Rationale Efficacy and Safety Evidence of Lenvatinib and Pembrolizumab Association in Anaplastic Thyroid Carcinoma
Abstract
1. Introduction
2. Methods
3. Preclinical Rationale
3.1. Preclinical Rationale of Pembrolizumab in Anaplastic Thyroid Carcinoma
3.2. Preclinical Rationale of Lenvatinib in Anaplastic Thyroid Carcinoma
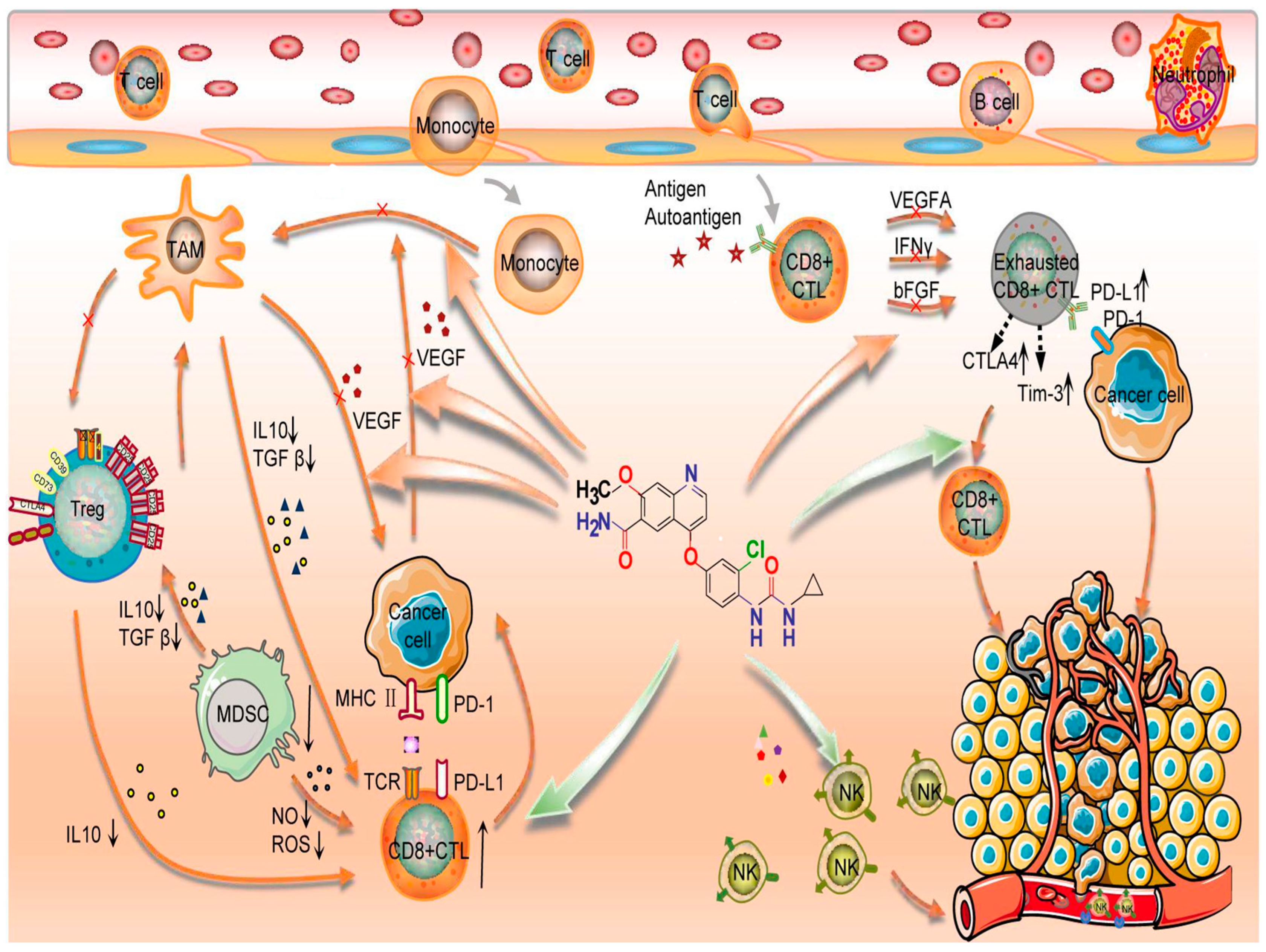
3.3. Preclinical Rationale of Pembrolizuman and Lenvatinib Association in Anaplastic Thyroid Carcinoma
4. Clinical Evidence
4.1. Clinical Evidence of Immunotherapy Alone in ATC
4.2. Clinical Evidence of Lenvatinib Alone in ATC
4.3. Clinical Evidence of Lenvatinib and Pembrolizumab Association in ATC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Cancer Statistics Review, 1975–2013—Previous Version—SEER Cancer Statistics Review. Available online: https://seer.cancer.gov/archive/csr/1975_2013/index.html (accessed on 1 September 2022).
