebec [2]. Patients diagnosed with lung cancer in Canada between 2015 and 2017 had a net survival rate of 22% [2]. Only 24% of all cases in Quebec diagnosed between 2014 to 2016 survived 5 years or more from any cause of death [3].
Patients with advanced cancer (i.e., locally advanced, or metastatic) with limited curative treatment options are typically given palliative treatment with chemotherapy, immunotherapy, or targeted therapy, the latter two being breakthrough therapies introduced in the past decade. Since 2011 in Quebec, oral EGFR tyrosine kinase inhibitors (EGFR-TKI) have been a standard targeted treatment for patients with tumors harboring EGFR-TKI sensitizing mutations, which include resistance mutations [4,5]. In Ontario, up to 20% of advanced patients with non-squamous, non-small-cell lung cancer have the most common EGFR-TKI sensitizing mutations (exon 19 deletions and exon 21-L858R) [6]. About 50–65% of patients progressing on first- and second-generation EGFR-TKIs develop an EGFR-TKI resistance mutation (T790M) that can be treated with third-generation EGFR-TKIs [7,8].
In Quebec, the Institut national d’excellence en santé et en services sociaux (INESSS) evaluates the evidence on new drugs, usually based on at least one randomized controlled trial (RCT), and provides recommendations to the Minister of Health and Social Services who regulates the drug formulary of Quebec’s universal public drug coverage program [9]. EGFR-TKIs are publicly covered in Quebec for specific indications (e.g., line of treatment, EGFR mutation status, etc…) and the clinical guidelines for treatment with these EGFR-TKIs are mainly based on benefits observed in RCTs in surrogate endpoints of overall survival (e.g., progression-free survival [PFS]) [5]. Despite the availability of mature data from these trials, some participants in the control arm received EGFR-TKIs following disease progression, which potentially masked overall survival (OS) benefits.
INESSS is exploring real-world data to assess the value of breakthrough therapies in Quebec’s clinical settings, given its potential for complementing evidence from small prospective studies and assessing the real-world value of interventions after their implementation into practice [10,11,12,13,14]. If results from real-world studies show smaller benefit than RCTs, review of the funding criteria or decision could be justified. This population-based retrospective study provides such evidence to policymakers and other stakeholders by drawing a portrait of advanced lung cancer patients who received EGFR-TKIs that are publicly covered in Quebec for specific indications and comparing estimates of OS in this population to those published in RCTs and real-world studies.
2. Materials and Methods
2.1. Study Design and Data
Our study included patients who had public coverage for prescriptions filled at community pharmacies in Quebec and were treated with EGFR-TKIs for indications included in Quebec’s drug formulary (Table 1): gefitinib or afatinib for 1st-line palliative treatment, or osimertinib as a second EGFR-TKI treatment following 1st-line palliative treatment with an EGFR-TKI. Since the public coverage of EGFR-TKIs requires a treating physician to confirm their patient’s status as advanced cancer with an activating EGFR mutation, payments of these drugs by the public insurer allowed us to infer this status for patients in our study. We did not investigate EGFR-TKIs that were (1) not in Quebec’s drug formulary (e.g., dacomitinib), (2) in Quebec’s drug formulary for indications included after our recruitment period (e.g., osimertinib for 1st-line treatment), and (3) in Quebec’s drug formulary without EGFR mutation status as an indication and reimbursement criterion (e.g., erlotinib for 2nd- or 3rd-line treatments).

Table 1.
Indications for gefitinib, afatinib, and osimertinib included in the drug formulary of Quebec’s universal public drug coverage program [15].
Inpatient and outpatient care administrative data (i.e., hospitalization [MED-ECHO], physician billing [SMOD], drugs dispensed in community pharmacies [SMED], health insurance registry [FIPA]) that is managed by the Régie de l’assurance maladie du Québec (RAMQ) and the Ministère de la Santé et des Services sociaux (MSSS) were used to create three EGFR-TKI cohorts: (1) 1st-line gefitinib, (2) 1st-line afatinib, and (3) post-EGFR-TKI osimertinib. For all cohorts, we selected patients who had their first lung cancer diagnostic code in MED-ECHO or SMOD between 1 April 2001, and 31 March 2019, (lung cancer index date) [3] with at least one gefitinib, afatinib, or osimertinib prescription in the same period. We also included in the osimertinib cohort patients with at least one osimertinib prescription before 31 March 2020, after receiving gefitinib, afatinib, or erlotinib between 1 April 2001, and 31 March 2019. International nonproprietary names and drug identification numbers were used to identify EGFR-TKI prescriptions in SMED (Appendix A). All patients were followed until death (captured in FIPA), or 31 March 2020, whichever came first.
We then excluded from each cohort patients that did not receive an EGFR-TKI for the line of treatment of interest. Since we had limited information on the line of palliative treatment and chemotherapy history, we developed an algorithm to identify the line of therapy associated with patients’ 1st EGFR-TKI (Appendix B). From each cohort, we first excluded patients who were covered by a public drug insurance plan in FIPA for less than 90% of the period from 3 months before the 1st EGFR-TKI treatment to the end of follow-up (targeted treatment observation period). From the gefitinib and afatinib cohorts, we also excluded patients whose 1st EGFR-TKI was not gefitinib or afatinib, and for the osimertinib cohort, we excluded those whose 2nd EGFR-TKI was not osimertinib after receiving gefitinib, afatinib, or erlotinib. All patients identified by the algorithm as receiving their 1st EGFR-TKI other than 1st-line palliative treatment were then excluded to form the final cohorts.
This study was carried out at INESSS as part of a Health System Impact Fellowship (SQ) of the Canadian Institutes of Health Research. It was part of an initiative to promote the use of real-world data for health technology assessment in line with INESSS’s work on real-world evidence in its 2019–2022 Three-Year Business Plan which aims to “Assess the value of interventions in real care settings using medico-administrative data” [16]. INESSS is not responsible for the content of this publication, however, the results reported here were part of a larger INESSS project to study EGFR-TKIs in Quebec [17]. Access to de-identified data for this study was made possible through a tripartite agreement between the MSSS, the RAMQ, and INESSS [18]. Clinical experts were consulted to select relevant intervention codes and develop the algorithm used to identify line of treatment. A scientific advisory committee and INESSS’ Comité de l’évolution des pratiques en oncologie (CEPO), a panel of experts in cancer (hemato- and radio-oncologists, surgeons and pharmacists), were consulted throughout the study [17].
2.2. Outcomes
For each cohort, we reported the annual number of new users and other estimates from the distributions of patient characteristics, total days of the EGFR-TKI supplied, and OS.
The date of the 1st EGFR-TKI prescription fill was used to count the number of new users by fiscal year (April to March) and calculate patients’ age. The age and sex distributions in each cohort were compared to distributions of a previous population-level lung cancer cohort created by INESSS with Quebec’s health administrative databases [3]. For the post-EGFR-TKI osimertinib cohort, we reported the EGFR-TKI that was also received as 1st-line treatment. The total number of days’ supply of EGFR-TKI per patient was obtained by adding the treatment duration (in days) of each EGFR-TKI prescription reimbursed by the RAMQ.
Patients’ overall survival time was calculated as months elapsed between patients’ first EGFR-TKI prescription of interest and the date of death from any cause. Administrative censoring occurred on 31 March 2020. Patients’ follow-up in the 1st-line gefitinib and afatinib cohorts was also censored at the time of receiving osimertinib, if applicable; osimertinib increases OS relative to standard chemotherapy when given as a subsequent line after another EGFR-TKI [19,20]. Censoring at the time of receiving osimertinib was applied to compare our survival results to studies in which patients did not receive osimertinib as a subsequent therapy, including studies submitted to INESSS for evaluation of gefitinib and afatinib.
2.3. Statistical Analyses
Frequencies and proportions, means and standard deviations, or medians and ranges (minimum to maximum), were reported for distributions of age, sex, the 1st-line EGFR-TKI drug, and new users. Boxplots were used to display the distribution of total days’ supply. Median follow-up time was estimated with the reverse Kaplan-Meier method in the prodlim package in R [21], and the survival package was used to plot Kaplan-Meier survival curves and estimate median overall survival times [22].
2.4. Comparison of Overall Survival
Our study could not include a comparator group of patients with EGFR mutated tumors who were not treated with EGFR-TKIs since we did not have information on mutation status, and more importantly, in the real-world clinical setting, it would be unethical to withhold EGFR-TKI treatment in the presence of an activating EGFR mutation. Therefore, we relied on the indirect comparison of the median OS in our cohorts to that of cohorts with similar EGFR-TKI use in previous studies. In May 2021, we conducted an extensive literature review to include all published experimental trials and real-world studies that reported OS estimates related to the three EGFR-TKIs for the indications of interest (Appendix C). The studies submitted to INESSS for evaluation of these treatments that reported estimates of median OS were automatically included in our review [20,23,24,25,26,27,28].
The similarity between our results and those of other studies (point estimates and 95% confidence intervals) was assessed with the degree of overlap that took into consideration the absolute difference between point estimates and the width of the confidence intervals, but without any formal statistical tests.
3. Results
Among 552 patients who received gefitinib, 457 (82.8%) were included in the 1st-line gefitinib cohort. Out of 117 patients receiving afatinib, 80 (68.4%) were included in the 1st-line afatinib cohort. Similarly, of 178 patients who received osimertinib, 119 (66.9%) were included in the post-EGFR-TKI osimertinib cohort (Figure 1). New users of 1st-line palliative treatments with gefitinib or afatinib started to accrue in the same year as their listing in Quebec’s drug formulary in November 2011 and May 2016, respectively. In contrast, 32 patients in the osimertinib cohort received the drug in the 2 years before it was listed on the formulary in November 2018, when the highest numbers of users were observed (Figure 2 and Table 1).
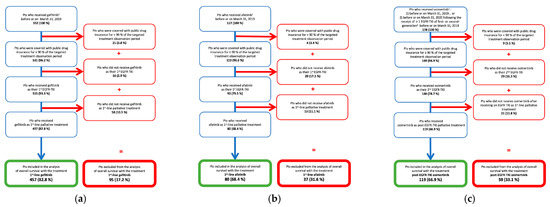
Figure 1.
Flowchart of study populations in EGFR-TKI cohorts: (a) 1st-line gefitinib cohort; (b) 1st-line afatinib cohort; (c) Post-EGFR-TKI osimertinib cohort. Abbreviations: Pts: Patients. 1 End of follow-up: 31 March 2020. 2 Gefitinib, afatinib, or erlotinib.
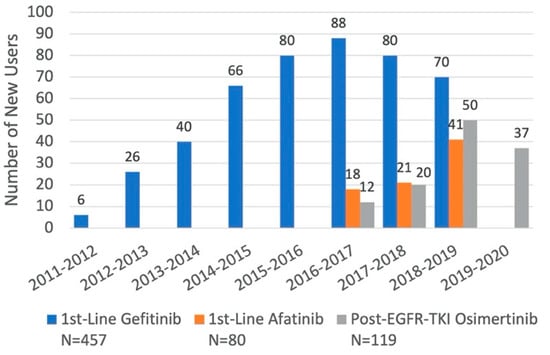
Figure 2.
Distribution of New Users of Each EGFR-TKI for Specific Indications, by Fiscal Year.
Compared to lung cancer patients in Quebec, there was a larger proportion of women in all three cohorts (60–71% vs. 49%) (Table 2 and Appendix D). The median age for the gefitinib cohort was the same as the overall cohort (71 years), whereas the median age for the afatinib cohort was lower (68 years) and that for the osimertinib cohort was higher (72 years). There was a much smaller portion of patients that were 80 years and over in the afatinib cohort in comparison to the overall cohort (7.5% vs 20.6%).

Table 2.
Characteristics of patients in EGR-TKI cohorts.
The median total days’ supply was 300 days (9.9 months) for gefitinib and osimertinib, and 274.5 days (9.0 months) for afatinib (Figure 3). Approximately 5% of patients had a total days’ supply of gefitinib that was greater than 3 years. Only 1 patient received a supply of afatinib for more than 3 years, while no one received a supply of osimertinib for this length of time.

Figure 3.
Distribution of total days’ supply: (a) 1st-line gefitinib cohort (N = 457); (b) 1st-line afatinib cohort (N = 80); (c) Post-EGFR-TKI osimertinib cohort (N = 119). Abbreviations: min: minimum; Q1: quartile 1; Q2: quartile 2 or median; Q3: quartile 3; max: maximum. The lower limit of the boxplot mustache represents the minimum, whereas the superior limit is equal to 1.5 times the interquartile range (Q3 minus Q1) plus Q3.
With a median follow-up of 29.6 months in the gefitinib cohort, we observed 295 (65%) deaths and a median OS of 18.9 months (95%CI: 16.3–21.9) (Figure 4). The afatinib cohort had a median follow-up of 17.6 months, 36 (45%) deaths and a median OS of 26.6 months (95%CI: 13.7-NE). With a median of 17.3 months of follow-up after receiving osimertinib in the post-EGFR-TKI osimertinib cohort, we observed 44 (38%) deaths and median OS was 19.9 months (95%CI: 17.4-NE). The upper bounds of the 95%CI for median OS in the afatinib and osimertinib groups were non-evaluable (NE) due to the immaturity of the data (i.e., less than 50% of patients in the cohort had the outcome).

Figure 4.
Overall survival: (a) 1st-line gefitinib cohort (N = 457); (b) 1st-line afatinib cohort (N = 80); (c) Post-EGFR-TKI osimertinib cohort (N = 119). Abbreviations: 95% CI: 95% confidence interval. The grey area around the curves represents the 95% confidence intervals.
From 2565 articles identified, we selected 10 RCTs (9 articles) and 17 real-world studies (18 articles) for indirect comparison with our OS results (Appendix C). We retained 7 RCTs [26,27,29,30,31,32,33] and 10 real-world studies [34,35,36,37,38,39,40,41,42,43] on gefitinib as 1st-line treatment (Figure 5). Among these studies, 5 RCTs and 6 real-world studies had median OS estimates similar to ours, 1 RCT [30] and 1 real-world study [40] had results superior to ours. We were limited in making comparisons with the estimates from other studies, which did not overlap our results and lacked confidence intervals. For afatinib as 1st-line treatment we retained 2 RCTs [28], and 1 study combining these RCTs [28], and 3 real-word studies [34,44,45]. All studies had similar results to ours, except for 1 real-world study for which we were limited in making a comparison due to a lack of overlap of results and the non-evaluable upper bound for our estimate’s 95%CI. We retained only 1 RCT [20] and 5 real-world studies [46,47,48,49,50] on osimertinib as a post-EGFR-TKI treatment. One real-world study [49] had a median inferior to ours and two others [47,50] had results similar to ours. Due to a lack of overlap of results and the non-evaluable upper bound of our estimate’s 95%CI, our comparisons with the other 2 real-world studies [46,48] were limited. Similarly, we were limited in the comparison with the single RCT, which had a longer median OS.

Figure 5.
Comparison of overall survival estimates with RCTs and real-world studies: (a) 1st-line gefitinib; (b) 1st-line afatinib; (c) Post-EGFR-TKI osimertinib cohort. * Studies submitted to INESSS for evaluation. Abbreviations: RCT: randomized controlled trial; NE: upper bound of 95% confidence interval is non-evaluable. The mustache around each estimate represents the 95% confidence interval. The orange or green square with a blue outline represents a median that is considered similar to Quebec’s median.
4. Discussion
In our study, the annual number of new users of gefitinib always exceeded the number of new patients using the second-generation EGFR-TKI afatinib as a 1st-line treatment. However, a decline in the use of gefitinib relative to afatinib was noted in the years following the introduction of afatinib in 2016. In contrast with other EGFR-TKIs, the use of osimertinib began before it was listed in Quebec’s drug formulary, which most likely occurred through the “exceptional patient program” that provides coverage for drugs that are not listed in Quebec’s drug formulary under exceptional circumstances. These trends indicate physicians’ proactiveness in integrating newer generations of EGFR-TKIs into clinical practice.
The high proportion of women (60–71%) we observed in all three of our cohorts is concordant with a previous study reporting that 57% of EGFR mutations in lung cancer tumours are found in women in Canada [6]. Our results are also concordant with the studies we selected from our literature review and one recent single-center study in Quebec that reported 71% of its 1st-line EGFR-TKI users being women [51]. Knowledge of a higher EGFR mutation rate in women could also drive a higher testing rate in women, as seen previously in Canada [52], and further contribute to the higher rates of EGFR-TKI use in women.
In general, all our cohorts included patients above the age of 80 years. Older patients may be less likely to receive cytotoxic chemotherapy due to a lack of clinical data on tolerability, but also due to clinical experience with the elderly who show more complications. In contrast, EGFR-TKIs have better tolerability than chemotherapy [19,32,33,53,54,55]. Furthermore, in RCTs on EGFR-TKIs, patients older than 80 years (maximum of 89 years) have been included [19,29,31,56,57], and studies on patients of 75 years or older, have reported median OS estimates similar to those of the overall population (19–35.2 months) [58,59,60,61,62,63]. However, we noted that patients in the afatinib cohort, compared to the gefitinib cohort, had a lower proportion of patients of 80 years and older (7.5% vs. 17.5%). Physicians may be hesitant in prescribing afatinib for the elderly due to its higher toxicity profile in comparison to gefitinib [56]. The trend of fewer older patients using afatinib in comparison to gefitinib has also been observed in other real-world studies [34,44,64].
When daily treatment is continuous until disease progression, estimates of total days’ supply should resemble time to treatment discontinuation, which is a proxy for PFS [65]. Indeed, the total days’ supply of EGFR-TKI in our cohorts were close to the PFS reported in the RCTs we selected from our literature review, and in one recent single-center study in Quebec. The studies reported a PFS of 8.4–11.9 months (median 10.4) for gefitinib as 1st-line treatment [27,29,31,32,33,51,53,54], and a PFS of 10.1 months for osimertinib as a post-EGFR-TKI treatment [20], while we found medians of total days’ supply of 9.9 months for both treatments. Two RCTs reported a PFS of 11.0–11.1 months for afatinib as a 1st-line treatment [28], while we found a total days’ supply of 9 months. Total days’ supply and PFS may not be equivalent when daily treatment is paused or discontinued for reasons other than progression, such as adverse events. Therefore, the small difference between both parameters that we observed for afatinib as a 1st-line treatment may have been led by its higher toxicity profile.
When indirect comparisons with our OS estimates were possible, we found 3 RCTs submitted to INESSS for evaluation of gefitinib and afatinib (IPASS, LUX-Lung 3, and LUX-Lung 6) to have results similar to ours. Among the 12 other RCT and real-world studies on gefitinib that we were able to indirectly compare with the current study, 10 studies had similar results. The 2 real-world studies on afatinib that we were able to compare with our study also had similar results. Similarly, among the 3 real-world studies on osimertinib that we were able to compare with, 2 had similar results. There were 7 out of 27 studies that reported longer median OS estimates than those in the current study, but these comparisons were limited because of a lack of overlap of results due to unreported or non-evaluable confidence intervals. Despite the uncertainty in these comparisons, it is likely that the OS gap between the RCT of osimertinib (AURA3) and the current study is real. Contrary to AURA3, patients in our post-EGFR-TKI osimertinib cohort may have received chemotherapy in between their 1st-line treatment with an EGFR-TKI and osimertinib as a second EGFR-TKI, which may have reduced the length of survival at the time of receiving osimertinib.
Based on the indirect comparisons, we conclude that the real-world estimates of OS in Quebec related to the use of the three EGFR-TKIs for specific indications are not different from most published studies that we selected from our literature review. This is further supported by one recent population-level study in Ontario that reported results similar to ours: a median OS of 21.6 months (95%CI: 19.3–23.3) for 1st-line gefitinib, and a median OS of 31.0 months (95%CI: 23.4–42.1) for 1st-line afatinib [64].
The limitations of this study include that we could not directly verify whether patients had a non-small cell type histology and an activating EGFR mutation. However, public coverage of EGFR-TKIs is conditional on these characteristics, making it unlikely for patients receiving public coverage of these medicines not to have them. The indirect comparisons in this study are subject to confounding by multiple factors such as age, smoking status, the type of EGFR mutation, and palliative treatments following EGFR-TKIs. For example, we did not include patients that had received EGFR-TKIs through private drug insurance, which likely rendered our cohorts slightly older than the ones in the RCTs submitted to INESSS for evaluations. However, we estimate the proportion of patients with private drug insurance among all EGFR-TKI users to be low since about 70% of lung cancer patients in Quebec are diagnosed at age 65 or older, when 90% of Quebec residents are registered for public drug coverage [3,66,67]. Finally, the survival estimates for the afatinib and osimertinib cohorts are less precise due to data immaturity, and the indirect comparisons of results from these cohorts with other studies imply a greater level of uncertainty.
Our study also has strengths. To our knowledge, this is the first population-level study on the use of EGFR-TKIs for specific indications in a Canadian province where public drug insurance offers full coverage of oral cancer drugs [66]. The involvement of clinical experts and a scientific committee helped us generate real-world evidence on these breakthrough therapies with analyses of Quebec’s health administrative data that are grounded in clinical and scientific expertise. Our holistic analytical approach through an extensive literature review also produced more meaningful results.
5. Conclusions
RCTs have shown that EGFR-TKIs are breakthrough therapies for advanced lung cancer patients. EGFR-TKIs have been included in Quebec’s drug formulary for specific indications, mainly based on the efficacy observed in surrogate endpoints of OS. Our study further confirms the value of these treatments in real-world clinical care, with OS rates in Quebec similar to those reported in most real-world studies and RCTs, including most RCTs used in the evaluation of these treatments for public drug coverage. Future studies should re-evaluate EGFR-TKIs with mature and richer data and, given their potential real-world value, further investigate inequities in access to these treatments.
Author Contributions
Conceptualization, S.Q., G.B., J.B. and E.S.; methodology, S.Q., G.B., C.L. and E.S.; validation, S.Q., G.B. and C.L.; formal analysis, S.Q., F.R. and K.T.G.; resources, C.T.; data curation, S.Q. and A.-C.G.; writing—original draft preparation, S.Q.; writing—review and editing, S.Q., G.B., J.B., A.-C.G., F.R., K.T.G., C.L., C.T. and E.S.; visualization, S.Q. and G.B.; supervision, J.B., E.S. and C.T.; funding acquisition, S.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was part of S.Q.’s doctoral Health System Impact Fellowship that was co-funded by the Canadian Institutes of Health Research (CIHR-Institutes of Health Services and Policy Research and Cancer Research) and the Institut national d’excellence en santé et services sociaux (INESSS), funding number HI9-166408. E.S.’s research is also supported by McGill University’s William Dawson Scholar Award.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to this study being part of a larger INESSS evaluation project stated in its 2019–2022 Three-Year Business Plan. As such, the use of de-identified secondary data is authorized by the tripartite agreement between the Ministère de la Santé et des Services sociaux (MSSS), the Régie de l’assurance maladie du Québec (RAMQ), and the Institut national d’excellence en santé et en services sociaux (INESSS).
Informed Consent Statement
This study was carried out as part of a larger evaluation project at INESSS and used de-identified secondary data made available from the tripartite agreement between the MSSS, the RAMQ, and the INESSS; informed consent was not required. Ethics review was requested before submitting to a peer-review journal, and granted by McGill University’s Faculty of Medicine and Health Sciences Institutional Review Board on 15 September 2022 (study number: A09-E38-22B).
Data Availability Statement
Restrictions apply to the availability of these data. Data are available to INESSS through RAMQ servers due to the tripartite agreement.
Acknowledgments
Nicole Bouchard, Alexis Bujold, and Catherine Labbé served as external collaborators for this project. They provided guidance, notably for developing an algorithm using health administrative databases to identify the line of palliative treatment associated with patients’ 1st EGFR-TKI treatment.
Conflicts of Interest
Among the authors listed above, some were INESSS employees. Their contributions are outlined in the contributions section. INESSS reviewed the article prior to submission for publication. The authors declare no other conflicts of interest.
Appendix A. Identification of EGFR-TKIs in Health Administrative Databases

Table A1.
International Non-Proprietary Name Codes and Drug Identification Numbers of EGFR-TKIs.
Table A1.
International Non-Proprietary Name Codes and Drug Identification Numbers of EGFR-TKIs.
| International Non-Proprietary Name (INN) | RAMQ’s Code for INN | Drug Identification Number (DIN) |
|---|---|---|
| Gefitinib | 47482 | 02468050 02248676 02487748 02491796 |
| Afatinib | 47998 48096 | 02415666 02415674 02415682 |
| Osimertinib | 48112 | 02456214 02456222 |
| Erlotinib | 47563 | 02461862 02461870 02461889 02483912 02483920 02483939 02454386 02454394 02269007 02269015 02269023 02377691 02377705 02377713 |
Appendix B. Algorithm to Identify the Line of Therapy Linked to Patients’ 1st EGFR-TKI Treatment
We first excluded patients who had a public drug insurance plan for less than 90% of the time covering 3 months before their 1st EGFR-TKI treatment to the time of death or 31 March 2020, whichever came first (i.e., targeted treatment observation period). This minimized the possibility of missing information on targeted treatments that patients may have received through private coverage. We then established the chronological sequence of all publicly reimbursed targeted therapies received between 1 April 2001, and 31 March 2020, including anaplastic lymphoma tyrosine kinase inhibitors (ALK-TKI). A new line of treatment was considered every time a patient switched targeted therapy drugs, with the possibility of an EGFR-TKI drug being assigned multiple lines.
History of cancer-related treatments (i.e., lung surgery, chemotherapy supervision, and radiotherapy) received between patients’ diagnosis date [3] and their 1st EGFR-TKI was retrieved from intervention codes in hospitalization and physician billing databases: MED-ECHO and SMOD. Patients were first grouped into 6 treatment code scenarios, and their 1st EGFR-TKI was further classified as 1st-line palliative treatment or 2nd-line or more of palliative treatment based on the previous use of chemotherapy and its context (curative versus palliative). We considered neoadjuvant chemotherapy, adjuvant chemotherapy, and chemoradiation as curative intent treatments, and, in consultation with medical experts, rules were created to identify these chemotherapies. Patients’ 1st EGFR-TKIs were assigned 1st-line treatment only in the absence of palliative chemotherapy.
Table A2 and Figure A1 further describe the steps to identify the line of treatment of patients’ 1st EGFR-TKI treatment. Table A1 (Appendix A) and Table A3 (Appendix B) provide international non-proprietary names and drug identification numbers used to identify targeted treatments in SMED and Table A4 provides intervention codes used to identify cancer-related treatments in SMOD and MED-ECHO.
More details on this algorithm can be found in a previous report [17].

Table A2.
Treatment Scenarios and Rules to Identify Chemotherapy Context.
Table A2.
Treatment Scenarios and Rules to Identify Chemotherapy Context.
| When no chemotherapy code is found prior the 1st EGFR-TKI, the latter is considered as 1st-line palliative treatment. |
| Since lung resection is considered a curative treatment (except in some cases), chemotherapy that is received prior to this surgery is also considered a curative treatment and it usually corresponds to neoadjuvant chemotherapy. The EGFR-TKI treatment that follow this chemotherapy is considered 1st-line palliative treatment. |
| Chemotherapy that comes after lung resection can correspond to adjuvant chemotherapy, which is given with curative intent if certain temporal conditions are met. Firstly, the chemotherapy should be given within 6 weeks of surgery. In practice, there may be a longer delay, therefore, we selected a more realistic margin of 12 weeks (≤84 days) that will allow the identification of most patients that received adjuvant chemotherapy (experts’ opinion). Secondly, since this type of chemotherapy usually involves 4 treatment cycles (4 months) [68], it should occur within a limited timeframe within patient’s treatment trajectory. We selected a margin of 6 months after the 1st chemotherapy code to account for potential treatment delays. When the 1st temporal condition is met (chemotherapy ≤ 12 weeks or 84 days after surgery), the chemotherapy is considered as adjuvant (curative intent; scenario 3B). If no additional chemotherapy code is found after 6 months, the patient is considered to receive no palliative chemotherapy and their 1st EGFR-TKI is considered as 1st-line palliative treatment (scenario 3B1). If ≥1 chemotherapy code is found after the 6 months period (scenario 3B2), the patient is moved into scenario 6 (see scenario 6). When the 1st chemotherapy occurs >12 weeks after surgery (scenario 3A), the patient is moved into scenario 6 (see scenario 6). |
| The physician billing code 8519 corresponds to treatment verification of an irradiated site (under treatment), and, according to experts that were consulted, it is systematically billed on a weekly basis throughout the treatment. Therefore, this code allows the estimation of the duration of radiotherapy. In the absence of a lung surgery, a combination of chemotherapy and radiation therapy can be administered with curative intent (chemoradiation) or in palliative intent (chemotherapy + palliative radiotherapy). The difference between these two types of treatments is that curative chemoradiation involves a higher dose of radiotherapy (generally 60-66 Gy for 6–7 weeks according to standard fractionation) [4]. Identifying ≥4 weeks of radiotherapy (≥4 × code 8519) can be used as a marker of curative treatment, whereas the presence of ≤3 weeks of radiotherapy corresponds to a palliative treatment (experts’ opinion). If the radiotherapy is considered curative (scenario 4A), the algorithm verifies if all chemotherapies occur within 6 months. If no chemotherapy is found beyond 6 months of the 1st chemotherapy, the patient is considered to receive no palliative chemotherapy and their 1st EGFR-TKI is considered as 1st-line palliative treatment (scenario 4A1). If ≥1 chemotherapy code is found after the 6 months of the 1st chemotherapy (scenario 4A2), the patient is moved into scenario 6 (see scenario 6). |
| When chemotherapy and radiotherapy codes are observed after lung surgery, they could be related to (1) adjuvant/post-operative chemotherapy and radiotherapy treatments with curative intent, (2) chemotherapy and radiotherapy treatments with palliative intent, or (3) salvage chemoradiotherapy with curative intent. If the 1st chemotherapy code occurs in ≤12 weeks of the most recent lung resection (scenario 5B), it is considered as a curative treatment, irrespective of radiotherapy codes that may be present. If no chemotherapy is observed beyond 6 months after the 1st chemotherapy, the patient is considered to receive no palliative chemotherapy and their 1st EGFR-TKI is considered as 1st-line palliative treatment (scenario 5B1). If ≥1 chemotherapy code is found beyond 6 months of the 1st chemotherapy (scenario 5B2), the patient is assessed for receiving salvage chemoradiotherapy (curative) with the same steps as in scenario 4 (scenario 5B2A = 4A and scenario 5B2B = 4B). If the 1st chemotherapy code occurs in >12 weeks of the most recent lung resection (scenario 5A), the patient is assessed for receiving salvage chemoradiotherapy (curative) with the same steps as in scenario 4 (scenario 5A1 = 4A and scenario 5A2 = 4B). |
| When chemotherapy is the only treatment observed, it always administered with palliative intent. Patients with this chemotherapy are considered to receive their 1st EGFR-TKI as 2nd-line or more of palliative treatment. An exception to this rule is when only 1 chemotherapy code is found which is within 30 days prior to the 1st EGFR-TKI, the latter is considered as 1st-line palliative treatment (scenario 6A). According to the experts we consulted, it is possible in clinic that some patients received a chemotherapy as an immediate treatment due to long delays in receiving mutation test results and patients pressing health state. In this case, the experts recommended that patients’ 1st EGFR-TKI be considered as a 1st-line palliative treatment |
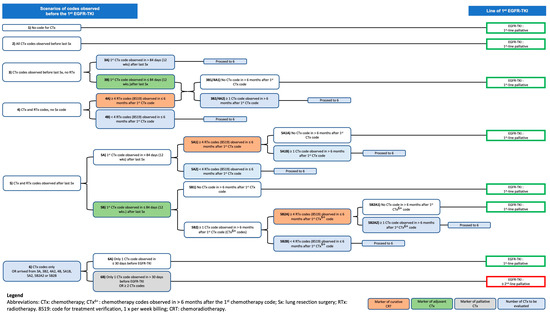
Figure A1.
Classification of patients according to the line of treatment with 1st EGFR-TKI.

Table A3.
International Non-Proprietary Names and Drug Identification Numbers of ALK-TKIs.
Table A3.
International Non-Proprietary Names and Drug Identification Numbers of ALK-TKIs.
| International Non-Proprietary Name (INN) | RAMQ’s Code for INN | Drug Identification Number (DIN) |
|---|---|---|
| Crizotinib | 47913 | 02384256 02384264 |
| Alectinib | 48129 | 02458136 |
| Céritinib | 48049 | 02436779 |

Table A4.
Cancer-Related Intervention Codes in SMOD and MED-ECHO.
Table A4.
