Outcomes of High-Dose Stereotactic Ablative Radiotherapy to All/Multiple Sites for Oligometastatic Renal Cell Cancer Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Population
2.2. Treatment Technique
2.3. Endpoints
2.4. Statistical Analysis
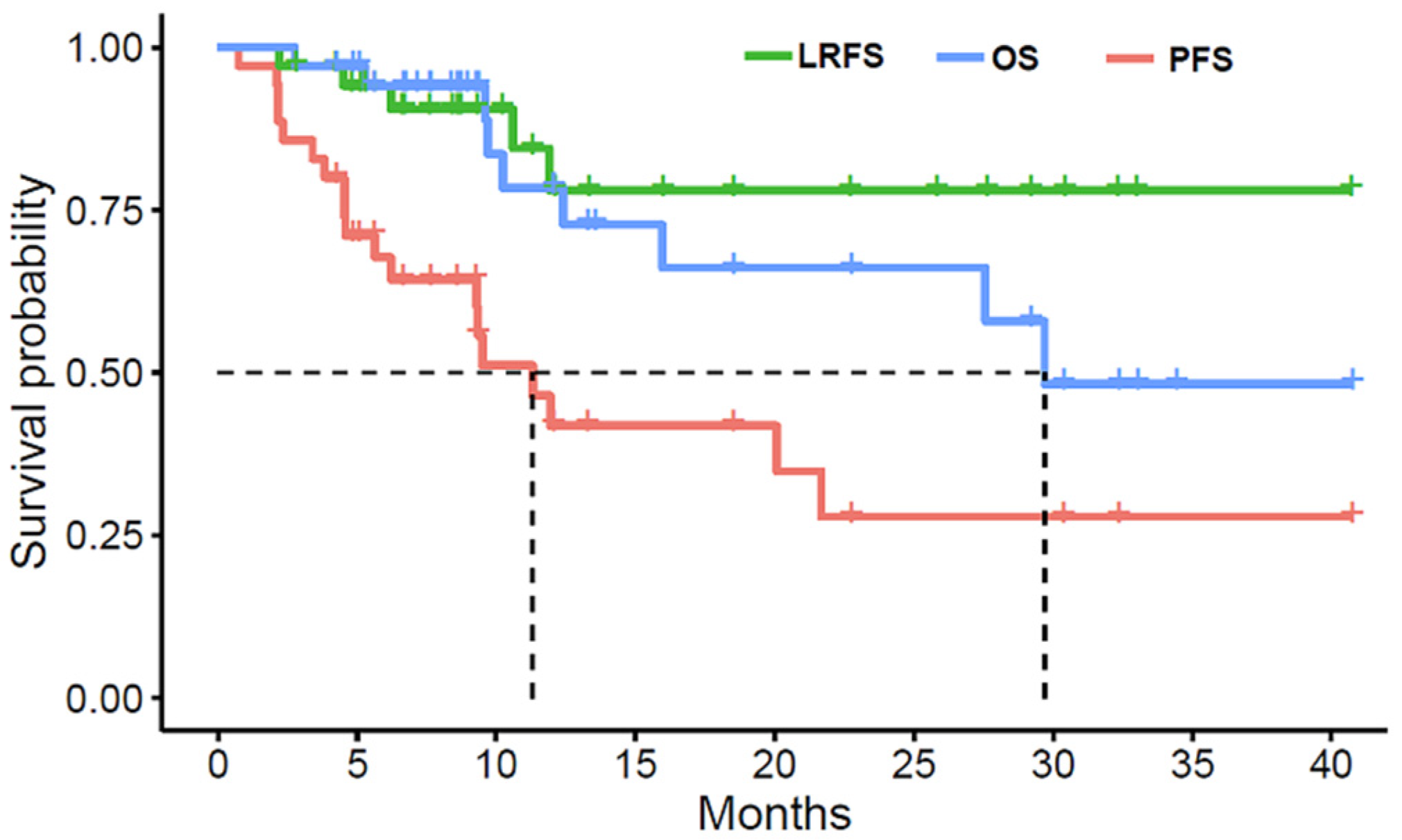
3. Results
3.1. Patients
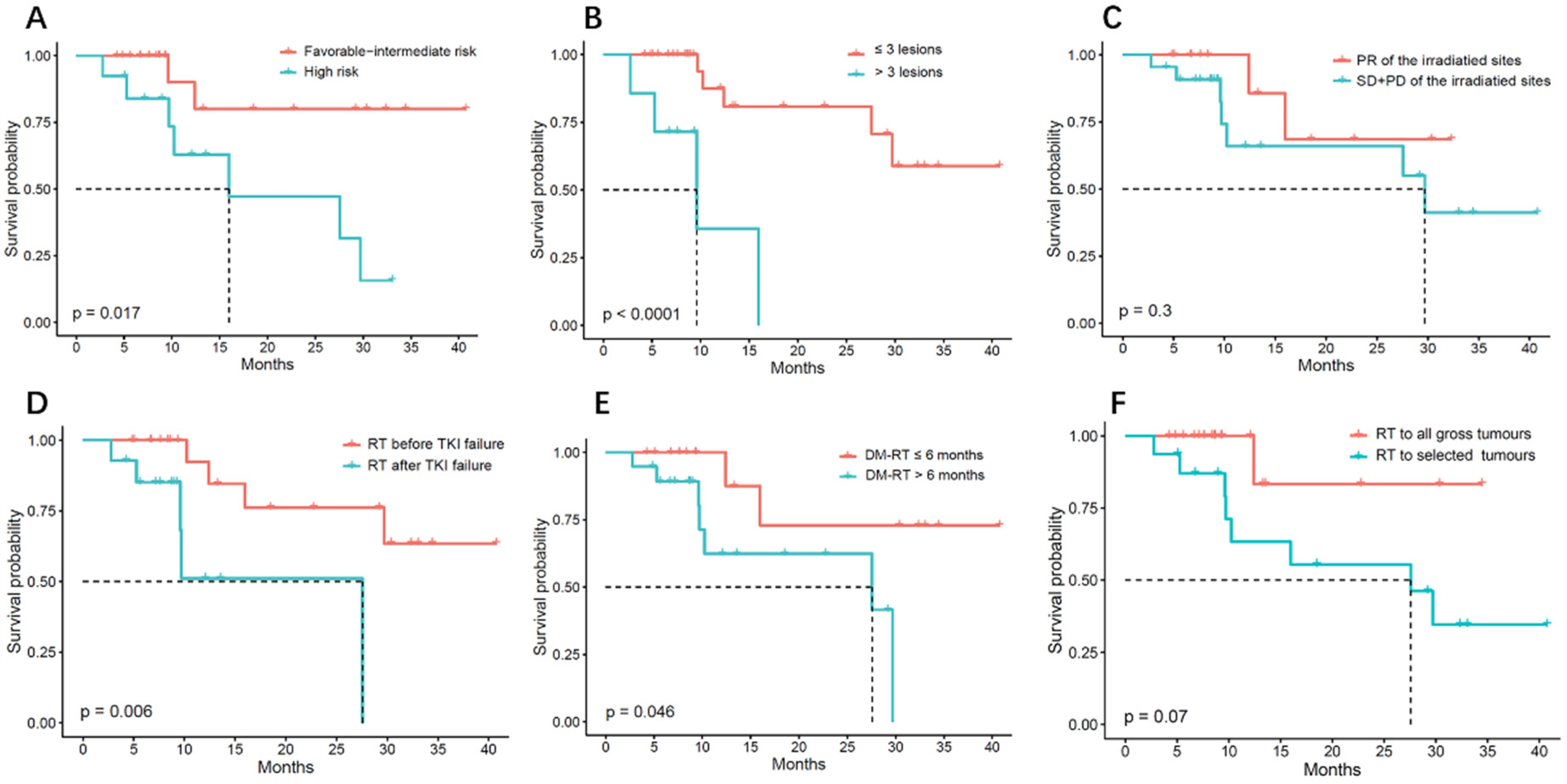
3.2. Treatment Results
3.3. Toxicity Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, J.S.; Shvarts, O.; Leppert, J.T.; Figlin, R.A.; Belldegrun, A.S. Renal cell carcinoma 2005: New frontiers in staging, prognostication and targeted molecular therapy. J. Urol. 2005, 173, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Fuks, Z.; Kolesnick, R. Engaging the vascular component of the tumor response. Cancer Cell 2005, 8, 89–91. [Google Scholar] [CrossRef]
- Chen, W.; Hill, H.; Christie, A.; Kim, M.S.; Holloman, E.; Pavia-Jimenez, A.; Homayoun, F.; Ma, Y.; Patel, N.; Yell, P.; et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 2016, 539, 112–117. [Google Scholar] [CrossRef]
- Francolini, G.; Detti, B.; Ingrosso, G.; Desideri, I.; Becherini, C.; Carta, G.; Pezzulla, D.; Caramia, G.; Dominici, L.; Maragna, V.; et al. Stereotactic body radiation therapy (SBRT) on renal cell carcinoma, an overview of technical aspects, biological rationale and current literature. Crit. Rev. Oncol. Hematol. 2018, 131, 24–29. [Google Scholar] [CrossRef]
- Bai, Y.; Gao, X.S.; Qin, S.B.; Chen, J.Y.; Su, M.M.; Liu, Q.; Qin, X.B.; Ma, M.W.; Zhao, B.; Gu, X.B.; et al. Partial stereotactic ablative boost radiotherapy in bulky non-small cell lung cancer: A retrospective study. Oncol. Targets Ther. 2018, 11, 2571–2579. [Google Scholar] [CrossRef]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef]
- Timmerman, R. A Story of Hypofractionation and the Table on the Wall. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 4–21. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Constine, L.S.; Deasy, J.O.; Eisbruch, A.; Jackson, A.; Marks, L.B.; Haken, R.K.T.; Yorke, E.D. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): An introduction to the scientific issues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. S3), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Hannan, R.; Christensen, M.; Hammers, H.; Christie, A.; Paulman, B.; Lin, D.; Garant, A.; Arafat, W.; Courtney, K.; Bowman, I.; et al. Phase II Trial of Stereotactic Ablative Radiation for Oligoprogressive Metastatic Kidney Cancer. Eur. Urol. Oncol. 2022, 5, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, P.G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Ingrosso, G.; Becherini, C.; Francolini, G.; Lancia, A.; Alì, E.; Caini, S.; Teriaca, M.A.; Marchionni, A.; Filippi, A.R.; Livi, L.; et al. Stereotactic body radiotherapy (SBRT) in combination with drugs in metastatic kidney cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2021, 159, 103242. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.; Pasquier, D.; Bernadou, G.; Calais, G.; Maroun, P.; Bossi, A.; Theodore, C.; Albiges, L.; Stefan, D.; De Crevoisier, R.; et al. Stereotactic radiation therapy in the strategy of treatment of metastatic renal cell carcinoma: A study of the Getug group. Eur. J. Cancer 2018, 98, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.J.M.; Louie, A.V.; Zaorsky, N.G.; Lehrer, E.J.; Ellis, R.; Ponsky, L.; Kaplan, I.; Mahadevan, A.; Chu, W.; Swaminath, A.; et al. The Emerging Role of Stereotactic Ablative Radiotherapy for Primary Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Eur. Urol. Focus 2019, 5, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Zaorsky, N.G.; Lehrer, E.J.; Kothari, G.; Louie, A.V.; Siva, S. Stereotactic ablative radiation therapy for oligometastatic renal cell carcinoma (SABR ORCA): A meta-analysis of 28 studies. Eur. Urol. Oncol. 2019, 2, 515–523. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Greco, C.; Motzer, R.; Magsanoc, J.M.; Pei, X.; Lovelock, M.; Mechalakos, J.; Zatcky, J.; Fuks, Z.; Yamada, Y. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1744–1748. [Google Scholar] [CrossRef]
- Masini, C.; Iotti, C.; De Giorgi, U.; Bellia, R.S.; Buti, S.; Salaroli, F.; Zampiva, I.; Mazzarotto, R.; Mucciarini, C.; Vitale, M.G.; et al. Nivolumab in Combination with Stereotactic Body Radiotherapy in Pretreated Patients with Metastatic Renal Cell Carcinoma. Results of the Phase II NIVES Study. Eur. Urol. 2021, 81, 274–282. [Google Scholar] [CrossRef]
- Hammers, H.J.; Vonmerveldt, D.; Ahn, C.; Nadal, R.M.; Carducci, M.A. Combination of dual immune checkpoint inhibition (ICI) with stereotactic radiation (SBRT) in metastatic renal cell carcinoma (mRCC) (RADVAX RCC). J. Clin. Oncol. 2020, 38 (Suppl. S6), 614. [Google Scholar] [CrossRef]
- Brooks, E.D.; Chang, J.Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat. Rev. Clin. Oncol. 2019, 16, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Bressel, M.; Wood, S.T.; Shaw, M.G.; Loi, S.; Sandhu, S.K.; Tran, B.; Azad, A.A.; Lewin, J.H.; Cuff, K.E.; et al. Stereotactic Radiotherapy and Short-course Pembrolizumab for Oligometastatic Renal Cell Carcinoma-The RAPPORT Trial. Eur. Urol. 2022, 81, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Correa, R.J.M.; Schneiders, F.; Haasbeek, C.J.A.; Rodrigues, G.B.; Lock, M.; Yaremko, B.P.; Bauman, G.S.; et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET-10): Study protocol for a randomized phase III trial. BMC Cancer 2019, 19, 816. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Han, H.; Liu, Z.; Huang, S.; Cao, W.; Liu, B.; Qin, Z.; Guo, S.; Zhang, Z.; et al. Survival Outcomes After Adding Stereotactic Body Radiotherapy to Metastatic Renal Cell Carcinoma Patients Treated With Tyrosine Kinase Inhibitors. Am. J. Clin. Oncol. 2020, 43, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Franzese, C.; Franceschini, D.; Di Brina, L.; D’Agostino, G.R.; Navarria, P.; Comito, T.; Mancosu, P.; Tomatis, S.; Scorsetti, M. Role of Stereotactic Body Radiation Therapy for the Management of Oligometastatic Renal Cell Carcinoma. J. Urol. 2019, 201, 70–75. [Google Scholar] [CrossRef]
- Stenman, M.; Sinclair, G.; Paavola, P.; Wersall, P.; Harmenberg, U.; Lindskog, M. Overall survival after stereotactic radiotherapy or surgical metastasectomy in oligometastatic renal cell carcinoma patients treated at two Swedish centres 2005–2014. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 127, 501–506. [Google Scholar] [CrossRef]
- Ranck, M.C.; Golden, D.W.; Corbin, K.S.; Hasselle, M.D.; Liauw, S.L.; Stadler, W.M.; Hahn, O.M.; Weichselbaum, R.R.; Salama, J.K. Stereotactic body radiotherapy for the treatment of oligometastatic renal cell carcinoma. Am. J. Clin. Oncol. 2013, 36, 589–595. [Google Scholar] [CrossRef]
- Marchand Crety, C.; Vigneau, E.; Invernizzi, C. Stereotactic Body Radiotherapy of a Solitary Metachronous Sphenoid Metastasis from Renal Cell Cancer: A Case Report. Case Rep. Oncol. 2021, 14, 269–273. [Google Scholar] [CrossRef]
- Ponsky, L.E.; Mahadevan, A.; Gill, I.S.; Djemil, T.; Novick, A.C. Renal radiosurgery: Initial clinical experience with histological evaluation. Surg. Innov. 2007, 14, 265–269. [Google Scholar] [CrossRef]
- Buti, S.; Bersanelli, M.; Viansone, A.; Leonetti, A.; Masini, C.; Ratta, R.; Procopio, G.; Maines, F.; Iacovelli, R.; Ciccarese, C.; et al. Treatment Outcome of metastatic lesions from renal cell carcinoma underGoing Extra-cranial stereotactic body radioTHERapy: The together retrospective study. Cancer Treat. Res. Commun. 2020, 22, 100161. [Google Scholar] [CrossRef]

| Characteristic | Value |
|---|---|
| Age (y), median (range) | 63 (32–82) |
| Sex | |
| Male | 29 (82.9) |
| Female | 6 (17.1) |
| ECOG a score | |
| 0 | 4 (11.4) |
| 1 | 31 (88.6) |
| Number of lesions | |
| ≤3 | 28 (80.0) |
| >3 | 7 (20.0) |
| Pathological type | |
| Clear-cell carcinoma | 29 (82.9) |
| Papillary renal carcinomas | 4 (17.1) |
| Others | 2 (5.7) |
| Sarcomatoid differentiation | 4 (11.4) |
| IMDC b score | |
| Favorable risk | 4 (17.1) |
| Intermediate risk | 18 (51.4) |
| High risk | 13 (37.1) |
| Synchronous metastases | 9 (25.7) |
| Surgery | |
| No surgery | 5 (14.3) |
| RN c or NSS d only | 22 (62.9) |
| Metastasectomy only | 1 (2.9) |
| Metastasectomy plus RN or NSS | 7 (20.0) |
| Tyrosine kinase inhibitor | 31 (88.6) |
| PD-1 e inhibitor | 10 (28.6) |
| Treatment Characteristics | |
|---|---|
| Time interval from first distant metastasis to radiation therapy | |
| Within 6 months | 16 (45.7) |
| Within 8 months | 21 (60.0) |
| Sequencing with TKI a | |
| Before failure of TKI | 14 (40.0) |
| After failure of TKI | 21 (60.0) |
| Coverage of irradiated sites | |
| Selected tumor sites irradiation | 16 (45.7) |
| All tumor sites irradiation | 19 (54.3) |
| Response of the irradiation sites | |
| PR b | 13 (37.1) |
| SD c or PD d | 22 (62.9) |
| Side Effects | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Anemia | 5 | 3 | 1 |
| Pneumonitis | 1 | 2 | - |
| Gastrointestinal toxicity | 12 | 3 | - |
| Dermatitis | 3 | - | - |
| Neutropenia | 1 | - | - |
| Thrombocytopenia | 1 | - | - |
| Fistula | - | 1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.-W.; Li, H.-Z.; Gao, X.-S.; Liu, M.-Z.; Yin, H.; Yang, K.-W.; Chen, J.-Y.; Ren, X.-Y.; Wang, D. Outcomes of High-Dose Stereotactic Ablative Radiotherapy to All/Multiple Sites for Oligometastatic Renal Cell Cancer Patients. Curr. Oncol. 2022, 29, 7832-7841. https://doi.org/10.3390/curroncol29100619
Ma M-W, Li H-Z, Gao X-S, Liu M-Z, Yin H, Yang K-W, Chen J-Y, Ren X-Y, Wang D. Outcomes of High-Dose Stereotactic Ablative Radiotherapy to All/Multiple Sites for Oligometastatic Renal Cell Cancer Patients. Current Oncology. 2022; 29(10):7832-7841. https://doi.org/10.3390/curroncol29100619
Chicago/Turabian StyleMa, Ming-Wei, Hong-Zhen Li, Xian-Shu Gao, Ming-Zhu Liu, Huan Yin, Kai-Wei Yang, Jia-Yan Chen, Xue-Ying Ren, and Dian Wang. 2022. "Outcomes of High-Dose Stereotactic Ablative Radiotherapy to All/Multiple Sites for Oligometastatic Renal Cell Cancer Patients" Current Oncology 29, no. 10: 7832-7841. https://doi.org/10.3390/curroncol29100619
APA StyleMa, M.-W., Li, H.-Z., Gao, X.-S., Liu, M.-Z., Yin, H., Yang, K.-W., Chen, J.-Y., Ren, X.-Y., & Wang, D. (2022). Outcomes of High-Dose Stereotactic Ablative Radiotherapy to All/Multiple Sites for Oligometastatic Renal Cell Cancer Patients. Current Oncology, 29(10), 7832-7841. https://doi.org/10.3390/curroncol29100619