- Onoda, N.; Sugitani, I.; Ito, K.; Suzuki, A.; Higashiyama, T.; Fukumori, T.; Suganuma, N.; Masudo, K.; Nakayama, H.; Uno, A.; et al. Evaluation of the 8th Edition TNM Classification for Anaplastic Thyroid Carcinoma. Cancers 2020, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Jannin, A.; Escande, A.; Al Ghuzlan, A.; Blanchard, P.; Hartl, D.; Chevalier, B.; Deschamps, F.; Lamartina, L.; Lacroix, L.; Dupuy, C.; et al. Anaplastic Thyroid Carcinoma: An Update. Cancers 2022, 14, 1061. [Google Scholar] [CrossRef]
- Maniakas, A.; Dadu, R.; Busaidy, N.L.; Wang, J.R.; Ferrarotto, R.; Lu, C.; Williams, M.D.; Gunn, G.B.; Hofmann, M.-C.; Cote, G.; et al. Evaluation of Overall Survival in Patients with Anaplastic Thyroid Carcinoma, 2000–2019. JAMA Oncol. 2020, 6, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, J.; Kukulska, A.; Jarzab, B. Efficacy of lenvatinib in treating thyroid cancer. Expert Opin. Pharmacother. 2016, 17, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Puliafito, I.; Esposito, F.; Prestifilippo, A.; Marchisotta, S.; Sciacca, D.; Vitale, M.P.; Giuffrida, D. Target Therapy in Thyroid Cancer: Current Challenge in Clinical Use of Tyrosine Kinase Inhibitors and Management of Side Effects. Front. Endocrinol. 2022, 13, 860671. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Thompson, J.C.; Carpenter, E.L. Plasma Tumor Mutation Burden and Response to Pembrolizumab-Response. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 1581. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Hatashima, A.; Archambeau, B.; Armbruster, H.; Xu, M.; Shah, M.; Konda, B.; Lott Limbach, A.; Sukrithan, V. An Evaluation of Clinical Efficacy of Immune Checkpoint Inhibitors for Patients with Anaplastic Thyroid Carcinoma. Thyroid Off. J. Am. Thyroid Assoc. 2022, 32, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Dierks, C.; Seufert, J.; Aumann, K.; Ruf, J.; Klein, C.; Kiefer, S.; Rassner, M.; Boerries, M.; Zielke, A.; la Rosee, P.; et al. Combination of Lenvatinib and Pembrolizumab Is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid Off. J. Am. Thyroid Assoc. 2021, 31, 1076–1085. [Google Scholar] [CrossRef]
- Kimura, T.; Kato, Y.; Ozawa, Y.; Kodama, K.; Ito, J.; Ichikawa, K.; Yamada, K.; Hori, Y.; Tabata, K.; Takase, K.; et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018, 109, 3993–4002. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.H.; Schmidt, E.V.; Dutcus, C.; Pinheiro, E.M.; Funahashi, Y.; Lubiniecki, G.; Rasco, D. The LEAP program: Lenvatinib plus pembrolizumab for the treatment of advanced solid tumors. Future Oncol. 2021, 17, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Colombo, N.; Casado Herráez, A.; Santin, A.D.; Colomba, E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- McCrary, H.C.; Aoki, J.; Huang, Y.; Chadwick, B.; Kerrigan, K.; Witt, B.; Hunt, J.P.; Abraham, D. Mutation based approaches to the treatment of anaplastic thyroid cancer. Clin. Endocrinol. 2022, 96, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Giannini, R.; Moretti, S.; Ugolini, C.; Macerola, E.; Menicali, E.; Nucci, N.; Morelli, S.; Colella, R.; Mandarano, M.; Sidoni, A.; et al. Immune Profiling of Thyroid Carcinomas Suggests the Existence of Two Major Phenotypes: An ATC-Like and a PDTC-Like. J. Clin. Endocrinol. Metab. 2019, 104, 3557–3575. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Wirth, L.J.; Ernst, T.; Aix, S.P.; Lin, C.-C.; Ramlau, R.; Butler, M.O.; Delord, J.-P.; Gelderblom, H.; Ascierto, P.A.; et al. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Schürch, C.M.; Roelli, M.A.; Forster, S.; Wasmer, M.-H.; Brühl, F.; Maire, R.S.; Di Pancrazio, S.; Ruepp, M.-D.; Giger, R.; Perren, A.; et al. Targeting CD47 in Anaplastic Thyroid Carcinoma Enhances Tumor Phagocytosis by Macrophages and Is a Promising Therapeutic Strategy. Thyroid Off. J. Am. Thyroid Assoc. 2019, 29, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kim, T.H.; Kim, S.W.; Ki, C.S.; Jang, H.W.; Kim, J.S.; Kim, J.H.; Choe, J.-H.; Shin, J.H.; Hahn, S.Y.; et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr. Relat. Cancer 2017, 24, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Cameselle-García, S.; Abdulkader-Sande, S.; Sánchez-Ares, M.; Rodríguez-Carnero, G.; Garcia-Gómez, J.; Gude-Sampedro, F.; Abdulkader-Nallib, I.; Cameselle-Teijeiro, J.M. PD-L1 expression and immune cells in anaplastic carcinoma and poorly differentiated carcinoma of the human thyroid gland: A retrospective study. Oncol. Lett. 2021, 22, 553. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.W.; Gigliotti, B.J.; Pai, S.I.; Parangi, S.; Wachtel, H.; Mino-Kenudson, M.; Gunda, V.; Faquin, W.C. PD-L1 and IDO1 Are Expressed in Poorly Differentiated Thyroid Carcinoma. Endocr. Pathol. 2018, 29, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Cantara, S.; Bertelli, E.; Occhini, R.; Regoli, M.; Brilli, L.; Pacini, F.; Castagna, M.G.; Toti, P. Blockade of the programmed death ligand 1 (PD-L1) as potential therapy for anaplastic thyroid cancer. Endocrine 2019, 64, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Chintakuntlawar, A.V.; Rumilla, K.M.; Smith, C.Y.; Jenkins, S.M.; Foote, R.L.; Kasperbauer, J.L.; Morris, J.C.; Ryder, M.; Alsidawi, S.; Hilger, C.; et al. Expression of PD-1 and PD-L1 in Anaplastic Thyroid Cancer Patients Treated with Multimodal Therapy: Results from a Retrospective Study. J. Clin. Endocrinol. Metab. 2017, 102, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, A.; Hernando, J.; Carmona-Alonso, A.; Capdevila, J. What is the status of immunotherapy in thyroid neoplasms? