Long-Term Toxicities of Immune Checkpoint Inhibitor (ICI) in Melanoma Patients
Abstract
1. Introduction
2. Materials and Methods
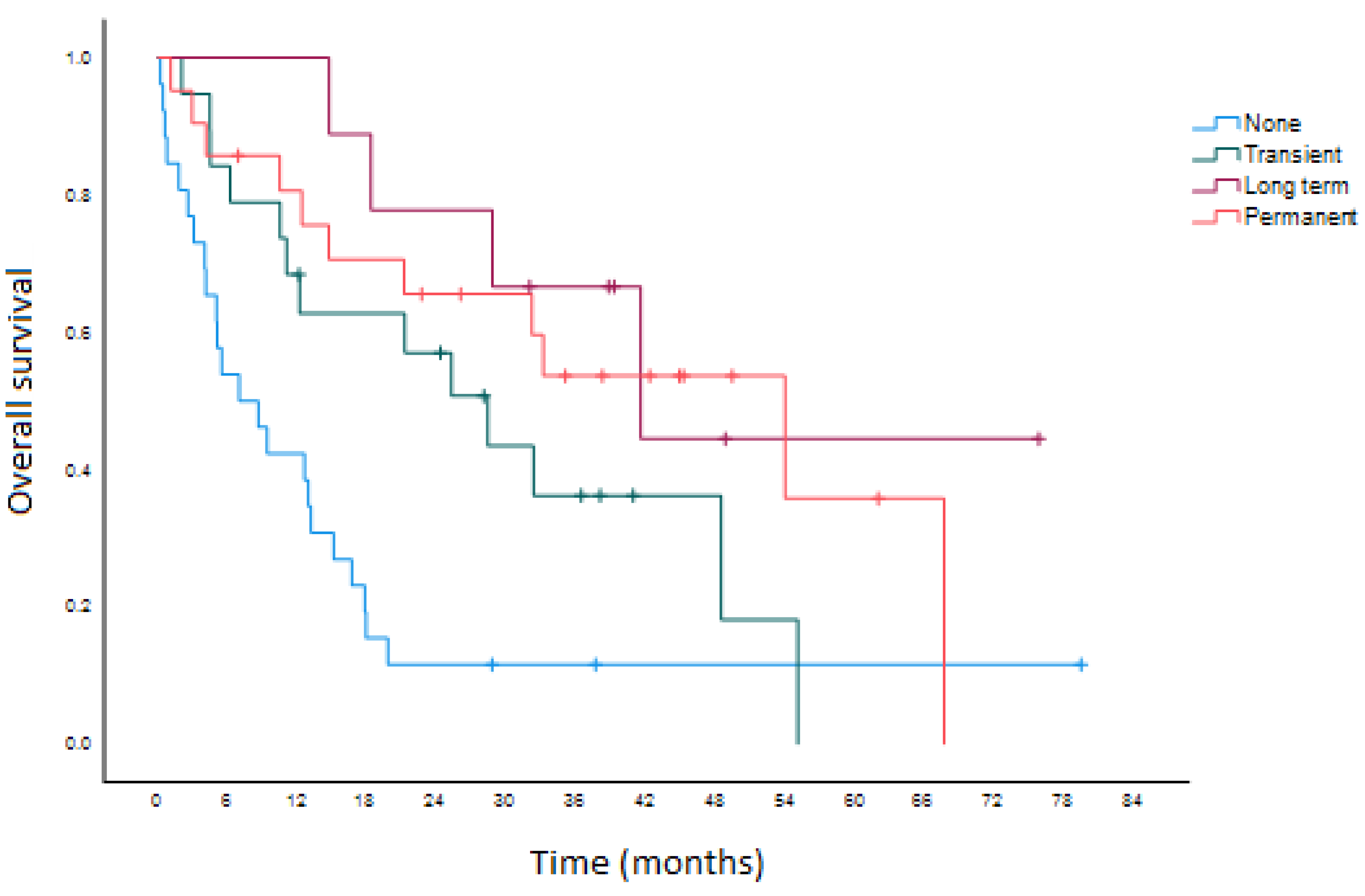
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Redman, J.M.; Gibney, G.T.; Atkins, M.B. Advances in immunotherapy for melanoma. BMC Med. 2016, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbe, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute (US). Common Terminology Criteria for Adverse Events (CTCAE); Version 4.0; National Institutes of Health: Bethesda, MD, USA, 2010; 10-5410.
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanan, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N. Engl. J. Med. 2016, 375, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Bello, D.M. Adjuvant immunotherapy for melanoma. J. Surg. Oncol. 2021, 123, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Friedman, D.L.; Berry, E.; Decker, I.; Ye, F.; Zhao, S.; Morgans, A.K.; Puzanov, I.; Sosman, J.A.; Lovely, C.M. Survivorship in Immune Therapy: Assessing Chronic Immune Toxicities, Health Outcomes, and Functional Status among Long-term Ipilimumab Survivors at a Single Referral Center. Cancer Immunol. Res. 2015, 3, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Patrinely, J.R., Jr.; Johnson, R.; Lawless, A.R.; Bhave, P.; Sawyers, A.; Dimitrova, M.; Yeoh, H.L.; Palmeri, M.; Ye, F.; Fan, R.; et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti-PD-1 Therapy for High-risk Resected Melanoma. JAMA Oncol. 2021, 7, 744–748. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (US). Duration of Anti-PD-1 Therapy in Metastatic Melanoma (STOP-GAP). Identifier NCT02821013. Available online: https://clinicaltrials.gov/ct2/show/NCT02821013 (accessed on 21 July 2022).
| Total | Permanent irAE | Long-Term irAE | Transient irAE | No irAE | p | |
|---|---|---|---|---|---|---|
| N | 161 | 66 | 15 | 34 | 46 | |
| Age | 0.172 | |||||
| <65 | 60 | 22 | 4 | 15 | 19 | |
| ≥65 | 101 | 44 | 11 | 19 | 27 | |
| Gender | 0.949 | |||||
| Male | 97 | 38 | 11 | 23 | 25 | |
| Female | 64 | 28 | 4 | 11 | 21 | |
| ECOG | 0.634 | |||||
| <2 | 137 | 58 | 12 | 32 | 35 | |
| ≥2 | 24 | 8 | 3 | 2 | 11 | |
| Baseline autoimmune history | 26 | 12 | 3 | 6 | 5 | 0.411 |
| Stage IV disease | 108 | 38 | 10 | 28 | 32 | 0.034 |
| BRAF mutant | 60 | 28 | 2 | 13 | 17 | 0.952 |
| Histology | 0.221 | |||||
| Cutaneous | 129 | 55 | 13 | 26 | 35 | |
| Non-cutaneous | 32 | 11 | 2 | 8 | 11 | |
| Brain metastases prior to ICI | 25 | 7 | 1 | 7 | 10 | 0.046 |
| Baseline NLR | 0.669 | |||||
| <5 | 127 | 52 | 13 | 26 | 36 | |
| ≥5 | 34 | 14 | 2 | 8 | 10 | |
| Baseline PLR | 0.126 | |||||
| <200 | 102 | 48 | 8 | 22 | 24 | |
| ≥200 | 59 | 18 | 7 | 12 | 22 | |
| Baseline LDH | 0.335 | |||||
| ≤ULN | 122 | 52 | 12 | 27 | 31 | |
| >ULN | 39 | 14 | 3 | 7 | 15 | |
| ICI regimen | ||||||
| Pembrolizumab | 106 | 32 | 13 | 23 | 38 | 0.006 |
| Nivolumab | 31 | 19 | 2 | 3 | 7 | 0.031 |
| Ipilimumab | 25 | 8 | 4 | 10 | 3 | 0.802 |
| Ipilimumab/Nivolumab | 32 | 21 | 0 | 8 | 3 | 0.053 |
| Other | 5 | 1 | 0 | 2 | 2 | 0.21 |
| Treatment intent/line | ||||||
| Adjuvant | 48 | 27 | 4 | 5 | 12 | 0.018 |
| Palliative | 123 | 46 | 11 | 32 | 34 | 0.07 |
| First-line | 102 | 39 | 9 | 27 | 27 | |
| Second-line | 41 | 12 | 5 | 10 | 14 | |
| Third-line | 9 | 2 | 1 | 4 | 2 | |
| Fourth-line | 1 | 1 | 0 | 0 | 0 |
| Total | Permanent irAE | Long-Term irAE | Transient irAE | |
|---|---|---|---|---|
| 283 | 111 | 34 | 138 | |
| irAE type | ||||
| Skin | 108 | 36 | 21 | 51 |
| Musculoskeletal | 25 | 12 | 0 | 13 |
| Endocrine | 45 | 40 | 2 | 3 |
| Neurological | 7 | 5 | 1 | 1 |
| Ocular | 8 | 0 | 1 | 7 |
| Gastrointestinal | 63 | 6 | 6 | 51 |
| Genitourinary | 2 | 2 | 0 | 0 |
| Respiratory | 20 | 6 | 2 | 12 |
| Cardiac | 2 | 2 | 0 | 0 |
| Rheumatologic, non-musculoskeletal | 3 | 2 | 1 | 0 |
| irAE severity | ||||
| Grade 1–2 | 223 | 85 | 27 | 111 |
| Grade 3–4 | 56 | 22 | 7 | 27 |
| Grade 5 | 4 | 4 | 0 | 0 |
| Corticosteroid use for irAE | 153 | 56 | 16 | 81 |
| High-dose corticosteroid use (≥1 mg/kg) | 53 | 22 | 6 | 25 |
| Additional immunosuppressant | 20 | 9 | 4 | 7 |
| ICI interruption/rechallenge | 64 | 7 | 7 | 50 |
| ICI permanent discontinuation | 38 | 18 | 4 | 16 |
| Subsequent irAE development | 167 | 61 | 20 | 86 |
| Same subsequent irAE type | 67 | 21 | 6 | 40 |
| Same or less subsequent irAE grade | 103 | 40 | 14 | 49 |
| Need for new long-term replacement therapy | 70 | 65 | 5 | 0 |
| Thyroid replacement | 25 | 24 | 1 | 0 |
| Maintenance corticosteroids | 22 | 22 | 0 | 0 |
| Additional immunosuppressant | 3 | 3 | 0 | 0 |
| Topical corticosteroid | 19 | 15 | 4 | 0 |
| Insulin | 1 | 1 | 0 | 0 |
| Need to increase existing replacement therapy | 4 | 4 | 0 | 0 |
| Any potential cosmetic issues (vitiligo, alopecia, poliosis) | 10 | 7 | 0 | 3 |
| Any previous transient irAE prior to permanent/long-term irAE | N/A | 37 | 11 | N/A |
| Total | Permanent irAE | Long-Term irAE | Transient irAE | |
|---|---|---|---|---|
| 204 | 80 | 32 | 92 | |
| irAE type | ||||
| Skin | 84 | 28 | 21 | 35 |
| Musculoskeletal | 20 | 10 | 0 | 10 |
| Endocrine | 36 | 31 | 2 | 3 |
| Neurological | 3 | 3 | 0 | 0 |
| Ocular | 2 | 0 | 1 | 1 |
| Gastrointestinal | 42 | 2 | 5 | 35 |
| Genitourinary | 2 | 2 | 0 | 0 |
| Respiratory | 12 | 2 | 2 | 8 |
| Cardiac | 1 | 1 | 0 | 0 |
| Rheumatologic, non-musculoskeletal | 2 | 1 | 1 | 0 |
| irAE severity | ||||
| Grade 1–2 | 171 | 67 | 26 | 78 |
| Grade 3–4 | 31 | 11 | 6 | 14 |
| Grade 5 | 2 | 2 | 0 | 0 |
| Corticosteroid use for irAE | 97 | 35 | 14 | 48 |
| High-dose corticosteroid use (≥1 mg/kg) | 23 | 8 | 4 | 11 |
| Additional immunosuppressant | 8 | 5 | 2 | 1 |
| ICI interruption/rechallenge | 41 | 5 | 6 | 30 |
| ICI permanent discontinuation | 21 | 10 | 3 | 8 |
| Subsequent irAE development | 116 | 46 | 18 | 52 |
| Same subsequent irAE type | 56 | 18 | 6 | 32 |
| Same or less subsequent irAE grade | 72 | 29 | 12 | 31 |
| Need for new long-term replacement therapy | 47 | 45 | 2 | 0 |
| Thyroid replacement | 18 | 18 | 0 | 0 |
| Maintenance corticosteroids | 13 | 13 | 0 | 0 |
| Additional immunosuppressant | 3 | 3 | 0 | 0 |
| Topical corticosteroid | 14 | 12 | 2 | 0 |
| Insulin | 0 | 0 | 0 | 0 |
| Need to increase existing replacement therapy | 3 | 3 | 0 | 0 |
| Any potential cosmetic issues (vitiligo, alopecia, poliosis) | 7 | 6 | 0 | 1 |
| Any previous transient irAE prior to permanent/long-term irAE | N/A | 19 | 10 | N/A |
| Total | Permanent irAE | Long-Term irAE | Transient irAE | |
|---|---|---|---|---|
| 79 | 31 | 2 | 46 | |
| irAE type | ||||
| Skin | 24 | 8 | 0 | 16 |