Cancer-Related Intervention Codes in SMOD and MED-ECHO.
| |
|---|---|
| A1. Chemotherapy (and Immunotherapy) 1 | |
| 734 | Supervision de l’administration de chimiothérapie |
| 15272 | (Forfait pour Clinique de chimiothérapie) Suivi et administration, le cas échéant, des traitements de chimiothérapie intraveineuse aux patients atteints d’un cancer et dont le chirurgien général a la charge. NOTE: Cet acte est accordé pour les activités réalisées au sein d’une clinique spécifique d’oncologie, pour un minimum de 5 patients par demi-journée. NOTE: Maximum quatre fois par médecin, par période de quatorze jours. NOTE: Aucun procédé diagnostique et thérapeutique, aucune visite ni aucune chirurgie ne peut être facturé avec le code 15272. |
| 15403 | (Forfait pour Clinique de chimiothérapie) Suivi et administration, le cas échéant, des traitements de chimiothérapie intraveineuse aux patients atteints d’un cancer et dont le médecin spécialiste en médecine interne a la charge. NOTE: Cet acte est accordé pour les activités réalisées au sein d’une clinique spécifique d’oncologie, pour un minimum de 5 patients par demi-journée. NOTE: Maximum quatre fois, par médecin, par période de quatorze jours. NOTE: Aucun procédé diagnostique et thérapeutique, aucune visite ni aucune chirurgie ne peut être facturé avec le code 15403. |
| 152 | Perfusion intraveineuse de gammaglobuline, incluant la surveillance |
| A2. Radiotherapy 2 | |
| 8503 | Injection intraveineuse de substance de contraste, supplément (Planification du traitement par radiations à l’aide de la tomodensitométrie—8553) |
| 8504 | Radiothérapie avec modulation d’intensité par planification inverse NOTE: Le code 08504 et le code 08564 sont mutuellement exclusifs. |
| 8511 | Évaluation et ajustement de la configuration des champs de radiation et de la collimation |
| 8512 | Soins médicaux à visée palliative, prodigués par un médecin spécialiste en radiooncologie, par site anatomique |
| 8518 | Vérification simulée de localisation à partir de documents radiologiques |
| 8519 | Vérification sous thérapie de site d’irradiation à partir de documents radiologiques. Maximum une fois par semaine, du lundi au dimanche, par patient, par site anatomique |
| 8520 | Étude de la dosimétrie à l’ordinateur (radiothérapie transcutanée) |
| 8553 | Planification du traitement par radiations à l’aide de la tomodensitométrie |
| 8554 | Irradiation stéréotaxique, incluant la planification et les séances de traitement, par site tumoral. Maximum 1 fois par patient, par site anatomique, par mois. Maximum 6 fois par patient à vie pour tous sites anatomiques. NOTE: Le code 08554 ne peut être facturé avec les codes 08511, 08518, 08520 et 08553 à la même séance. |
| 8564 | Radiothérapie avec modulation d’intensité |
| 8565 | Radio-oncologie/Fusion d’images |
| 15465 | Présentation à un ou plusieurs radiooncologues du dossier d’un patient avec évaluation formelle du plan de traitement élaboré, avec note au dossier. (centre hospitalier) NOTE: Le code 15465 n’est facturable que par le radiooncologue présentateur, et ce, par site anatomique. |
| 20158 | Planification du traitement par radiations avec imagerie multimodalité. NOTE: Le code 20158 ne peut être facturé avec le code 08565 à la même séance. |
| 20159 | Planification d’un site de réirradiation comportant un risque de chevauchement de champ d’irradiation utilisé dans le passé. NOTE: Le code 20159 ne peut être facturé plus d’une fois par patient, par trimestre. |
| 20160 | Planification du traitement par radiations à l’aide de la tomodensitométrie en 4D incluant la synchronisation respiratoire, le cas échéant. NOTE: Le code 20160 ne peut être facturé avec le code 20158, si effectué quinze jours avant ou après. NOTE: Le code 20160 ne peut être facturé avec le code 08565, le même jour. |
| 20181 | Bronchoscopie—avec insertion de marqueurs (grains d’or) au niveau pulmonaire (transbronchique) pour radiothérapie stéréotaxique, supplément |
| 8507 | Planification du traitement par radiations lésions non cutanées—plus de 30 min mais moins de 45 min, supplément |
| 8508 | Planification du traitement par radiations lésions non cutanées—45 min ou plus, supplément |
| 8509 | Planification du traitement par radiations à l’aide de la tomodensitométrie—plus de 45 min, supplément |
| A3. Surgery—Lung Resection | |
| 3078 | avec angioplastie, supplément (3125) |
| 3079 | Pneumonectomie, réintervention plus de 30 jours après l’intervention initiale, supplément |
| 3122 | Résection cunéiforme (Wedge) |
| 3124 | Segmentectomie simple incluant bronches et artère segmentaire |
| 3125 | Lobectomie simple avec ou sans évidement ganglionnaire |
| 3126 | segmentectomie additionnelle, supplément (3125) |
| 3127 | lobectomie moyenne (côté droit), supplément (3125) |
| 3128 | avec résection en manchon d’une bronche, supplément (3125) |
| 3129 | avec bronchoplastie, supplément (3125) |
| 3130 | résection de paroi thoracique, sans reconstruction, supplément (3125) |
| 3131 | résection de paroi thoracique, avec reconstruction prosthétique, tout type, supplément (3125) |
| 3132 | Lobectomie avec ou sans évidement ganglionnaire incluant résection de la paroi, pour tumeur de Pancoast |
| 3133 | Pneumonectomie simple avec ou sans évidement ganglionnaire |
| 3134 | Pneumonectomie simple—Péricardectomie (résection intrapéricardique), supplément |
| 3135 | Pneumonectomie simple avec résection de paroi thoracique sans reconstruction, supplément |
| 3136 | Pneumonectomie simple avec résection de paroi thoracique avec reconstruction, supplément |
| 3137 | Pneumonectomie simple avec résection de l’éperon trachéal incluant la réparation, supplément |
| 3139 | Réintervention pour lobectomie plus de 30 jours après l’intervention initiale, supplément |
| 3140 | Excision—chaque résection additionnelle (maximum 3), supplément (3122) |
| 3162 | pneumonectomie complémentaire si envahissement de la marge de résection, supplément (3125) |
| |
| B1. Chemotherapy | |
| 1ZZ35CAM0 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche par voie naturelle (orale) utilisation d’agents anticancéreux SAI |
| 1ZZ35CAM1 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche par voie naturelle (orale) utilisation d’un agent alcoylant |
| 1ZZ35CAM2 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche par voie naturelle (orale) utilisation d’agents antimétabolites |
| 1ZZ35CAM3 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche par voie naturelle (orale) utilisation d’agents alcaloïdes et d’autres produits naturels |
| 1ZZ35CAM4 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche par voie naturelle (orale) utilisation d’agents antibiotiques cytotoxiques et de substances similaires |
| 1ZZ35CAM5 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche par voie naturelle (orale) utilisation d’autres agents anticancéreux |
| 1ZZ35CAM7 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche par voie naturelle (orale) utilisation d’agents immunostimulants |
| 1ZZ35CAM8 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche par voie naturelle (orale) utilisation d’agents immunosuppresseurs |
| 1ZZ35CAM9 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche par voie naturelle (orale) utilisation d’agents anticancéreux combinés [multiples] |
| 1ZZ35HAM0 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche percutanée [intramusculaire, intraveineuse, souscutanée, intradermique] utilisation d’agents anticancéreux SAI |
| 1ZZ35HAM1 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche percutanée [intramusculaire, intraveineuse, souscutanée, intradermique] utilisation d’un agent alcoylant |
| 1ZZ35HAM2 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche percutanée [intramusculaire, intraveineuse, souscutanée, intradermique] utilisation d’agents antimétabolites |
| 1ZZ35HAM3 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche percutanée [intramusculaire, intraveineuse, souscutanée, intradermique] utilisation d’agents alcaloïdes et d’autres produits naturels |
| 1ZZ35HAM4 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche percutanée [intramusculaire, intraveineuse, souscutanée, intradermique] utilisation d’agents antibiotiques cytotoxiques et de substances similaires |
| 1ZZ35HAM5 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche percutanée [intramusculaire, intraveineuse, souscutanée, intradermique] utilisation d’autres agents anticancéreux |
| 1ZZ35HAM7 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche percutanée [intramusculaire, intraveineuse, souscutanée, intradermique] utilisation d’agents immunostimulants |
| 1ZZ35HAM8 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche percutanée [intramusculaire, intraveineuse, souscutanée, intradermique] utilisation d’agents immunosuppresseurs |
| 1ZZ35HAM9 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs approche percutanée [intramusculaire, intraveineuse, souscutanée, intradermique] utilisation d’agents anticancéreux combinés [multiples] |
| 1ZZ35YAM0 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs voie NCA [transdermique, etc.] utilisation d’agents anticancéreux SAI |
| 1ZZ35YAM2 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs voie NCA [transdermique, etc.] utilisation d’agents antimétabolites |
| 1ZZ35YAM3 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs voie NCA [transdermique, etc.] utilisation d’agents alcaloïdes et d’autres produits naturels |
| 1ZZ35YAM4 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs voie NCA [transdermique, etc.] utilisation d’agents antibiotiques cytotoxiques et de substances similaires |
| 1ZZ35YAM5 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs voie NCA [transdermique, etc.] utilisation d’autres agents anticancéreux |
| 1ZZ35YAM9 | Pharmacothérapie, corps entier, agents anticancéreux et immunomodulateurs voie NCA [transdermique, etc.] utilisation d’agents anticancéreux combinés [multiples] |
| B2. Radiotherapy 2 | |
| 1GT27JA | Rayonnements, poumon NCA, faisceau externe |
| B3. Surgery—Lung Resection | |
| 1GM80DA | Réparation, bronches NCA, avec technique d’apposition simple [par exemple, suture] approche endoscopique (percutanée) |
| 1GM80DAXXE | Réparation, bronches NCA, avec lambeau local approche endoscopique (percutanée) |
| 1GM80LA | Réparation, bronches NCA, avec technique d’apposition simple [par exemple, suture] approche ouverte |
| 1GM80LAXXE | Réparation, bronches NCA, avec lambeau local approche ouverte |
| 1GM80LAXXG | Réparation, bronches NCA, avec lambeau pédiculé à distance approche ouverte |
| 1GM87DA | Excision partielle, bronches NCA, approche endoscopique (percutanée) |
| 1GM87LA | Excision partielle, bronches NCA, approche ouverte |
| 1GN92LA | Excision radicale avec reconstruction, éperon trachéal, approche ouverte |
| 1GR87DA | Excision partielle, lobe du poumon, approche endoscopique [chirurgie thoracique vidéo assistée] |
| 1GR87NW | Excision partielle, lobe du poumon, approche intrapéricardique [transpéricardique] |
| 1GR87PN | Excision partielle, lobe du poumon, télémanipulation robotisée d’outils |
| 1GR87QB | Excision partielle, lobe du poumon, approche thoracique ouverte |
| 1GR89DA | Excision totale, lobe du poumon, approche endoscopique [chirurgie thoracique vidéo assistée] |
| 1GR89NW | Excision totale, lobe du poumon, approche intrapéricardique [transpéricardique] |
| 1GR89QB | Excision totale, lobe du poumon, approche thoracique ouverte |
| 1GR91NW | Excision radicale, lobe du poumon, approche intrapéricardique ouverte [transpéricardique] avec fermeture simple |
| 1GR91NWXXA | Excision radicale, lobe du poumon, approche intrapéricardique ouverte [transpéricardique] avec autogreffe [péricarde] |
| 1GR91NWXXF | Excision radicale, lobe du poumon, approche intrapéricardique ouverte [transpéricardique] avec lambeau libre |
| 1GR91NWXXG | Excision radicale, lobe du poumon, approche intrapéricardique ouverte [transpéricardique] avec lambeau pédiculé à distance |
| 1GR91NWXXL | Excision radicale, lobe du poumon, approche intrapéricardique ouverte [transpéricardique] avec xénogreffe |
| 1GR91NWXXN | Excision radicale, lobe du poumon, approche intrapéricardique ouverte [transpéricardique] avec matériel synthétique |
| 1GR91NWXXQ | Excision radicale, lobe du poumon, approche intrapéricardique ouverte [transpéricardique] avec source combinée de tissus |
| 1GR91QB | Excision radicale, lobe du poumon, approche thoracique ouverte avec fermeture simple |
| 1GR91QBXXA | Excision radicale, lobe du poumon, approche thoracique ouverte avec autogreffe [péricarde] |
| 1GR91QBXXF | Excision radicale, lobe du poumon, approche thoracique ouverte avec lambeau libre |
| 1GR91QBXXG | Excision radicale, lobe du poumon, approche thoracique ouverte avec lambeau pédiculé à distance |
| 1GR91QBXXN | Excision radicale, lobe du poumon, approche thoracique ouverte avec matériel synthétique |
| 1GR91QBXXQ | Excision radicale, lobe du poumon, approche thoracique ouverte avec source combinée de tissus |
| 1GT80LA | Réparation, poumon NCA, approche ouverte |
| 1GT87DA | Excision partielle, poumon NCA, approche endoscopique [chirurgie thoracique vidéo assistée] |
| 1GT87NW | Excision partielle, poumon NCA, approche intrapéricardique [transpéricardique] |
| 1GT87QB | Excision partielle, poumon NCA, approche thoracique ouverte |
| 1GT89DA | Excision totale, poumon NCA, approche endoscopique [chirurgie thoracique vidéo assistée] |
| 1GT89NW | Excision totale, poumon NCA, approche intrapéricardique [transpéricardique] |
| 1GT89QB | Excision totale, poumon NCA, approche thoracique ouverte |
| 1GT91NW | Excision radicale, poumon NCA, avec fermeture simple approche intrapéricardique ouverte [transpéricardique] |
| 1GT91NWXXF | Excision radicale, poumon NCA, avec lambeau libre approche intrapéricardique ouverte [transpéricardique] |
| 1GT91NWXXG | Excision radicale, poumon NCA, avec lambeau pédiculé à distance approche intrapéricardique ouverte [transpéricardique] |
| 1GT91NWXXN | Excision radicale, poumon NCA, avec matériel synthétique approche intrapéricardique ouverte [transpéricardique] |
| 1GT91NWXXQ | Excision radicale, poumon NCA, avec source combinée de tissus approche intrapéricardique ouverte [transpéricardique] |
| 1GT91QB | Excision radicale, poumon NCA, avec fermeture simple approche thoracique ouverte |
| 1GT91QBXXF | Excision radicale, poumon NCA, avec lambeau libre approche thoracique ouverte |
| 1GT91QBXXG | Excision radicale, poumon NCA, avec lambeau pédiculé à distance approche thoracique ouverte |
| 1GT91QBXXN | Excision radicale, poumon NCA, avec matériel synthétique approche thoracique ouverte |
| 1GT91QBXXQ | Excision radicale, poumon NCA, avec source combinée de tissus approche thoracique ouverte |
| |
| C1. Chemotherapy | |
| 1355 | Injection/infusion d’agents chimiotherap.du cancer/Injection agents chimiothérapeutiques |
| C2. Radiotherapy 2 | |
| 621 | Teleradiotherapie |
| 623 | Teleradiotherapie par electrons |
| 624 | Teleradiotherapie par autres particules |
| 639 | Autre procede radiotherapeutique |
| C3. Surgery—Lung Resection | |
| 4411 | Resection en bloc des bronches |
| 4419 | Autres excision des bronches nca/Exérèse bronches |
| 4439 | Resection segmentaire du poumon (basilaire) (superieur)/Segmentectomie pulmonaire |
| 4449 | Lobectomie du poumon/Lobectomie pulmonaire |
| 4459 | Pneumonectomie/Pneumonectomie complete |
| 4499 | Autre excision du poumon |
| 4543 | Autre reparation et plastie des bronches/Réparation et plastie bronches |
| 4545 | Autre reparation et plastie du poumon |
| 4593 | Aut. op. sur les bronches |
| 4595 | Aut. op. sur le poumon |
| 4639 | Exérèse lésion cage paroi thoracique/Exc./destruction de lesion de la cage thoracique |
| 4669 | Réparation paroi thoracique |
1 Even though palliative immunotherapy is not indicated for treating lung cancer patients whose tumors harbor an EGFR mutation, the billing code for intravenous infusion of gamma globulins (152) was included in the chemotherapy category to account for situations where it may have been prescribed prior to an EGFR-TKI treatment. 2 All radiotherapy codes were used to group patients into 1 of 6 initial treatment code scenarios, however, only the code 8519 (in bold) was used for subsequent steps of the algorithm.
Appendix C. Literature Review on Experimental and Real-World Studies Reporting Survival Data for EGFR-TKIs for Indications of Interest
A preliminary literature search of the “snowball” type was carried out through the MEDLINE (PubMed) database. The goal of this preliminary search was to explore the literature to develop the search strategy and inclusion and exclusion criteria of our final literature review. PubMed’s “similar articles” function allowed us to consult randomized controlled trials (i.e., experimental trials) on EGFR-TKIs similar to those presented in the summary tables of the algorithm for care of lung cancer patients [69].
The final systematic search of the literature was conducted to include all experimental trials and real-world studies reporting overall survival data related to the three EGFR-TKIs for indications of interest. In May 2021, we searched for articles in French or English that were published since 2005 in the following bibliographic databases: MEDLINE (Ovid), Embase (Ovid) et EBM Reviews: Cochrane Database of Systematic Reviews (EBSCO). Table A5 and Table A6 provide information on the PICO-based (i.e., population, intervention, comparator, outcome) inclusion/exclusion criteria and search strategy, respectively. The studies that were submitted to INESSS for evaluation of the EGFR-TKI for indications of interest and reported overall survival results were included in our review, irrespective of inclusion/exclusion criteria. [20,23,24,25,26,27,28] The results from the article selection process are presented in a flow diagram in Figure A2.
Two reviewers (SQ and GB) were each responsible for a part of the abstract and full-text screening. The references of retained articles were also searched. Data extraction was divided between the two reviewers and particular attention was given to subsequent treatments (i.e., osimertinib) in studies on 1st-line treatment with gefitinib or afatinib. Table A7 provides the data abstracted from each study, in addition to the median overall survival results that are presented in Figure 5 of the main text.

Table A5.
Inclusion and Exclusion Criteria.
Table A5.
Inclusion and Exclusion Criteria.
| PICO | Criteria | |
|---|---|---|
| Inclusion | Exclusion | |
| Population (P) |
|
|
| Intervention (I) |
| For the gefitinib and afatinib: the use of osimertinib in a subsequent line (when mentioned) unless it involves less than 5% of all patients.2 |
| Comparator (C) |
| - |
| Outcomes (O) | Median overall survival | - |
| Study Design | Experimental trials, real-world studies, systematic reviews 1, meta-analyses 1 | Narrative reviews |
1 Systematic reviews and meta-analyses were only included to search for additional studies that may have been missed by the literature review. 2 Osimertinib is known to increase overall survival relative to standard chemotherapy when given as a subsequent EGFR-TKI [19,20].

Table A6.
Search Strategy.
Table A6.
Search Strategy.
| MEDLINE (Ovid) Retrieval date: May 2021 Restrictions: 2005-; English, French | |
|---|---|
| 1 | (gefitinibOR iressa* OR ZD1839* OR ZD-1839*).ti,ab,hw,kf,kw |
| 2 | (afatinib* OR giotrif OR gilotrif OR BIBW-2992* OR BIBW2992*).ti,ab,hw,kf,kw |
| 3 | (osimertinib OR tagrisso OR AZD9291* OR AZD-9291*).ti,ab,hw,kf,kw |
| 4 | (adenocarcinoma* OR non-squamous OR non-small-cell lung cancer OR NSCLC).ti,ab,hw,kf,kw |
| 5 | survival.ti,ab |
| 6 | lung.ti,ab |
| 7 | 1 AND 4 AND 5 AND 6 |
| 8 | 2 AND 4 AND 5 AND 6 |
| 9 | 3 AND 4 AND 5 AND 6 |
| 10 | (animal* OR cell line* OR vitro OR invitro OR rat OR rats OR mouse OR mice).ti,ab |
| 11 | (case report* OR comment* OR reply OR replies OR editorial* OR letter* OR cost* OR econom* OR conference* OR opinion* OR consensus).ti |
| 12 | (Case Reports OR Comment OR Editorial OR Letter OR Review OR Guideline OR Practice Guideline OR Consensus Development Conference).pt |
| 13 | (Meta-Analysis OR Randomized Controlled Trial OR Systematic Review).pt |
| 14 | 12 AND 13 |
| 15 | 12 NOT 14 |
| 16 | 7 NOT 10 |
| 17 | 16 NOT 11 |
| 18 | 17 NOT 15 |
| 19 | (random* OR meta-analy* OR real-world OR real-life OR retrospect* OR observational).ti,ab,hw,kf,kw |
| 20 | 17 AND 19 |
| 21 | 18 OR 20 |
| 22 | 8 NOT 10 |
| 23 | 22 NOT 11 |
| 24 | 23 NOT 15 |
| 25 | 19 AND 23 |
| 26 | 24 OR 25 |
| 27 | 9 NOT 10 |
| 28 | 27 NOT 11 |
| 29 | 28 NOT 15 |
| 30 | 19 AND 27 |
| 31 | 29 OR 30 |
| Embase (Ovid) Retrieval date: May 2021 Restrictions: 2005-; English, French | |
| 1 | (gefitinib* OR iressa* OR ZD1839* OR ZD-1839*).ti,ab, |
| 2 | (afatinib* OR giotrif OR gilotrif OR BIBW-2992* OR BIBW2992*).ti,ab |
| 3 | (osimertinib OR tagrisso OR AZD9291* OR AZD-9291*).ti,ab |
| 4 | (adenocarcinoma* OR non-squamous OR non-small-cell lung cancer OR NSCLC).ti,ab |
| 5 | survival.ti,ab |
| 6 | 1 AND 4 AND 5 |
| 7 | 2 AND 4 AND 5 |
| 8 | 3 AND 4 AND 5 |
| 9 | (animal* OR cell line* OR vitro OR invitro OR rat OR rats OR mouse OR mice).ti,ab |
| 10 | (case report* OR comment* OR reply OR replies OR editorial* OR letter* OR cost* OR econom* OR conference* OR opinion* OR consensus).ti |
| 11 | (Case Report OR Comment OR Editorial OR Letter OR “Review” OR Practice Guideline OR Consensus Development).pt |
| 12 | Meta Analysis/OR Randomized Controlled Trial/OR “Systematic Review”/ |
| 13 | 11 AND 12 |
| 14 | 11 NOT 13 |
| 15 | 6 NOT 9 |
| 16 | 15 NOT 10 |
| 17 | 16 NOT 14 |
| 18 | (random* OR meta-analy* OR real-world OR real-life OR retrospect* OR observational).ti,ab,hw,kw |
| 19 | 16 AND 18 |
| 20 | 17 OR 19 |
| 21 | limit 20 to embase |
| 22 | limit 20 to exclude medline journals |
| 23 | 21 OR 22 |
| 24 | 7 NOT 9 |
| 25 | 24 NOT 10 |
| 26 | 25 NOT 14 |
| 27 | 18 AND 25 |
| 28 | 26 OR 27 |
| 29 | limit 28 to embase |
| 30 | limit 28 to exclude medline journals |
| 31 | 29 OR 30 |
| 32 | 8 NOT 9 |
| 33 | 32 NOT 10 |
| 34 | 33 NOT 14 |
| 35 | 18 AND 33 |
| 36 | 34 OR 35 |
| 37 | limit 36 to embase |
| 38 | limit 36 to exclude medline journals |
| 39 | 37 OR 38 |
| EBM Reviews: Cochrane Database of Systematic Reviews (EBSCO) Retrieval date: May 2021 Restrictions: 2005-; English, French | |
| 1 | (gefitinib* OR iressa* OR ZD1839* OR ZD-1839*).mp |
| 2 | (afatinib* OR giotrif OR gilotrif OR BIBW-2992* OR BIBW2992*).mp |
| 3 | (osimertinib OR tagrisso OR AZD9291* OR AZD-9291*).mp |
| 4 | (adenocarcinoma* OR non-squamous OR non-small-cell lung cancer OR NSCLC).mp |
| 5 | survival.mp |
| 6 | 1 AND 4 AND 5 |
| 7 | 2 AND 4 AND 5 |
| 8 | 3 AND 4 AND 5 |
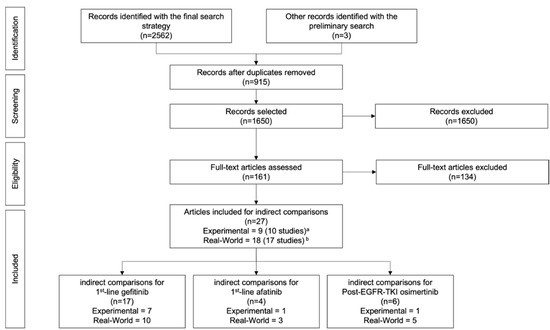
Figure A2.
Flow Diagram of the Article Selection Process. a 1 article [28] included 2 randomized controlled trials (i.e., 2 studies) on afatinib as 1st-line treatment. b 1 article [34], which included 1 real-world study, was counted twice since it was used in the indirect comparisons of EGFR-TKIs for 2 indications: 1st-line gefitinib treatment and 1st-line afatinib treatment.

Table A7.
Articles Included for Indirect Comparison of Median Overall Survival Results.
Table A7.
Articles Included for Indirect Comparison of Median Overall Survival Results.
| Author(s) and Year [Study Name] | Geographical Region | Single Center/Multicenter | n (Group with EGFR-TKI of Interest) | Median Follow-Up (Months) | Osimertinib as a Subsequent Treatment |
|---|---|---|---|---|---|
| 1st-line gefitinib treatment | |||||
| Experimental Trials | |||||
| Garcia-Campelo et al., 2020 [29] [GOAL] | Spain and Mexico | Multicenter | 91 | 26.2 |
|
| Yoshioka et al., 2019 [30] [WJTOG3405] | Japan | Multicenter | 88 | 59.1 | No patient received osimertinib |
| Yang et al., 2018 [31] | China | Single center | 35 | N/A |
|
| Han et al., 2017 [32] | China | Single center | 81 | N/A |
|
| Patil et al., 2017 [33] | India | Single center | 145 | 14.2 |
|
| Inoue et al., 2013 [26] [NEJ002] a | Japan | Multicenter | 114 | 23.2 | No patient could have received osimertinib before the end of study follow-up (December 2010) |
| Fukuoka et al., 2011 [27] [IPASS] a,b | East Asia | Multicenter | 132 | 17.0 | No patient could have received osimertinib before the end of study follow-up (June 2010) |
| Real-World Studies | |||||
| Brat et al., 2020c [34] | Czech Republic | Multicenter | 325 | N/A |
|
| Hsieh et al., 2020 [35] | Taiwan | Multicenter | 3982 | 20.3 |
|
| Choi et al., 2018 [36] | South Korea | Single center | 60 | 18 |
|
| Imai et al., 2017 [37] | Japan | Multicenter | 138 | N/A |
|
| Krawczyk et al., 2017 [38] | Poland | Multicenter | 66 | N/A | No patient received osimertinib |
| Sutiman et al., 2017 [39] | Singapore | Single center | 383 | N/A |
|
| Inoue et al., 2016 [40] | Japan | Multicenter | 929 | N/A |
|
| Vavala et al., 2016 [41] [BE-POSITIVE] | Italy | Multicenter | 288 | N/A |
|
| Lai et al., 2014 [42] | Taiwan | Single center | 85 | N/A |
|
| Park et al., 2013 [43] | South Korea | Single center | 50 | N/A |
|
| 1st-line afatinib treatment | |||||
| Experimental Trials | |||||
| Yang et al., 2015 d [28] [LUX-Lung 3] e | International (Asian: 71.7%) | Multicenter | 230 | 41 | 1% of patients received osimertinib after afatinib (threshold for study exclusion: >5%) |
| Yang et al., 2015 d [28] [LUX-Lung 6] e | China, South Korea, and Thailand | Multicenter | 242 | 33 | No patient received osimertinib |
| Yang et al., 2015 d [28] [LUX-Lung-3 and 6 combined] | International | Multicenter | 472 | N/A | See individual studies above |
| Real-World Studies | |||||
| Brat et al., 2020 c [34] | Czech Republic | Multicenter | 147 | N/A |
|
| Li et al., 2019 [44] | United States | Multicenter | 87 | 12 | No patient received osimertinib |
| Liang et al., 2018 [45] | Taiwan | Single center | 259 | 22 |
|
| Post-EGFR-TKI osimertinib treatment | |||||
| Experimental Trials | |||||
| Papadimitrakopoulou et al., 2020 [20] [AURA3] f | International | Multicenter | 279 | 23.5 | - |
| Real-World Studies | |||||
| Chen et al., 2020 [46] | China | Single center | 246 | 42.83 | - |
| Kishikawa et al., 2020 [47] | Japan | Multicenter | 56 | 15.1 | - |
| Lin et al., 2020 [48] | Taiwan | Single center | 162 | 15.8 | - |
| Cao et al., 2019 [49] | China | Single center | 74 | 9 | - |
| Kato et al., 2019 [50] | Japan | Single center | 31 | 12 | - |
a Study submitted to INESSS for evaluation of gefitinib for enlistment on Quebec’s drug formulary. b According to the literature review inclusion/exclusion criteria, the IPASS study should have been excluded since the study is restricted to a non-smoking or light smoking population. However, this study was included since all studies submitted to INESSS for drug evaluation were automatically included in the review. c This article was included twice for overall survival comparisons, once in relation to 1st-line gefitinib treatment and once to 1st-line afatinib treatment. d This article included overall survival results from two RCTs, including their combined results. e Study was submitted to INESSS for evaluation of afatinib for enlistment on Quebec’s drug formulary. f Study was submitted to INESSS for evaluation of osimertinib for enlistment on Quebec’s drug formulary. However, the overall survival results were not mature at the time of submission in the article by Mok and colleagues [19].
Appendix D. Additional Patient Characteristics

Table A8.
Characteristics of Patients in Each EGR-TKI Cohort.
Table A8.
Characteristics of Patients in Each EGR-TKI Cohort.
| Characteristics | 1st-Line Gefitinib Cohort 1 | 1st-Line Afatinib Cohort 1 | Post-EGFR-TKI Osimertinib Cohort 1 | Lung Cancer Cohort 2016 2 | ||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Sex | ||||||||
| Male | 313 | (68.5) | 48 | (60.0) | 84 | (70.6) | 4803 | (49.3) |
| Female | 144 | (31.5) | 32 | (40.0) | 35 | (29.4) | 4936 | (50.6) |
| Age in years | ||||||||
| Median (range) 3 | 70.6 | (34; 93) | 68.4 | (22; 93) | 72.3 | (24; 92) | 70.7 | (NR) |
| < 50 years | 15 | (3.3) | 4 | (5.0) | 5 | (4.2) | 249 | (2.6) |
| 50–64 years | 91 | (19.9) | 24 | (30.0) | 26 | (21.8) | 2716 | (27.9) |
| 65–79 years | 271 | (59.3) | 46 | (57.5) | 64 | (53.8) | 4762 | (48.8) |
| ≥80 | 80 | (17.5) | 6 | (7.5) | 24 | (20.2) | 2012 | (20.6) |
| 1st-line EGFR-TKI | ||||||||
| Gefitinib | 457 | (100) | - | 92 | (77.5) | - | ||
| Afatinib | - | 80 | (100) | 25 | (21.0) | - | ||
| Erlotinib | - | - | 2 | (1.7) | - | |||
| Material Vulnerability Index 4 | ||||||||
| 0 | 5 | (1.1) | 0 | (0.0) | 1 | (0.8) | 98 | (1.0) |
| (− vuln.) Quintile 1 | 82 | (17.9) | 12 | (15.0) | 27 | (22.7) | 1217 | (12.5) |
| Quintile 2 | 92 | (20.1) | 12 | (15.0) | 22 | (18.5) | 1594 | (16.3) |
| Quintile 3 | 81 | (17.7) | 16 | (20.0) | 22 | (18.5) | 1916 | (19.6) |
| Quintile 4 | 88 | (19.3) | 19 | (23.8) | 15 | (12.6) | 2288 | (23.5) |
| (+ vuln.) Quintile 5 | 107 | (23.4) | 18 | (22.5) | 31 | (26.1) | 2473 | (25.4) |
| Missing | 2 | (0.4) | 3 | (3.8) | 1 | (0.8) | 166 | (1.7) |
| Number of comorbidities 5 | ||||||||
| Median | 6 | NR | 7 | (NR) | 6 | (NR) | NR | (NR) |
| 0–4 | 169 | (37.0) | 27 | (33.8) | 42 | (35.3) | NR | (NR) |
| 5–9 | 160 | (35.0) | 30 | (37.5) | 50 | (42.0) | NR | (NR) |
| 10–14 | 80 | (17.5) | 17 | (21.3) | 19 | (16.0) | NR | (NR) |
| 15–19 | 34 | (7.4) | 4 | (5.0) | 8 | (6.7) | NR | (NR) |
| ≥ 20 | 13 | (2.8) | 2 | (2.5) | 0 | (0) | NR | (NR) |
| N/AV | 1 | (0.2) | 0 | (0) | 0 | (0) | NR | (NR) |
| Total | 457 | 80 | 119 | 9752 | ||||
Abbreviations: NR: not reported. 1 Age measured at the start of the EGFR-TKI of interest. 2 Quebec lung cancer cohort developed by INESSS, in which age was measured at the diagnosis date [3]. 3 Range: minimum; maximum. 4 Patients’ dissemination areas (DA: smallest Canada-wide geographic unit with a standard population size of 400–700) were obtained from the health insurance registry (FIPA) and linked to a DA-level material vulnerability index that was developed at INESSS with the 2016 Census data (DA-level unemployment ratio, median income, and low education). The index was adapted from a previous method and quintiles were created based on the total RAMQ population (Q1: least deprived and Q5: most deprived [70]. 5 The Population Grouping Methodology was applied to obtain the total number of comorbidities for each patient [71,72]. Patients’ hospitalization data was screened for diagnostic codes for 226 possible health conditions (excluding lung cancer) in the three years prior to patients’ 1st EGFR-TKI.
References
- Canadian Cancer Society’s Advisory Committee. Canadian Cancer Statistics: A 2020 Special Report on Lung Cancer; Canadian Cancer Society: Toronto, ON, Canada, 2020. [Google Scholar]
- Canadian Cancer Society’s Advisory Committee in Collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada. Canadian Cancer Statistics 2021; Canadian Cancer Society: Toronto, ON, Canada, 2021. [Google Scholar]
- Institut National d’Excellence en Santé et en Services Sociaux (INESSS). Création et Caractérisation d’une Cohorte Québécoise de Patients Atteints d’un Cancer du Poumon aà L’aide de Données Clinico-Administratives; Institut National d’Excellence en Santé et en Services Sociaux: Québec, QC, Canada, 2021; p. 152.
- Institut National d’Excellence en Santé et en Services Sociaux (INESSS); Groupe d’étude en oncologie du Québec (GEOQ). Algorithmes D’investigation, de Traitement et de Suivi Cancer du Poumon; Institut National d’Excellence en Santé et en Services Sociaux: Québec, QC, Canada, 2014; p. 269.
- Institut National d’Excellence en Santé et en Services Sociaux (INESSS); Groupe d’étude en oncologie du Québec (GEOQ). Algorithme: Cancer du Poumon; 2550712501; Institut National d’Excellence en Santé et en Services Sociaux: Québec, QC, Canada, 2020.
- Shiau, C.J.; Babwah, J.P.; da Cunha Santos, G.; Sykes, J.R.; Boerner, S.L.; Geddie, W.R.; Leighl, N.B.; Wei, C.; Kamel-Reid, S.; Hwang, D.M.; et al. Sample features associated with success rates in population-based EGFR mutation testing. J. Thorac. Oncol. 2014, 9, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Westover, D.; Zugazagoitia, J.; Cho, B.C.; Lovly, C.M.; Paz-Ares, L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann. Oncol. 2018, 29, i10–i19. [Google Scholar] [CrossRef] [PubMed]
- Institut National d’Excellence en Santé et en Services Sociaux (INESSS). Approche, Modalités et Processus D’éValuation. Available online: https://www.inesss.qc.ca/thematiques/medicaments/approche-modalites-et-processus-devaluation.html (accessed on 17 May 2022).
- Tadrous, M.; Ahuja, T.; Ghosh, B.; Kropp, R. Developing a Canadian Real-World Evidence Action Plan across the Drug Life Cycle. Healthc. Policy 2020, 15, 41–47. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. NICE strategy 2021 to 2026: Dynamic, Collaborative, Excellent; National Institute for Health and Care Excellence (NICE): London, UK, April 2021; p. 35. [Google Scholar]
- Haute Autorité de Santé. Real-World Studies for the Assessment of Medicinal Products and Medical Devices; Haute Autorité de Santé (HAS): Saint-Denis, France, 10 June 2021; p. 50. [Google Scholar]
- Food Drug Administration. Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices: Guidance for Industry and Food and Drug Administration staff; Food and Drug Administration (FDA): Silver Spring, MD, USA, 31 August 2017. [Google Scholar]
- Chan, K.; Nam, S.; Evans, B.; de Oliveira, C.; Chambers, A.; Gavura, S.; Hoch, J.; Mercer, R.E.; Dai, W.F.; Beca, J. Developing a framework to incorporate real-world evidence in cancer drug funding decisions: The Canadian Real-world Evidence for Value of Cancer Drugs (CanREValue) collaboration. BMJ Open 2020, 10, e032884. [Google Scholar] [CrossRef]
- Régie de L’Assurance Maladie du Québec (RAMQ). Liste des Médicaments, 26 May 2022 ed.; Bibliothèque et Archives Nationales du Québec: Montréal, QC, Canada, 2022. [Google Scholar]
- Institut National d’Excellence en Santé et en Services Sociaux (INESSS). Plan Triennal D’activités 2019–2020. Des Évaluations Axées Sur la Création de Valuer en santé Et en Services Sociaux.; Institut National d’Excellence en Santé et en Services Sociaux: Québec, QC, Canada, 2021.
- Institut National d’Excellence en Santé et en Services Sociaux (INESSS). Utilisation en Contexte Québécois des Inhibiteurs de la Tyrosine Kinase du Récepteur du Facteur de Croissance épidermique (EGFR) Pour le Traitement du Cancer du Poumon; Institut National d’Excellence en Santé et en Services Sociaux: Québec, QC, Canada, 2022; p. 109.
- Institut National d’Excellence en Santé et en Services Sociaux (INESSS). Rapport D’activités Scientifiques 2016/2017; Institut National d’Excellence en Santé et en Services Sociaux: Québec, QC, Canada, 2017.
- Mok, T.S.; Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Papadimitrakopoulou, V.A.; Mok, T.S.; Han, J.Y.; Ahn, M.J.; Delmonte, A.; Ramalingam, S.S.; Kim, S.W.; Shepherd, F.A.; Laskin, J.; He, Y.; et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann. Oncol. 2020, 31, 1536–1544. [Google Scholar] [CrossRef]
- Korn, E.L. Censoring distributions as a measure of follow-up in survival analysis. Stat. Med. 1986, 5, 255–260. [Google Scholar] [CrossRef]
- Therneau, T. A Package for Survival Analysis in R. 2021. Available online: https://cran.r-project.org/web/packages/survival/citation.html (accessed on 23 May 2021).
- Institut National d’Excellence en Santé et en Services Sociaux (INESSS). Extrait d’Avis au Ministre: Iressa. Available online: https://www.inesss.qc.ca/thematiques/medicaments/medicaments-evaluation-aux-fins-dinscription/extrait-davis-au-ministre/iressa-1341.html (accessed on 23 May 2021).
- Institut National d’Excellence en Santé et en Services Sociaux (INESSS). Extrait d’Avis au Ministre: Giotrif. Available online: https://www.inesss.qc.ca/thematiques/medicaments/medicaments-evaluation-aux-fins-dinscription/extrait-davis-au-ministre/giotrif-2980.html (accessed on 23 May 2021).
- Institut National d’Excellence en Santé et en Services Sociaux (INESSS). Extrait d’Avis au Ministre: Tagrisso (Cancer Poumon). Available online: https://www.inesss.qc.ca/thematiques/medicaments/medicaments-evaluation-aux-fins-dinscription/extrait-davis-au-ministre/tagrisso-cancer-poumon-4044.html (accessed on 23 May 2021).
- Inoue, A.; Kobayashi, K.; Maemondo, M.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann. Oncol. 2013, 24, 54–59. [Google Scholar] [CrossRef]
- Fukuoka, M.; Wu, Y.L.; Thongprasert, S.; Sunpaweravong, P.; Leong, S.S.; Sriuranpong, V.; Chao, T.Y.; Nakagawa, K.; Chu, D.T.; Saijo, N.; et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 2011, 29, 2866–2874. [Google Scholar] [CrossRef]
- Yang, J.C.; Wu, Y.L.; Schuler, M.; Sebastian, M.; Popat, S.; Yamamoto, N.; Zhou, C.; Hu, C.P.; O’Byrne, K.; Feng, J.; et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015, 16, 141–151. [Google Scholar] [CrossRef]
- Garcia-Campelo, R.; Arrieta, O.; Massuti, B.; Rodriguez-Abreu, D.; Granados, A.L.O.; Majem, M.; Vicente, D.; Lianes, P.; Bosch-Barrera, J.; Insa, A.; et al. Combination of gefitinib and olaparib versus gefitinib alone in EGFR mutant non-small-cell lung cancer (NSCLC): A multicenter, randomized phase II study (GOAL). Lung Cancer 2020, 150, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Shimokawa, M.; Seto, T.; Morita, S.; Yatabe, Y.; Okamoto, I.; Tsurutani, J.; Satouchi, M.; Hirashima, T.; Atagi, S.; et al. Final overall survival results of WJTOG3405, a randomized phase III trial comparing gefitinib versus cisplatin with docetaxel as the first-line treatment for patients with stage IIIB/IV or postoperative recurrent EGFR mutation-positive non-small-cell lung cancer. Ann. Oncol. 2019, 30, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.B.; Chai, X.S.; Wu, W.Y.; Long, S.Q.; Deng, H.; Pan, Z.Q.; He, W.F.; Zhou, Y.S.; Liao, G.Y.; Xiao, S.J. Gefitinib plus Fuzheng Kang’ai Formula () in Patients with Advanced Non-Small Cell Lung Cancer with Epidermal Growth Factor Receptor Mutation: A Randomized Controlled Trial. Chin. J. Integr. Med. 2018, 24, 734–740. [Google Scholar] [CrossRef]
- Han, B.; Jin, B.; Chu, T.; Niu, Y.; Dong, Y.; Xu, J.; Gu, A.; Zhong, H.; Wang, H.; Zhang, X.; et al. Combination of chemotherapy and gefitinib as first-line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: A randomized controlled trial. Int. J. Cancer 2017, 141, 1249–1256. [Google Scholar] [CrossRef]
- Patil, V.M.; Noronha, V.; Joshi, A.; Choughule, A.B.; Bhattacharjee, A.; Kumar, R.; Goud, S.; More, S.; Ramaswamy, A.; Karpe, A.; et al. Phase III study of gefitinib or pemetrexed with carboplatin in EGFR-mutated advanced lung adenocarcinoma. ESMO Open 2017, 2, e000168. [Google Scholar] [CrossRef]
- Brat, K.; Bratova, M.; Skrickova, J.; Barinova, M.; Hurdalkova, K.; Pesek, M.; Havel, L.; Koubkova, L.; Hrnciarik, M.; Krejci, J.; et al. Real-life effectiveness of first-line anticancer treatments in stage IIIB/IV NSCLC patients: Data from the Czech TULUNG Registry. Thorac. Cancer 2020, 11, 3346–3356. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Fang, W.T.; Lo, Y.W.; Chen, Y.H.; Chien, L.N. Comparing the effectiveness of different EGFR-TKIs in patients with EGFR mutant non-small-cell lung cancer: A retrospective cohort study in Taiwan. Int. J. Cancer 2020, 147, 1107–1116. [Google Scholar] [CrossRef]
- Choi, Y.W.; Jeon, S.Y.; Jeong, G.S.; Lee, H.W.; Jeong, S.H.; Kang, S.Y.; Park, J.S.; Choi, J.H.; Koh, Y.W.; Han, J.H.; et al. EGFR Exon 19 Deletion is Associated With Favorable Overall Survival After First-line Gefitinib Therapy in Advanced Non-Small Cell Lung Cancer Patients. Am. J. Clin. Oncol. 2018, 41, 385–390. [Google Scholar] [CrossRef]
- Imai, H.; Kuwako, T.; Kaira, K.; Masuda, T.; Miura, Y.; Seki, K.; Sakurai, R.; Utsugi, M.; Shimizu, K.; Sunaga, N.; et al. Evaluation of gefitinib efficacy according to body mass index, body surface area, and body weight in patients with EGFR-mutated advanced non-small cell lung cancer. Cancer Chemother Pharm. 2017, 79, 497–505. [Google Scholar] [CrossRef][Green Version]
- Krawczyk, P.; Kowalski, D.M.; Ramlau, R.; Kalinka-Warzocha, E.; Winiarczyk, K.; Stencel, K.; Powrozek, T.; Reszka, K.; Wojas-Krawczyk, K.; Bryl, M.; et al. Comparison of the effectiveness of erlotinib, gefitinib, and afatinib for treatment of non-small cell lung cancer in patients with common and rare EGFR gene mutations. Oncol. Lett. 2017, 13, 4433–4444. [Google Scholar] [CrossRef] [PubMed]
- Sutiman, N.; Tan, S.W.; Tan, E.H.; Lim, W.T.; Kanesvaran, R.; Ng, Q.S.; Jain, A.; Ang, M.K.; Tan, W.L.; Toh, C.K.; et al. EGFR Mutation Subtypes Influence Survival Outcomes following First-Line Gefitinib Therapy in Advanced Asian NSCLC Patients. J. Thorac. Oncol. 2017, 12, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Yoshida, K.; Morita, S.; Imamura, F.; Seto, T.; Okamoto, I.; Nakagawa, K.; Yamamoto, N.; Muto, S.; Fukuoka, M. Characteristics and overall survival of EGFR mutation-positive non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors: A retrospective analysis for 1660 Japanese patients. Jpn. J. Clin. Oncol. 2016, 46, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Vavala, T.; Follador, A.; Tiseo, M.; Galetta, D.; Morabito, A.; Di Maio, M.; Martelli, O.; Caffo, O.; Piovano, P.L.; Cortinovis, D.; et al. BE-POSITIVE: Beyond progression after tyrosine kinase inhibitor in EGFR- positive non small cell lung cancer patients: Results from a multicenter Italian observational study. Lung Cancer 2016, 95, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.W.; Ho, C.L.; Dai, M.S.; Chen, W.L.; Chang, P.Y.; Wu, Y.Y.; Perng, C.L.; Lai, C.Y. Gefitinib as first-line treatment for patients with epidermal growth factor receptor-mutated advanced lung adenocarcinoma: A single institution experience in Taiwan. J. BUON 2014, 19, 459–465. [Google Scholar]
- Park, J.H.; Kim, T.M.; Keam, B.; Jeon, Y.K.; Lee, S.H.; Kim, D.W.; Chung, D.H.; Kim, Y.T.; Kim, Y.W.; Heo, D.S. Tumor burden is predictive of survival in patients with non-small-cell lung cancer and with activating epidermal growth factor receptor mutations who receive gefitinib. Clin. Lung Cancer 2013, 14, 383–389. [Google Scholar] [CrossRef]
- Li, Y.; Appius, A.; Pattipaka, T.; Feyereislova, A.; Cassidy, A.; Ganti, A.K. Real-world management of patients with epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer in the USA. PLoS ONE 2019, 14, e0209709. [Google Scholar] [CrossRef]
- Liang, S.K.; Lee, M.R.; Liao, W.Y.; Ho, C.C.; Ko, J.C.; Shih, J.Y. Prognostic factors of afatinib as a first-line therapy for advanced EGFR mutation-positive lung adenocarcinoma: A real-world, large cohort study. Oncotarget 2018, 9, 23749–23760. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Zhang, B.; Zhao, Y.; Zhang, L.; Hu, M.; Zhang, W.; Han, B. Clinical Factors Affecting the Response to Osimertinib in Non-Small Cell Lung Cancer Patients with An Acquired Epidermal Growth Factor Receptor T790M Mutation: A Long-Term Survival Analysis. Target 2020, 15, 337–345. [Google Scholar] [CrossRef]
- Kishikawa, T.; Kasai, T.; Okada, M.; Nakachi, I.; Soda, S.; Arai, R.; Takigami, A.; Sata, M. Osimertinib, a third-generation EGFR tyrosine kinase inhibitor: A retrospective multicenter study of its real-world efficacy and safety in advanced/recurrent non-small cell lung carcinoma. Thorac. Cancer 2020, 11, 935–942. [Google Scholar] [CrossRef]
- Lin, Y.T.; Tsai, T.H.; Wu, S.G.; Liu, Y.N.; Yu, C.J.; Shih, J.Y. Complex EGFR mutations with secondary T790M mutation confer shorter osimertinib progression-free survival and overall survival in advanced non-small cell lung cancer. Lung Cancer 2020, 145, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qiu, X.; Xiao, G.; Hu, H.; Lin, T. Effectiveness and safety of osimertinib in patients with metastatic EGFR T790M-positive NSCLC: An observational real-world study. PLoS ONE 2019, 14, e0221575. [Google Scholar] [CrossRef]
- Kato, Y.; Hosomi, Y.; Watanabe, K.; Yomota, M.; Kawai, S.; Okuma, Y.; Kubota, K.; Seike, M.; Gemma, A.; Okamura, T. Impact of clinical features on the efficacy of osimertinib therapy in patients with T790M-positive non-small cell lung cancer and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J. Thorac. Dis. 2019, 11, 2350–2360. [Google Scholar] [CrossRef] [PubMed]
- Agulnik, J.S.; Kasymjanova, G.; Pepe, C.; Hurry, M.; Walton, R.N.; Sakr, L.; Cohen, V.; Small, D. Real-World Pattern of Treatment and Clinical Outcomes of EGFR-Mutant Non-Small Cell Lung Cancer in a Single Academic Centre in Quebec. Curr. Oncol. 2021, 28, 5179–5191. [Google Scholar] [CrossRef]
- Ellis, P.M.; Verma, S.; Sehdev, S.; Younus, J.; Leighl, N.B. Challenges to implementation of an epidermal growth factor receptor testing strategy for non–small-cell lung cancer in a publicly funded health care system. J. Thorac. Oncol. 2013, 8, 1136–1141. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.M.; Boyer, M.; et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef]
- Minegishi, Y.; Yamaguchi, O.; Sugawara, S.; Kuyama, S.; Watanabe, S.; Usui, K.; Mori, M.; Hataji, O.; Nukiwa, T.; Morita, S. A phase II study of first-line afatinib for patients aged ≥ 75 years with EGFR mutation-positive advanced non-small cell lung cancer: North East Japan Study Group trial NEJ027. BMC Cancer 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Hiranuma, O.; Uchino, J.; Sakaguchi, C.; Araya, T.; Hiraoka, N.; Ishizuka, T.; Takeda, T.; Kawasaki, M.; Goto, Y. Final results from a phase II trial of osimertinib for elderly patients with epidermal growth factor receptor t790m-positive non-small cell lung cancer that progressed during previous treatment. J. Clin. Med. 2020, 9, 1762. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Sequist, L.V.; Tan, E.-H.; Geater, S.L.; Orlov, S.; Zhang, L.; Lee, K.H.; Tsai, C.-M.; Kato, T.; Barrios, C.H. Afatinib as first-line treatment of older patients with EGFR mutation-positive non-small-cell lung cancer: Subgroup analyses of the LUX-Lung 3, LUX-Lung 6, and LUX-Lung 7 trials. Clin. Lung Cancer 2018, 19, e465–e479. [Google Scholar] [CrossRef]
- Kuwako, T.; Imai, H.; Masuda, T.; Miura, Y.; Seki, K.; Yoshino, R.; Kaira, K.; Utsugi, M.; Shimizu, K.; Sunaga, N. First-line gefitinib treatment in elderly patients (aged ≥ 75 years) with non-small cell lung cancer harboring EGFR mutations. Cancer Chemother. Pharmacol. 2015, 76, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Ichiyama, T.; Hirai, K.; Agatsuma, T.; Koyama, S.; Hachiya, T.; Morozumi, N.; Shiina, T.; Koizumi, T. Clinical outcomes in elderly patients administered gefitinib as first-line treatment in epidermal growth factor receptor-mutated non-small-cell lung cancer: Retrospective analysis in a Nagano Lung Cancer Research Group study. Med. Oncol. 2013, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Maemondo, M.; Minegishi, Y.; Inoue, A.; Kobayashi, K.; Harada, M.; Okinaga, S.; Morikawa, N.; Oizumi, S.; Tanaka, T.; Isobe, H. First-line gefitinib in patients aged 75 or older with advanced non–small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J. Thorac. Oncol. 2012, 7, 1417–1422. [Google Scholar] [CrossRef]
- Yong-Jin, K.; Oremus, M.; Chen, H.H.; McFarlane, T.; Fearon, D.; Horton, S. Factors affecting treatment selection and overall survival for first-line EGFR-tyrosine kinase inhibitor therapy in non-small-cell lung cancer. J. Comp. Eff. Res. 2021, 10, 193–206. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Gong, Y.; Kehl, K.; Mishra-Kalyani, P.; Goldberg, K.B.; Khozin, S.; Kluetz, P.G.; Oxnard, G.R.; Pazdur, R. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann. Oncol. 2019, 30, 830–838. [Google Scholar] [CrossRef]
- Sorin, M.; Franco, E.L.; Quesnel-Vallée, A. Inter- and intraprovincial inequities in public coverage of cancer drug programs across Canada: A plea for the establishment of a pan-Canadian pharmacare program. Curr. Oncol. 2019, 26, 266–269. [Google Scholar] [CrossRef]
- Institut National de Santé Publique du Québec. Portrait de Santé du Québec et de ses Régions 2006: Deuxième Rapport National sur L’état de Santé de la Population du Québec LES Statistiques; Institut National de Santé Publique du Québec: Québec, QC, Canada, 2006. [Google Scholar]
- Winton, T.; Livingston, R.; Johnson, D.; Rigas, J.; Johnston, M.; Butts, C.; Cormier, Y.; Goss, G.; Inculet, R.; Vallieres, E.; et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N. Engl. J. Med. 2005, 352, 2589–2597. [Google Scholar] [CrossRef]
- Institut National d’Excellence en Santé et en Services Sociaux (INESSS) et Groupe d’étude en Oncologie du Québec (GEOQ). Algorithme: Cancer du Poumon; Institut National d’Excellence en Santé et en Services Sociaux: Québec, QC, Canada, 2020. [Google Scholar]
- Gamache, P.; Hamel, D.; Blaser, C. Material and Social Deprivation Index: A Summary; Bureau D’information et D’études en Santé des Populations, Ed.; Institut National de Santé Publique du Québec (INSPQ): Québec, QC, Canada, 2019; p. 16. [Google Scholar]
- Canadian Institutes for Health Information. Population Grouping Methodology [Information Sheet]; Canadian Institute for Health Information: Ottawa, ON, Canada, 2020.
- Weir, S.; Steffler, M.; Li, Y.; Shaikh, S.; Wright, J.G.; Kantarevic, J. Use of the Population Grouping Methodology of the Canadian Institute for Health Information to predict high-cost health system users in Ontario. CMAJ 2020, 192, E907–E912. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).