| Musculoskeletal | 5 | 2 | 0 | 3 |
| Endocrine | 9 | 9 | 0 | 0 |
| Neurological | 4 | 2 | 1 | 1 |
| Ocular | 6 | 0 | 0 | 6 |
| Gastrointestinal | 21 | 4 | 1 | 16 |
| Genitourinary | 0 | 0 | 0 | 0 |
| Respiratory | 8 | 4 | 0 | 4 |
| Cardiac | 1 | 1 | 0 | 0 |
| Rheumatologic, non-musculoskeletal | 1 | 1 | 0 | 0 |
| irAE severity | ||||
| Grade 1–2 | 52 | 18 | 1 | 33 |
| Grade 3–4 | 25 | 11 | 1 | 13 |
| Grade 5 | 2 | 2 | 0 | 0 |
| Corticosteroid use for irAE | 56 | 21 | 2 | 33 |
| High-dose corticosteroid use (≥1 mg/kg) | 30 | 14 | 2 | 14 |
| Additional immunosuppressant | 12 | 4 | 2 | 6 |
| ICI interruption/rechallenge | 23 | 2 | 1 | 20 |
| ICI permanent discontinuation | 17 | 8 | 1 | 8 |
| Subsequent irAE development | 51 | 15 | 2 | 34 |
| Same subsequent irAE type | 11 | 3 | 0 | 8 |
| Same or less subsequent irAE grade | 31 | 11 | 2 | 18 |
| Need for new long-term replacement therapy | 19 | 18 | 0 | 1 |
| Thyroid replacement | 6 | 6 | 0 | 0 |
| Maintenance corticosteroids | 9 | 9 | 0 | 0 |
| Additional immunosuppressant | 1 | 1 | 0 | 0 |
| Topical corticosteroid | 2 | 2 | 0 | 0 |
| Insulin | 1 | 1 | 0 | 0 |
| Need to increase existing replacement therapy | 1 | 1 | 0 | 0 |
| Any potential cosmetic issues (vitiligo, alopecia, poliosis) | 3 | 1 | 0 | 2 |
| Any previous transient irAE prior to permanent/long-term irAE | N/A | 18 | 1 | N/A |
| Sig. | Odds Ratio | 95% Confidence Interval | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age ≥ 65 | 0.070 | 1.999 | 0.946 | 4.224 |
| Stage IV disease | 0.020 | 0.409 | 0.192 | 0.870 |
| Baseline PLR ≥ 200 | 0.101 | 0.536 | 0.255 | 1.129 |
| Dual ICI therapy | 0.245 | 1.798 | 0.669 | 4.835 |
| Highest grade of irAE ≥ 3 | <0.001 | 6.055 | 2.481 | 14.777 |
| Total | Permanent irAE | Long-Term irAE | Transient irAE | No irAE | p-Value | |
|---|---|---|---|---|---|---|
| 75 | 21 | 9 | 19 | 26 | ||
| CR/PR | 31 (41.3%) | 13 (61.9%) | 5 (55.6%) | 7 (36.8%) | 6 (23.1%) | 0.042 |
| Stable/Mixed/DP | 44 (58.7%) | 8 (38.1%) | 4 (44.4%) | 12 (63.2%) | 20 (76.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, J.; Kartolo, A.; Yeung, C.; Hopman, W.; Baetz, T. Long-Term Toxicities of Immune Checkpoint Inhibitor (ICI) in Melanoma Patients. Curr. Oncol. 2022, 29, 7953-7963. https://doi.org/10.3390/curroncol29100629
Tong J, Kartolo A, Yeung C, Hopman W, Baetz T. Long-Term Toxicities of Immune Checkpoint Inhibitor (ICI) in Melanoma Patients. Current Oncology. 2022; 29(10):7953-7963. https://doi.org/10.3390/curroncol29100629
Chicago/Turabian StyleTong, Justin, Adi Kartolo, Cynthia Yeung, Wilma Hopman, and Tara Baetz. 2022. "Long-Term Toxicities of Immune Checkpoint Inhibitor (ICI) in Melanoma Patients" Current Oncology 29, no. 10: 7953-7963. https://doi.org/10.3390/curroncol29100629
APA StyleTong, J., Kartolo, A., Yeung, C., Hopman, W., & Baetz, T. (2022). Long-Term Toxicities of Immune Checkpoint Inhibitor (ICI) in Melanoma Patients. Current Oncology, 29(10), 7953-7963. https://doi.org/10.3390/curroncol29100629





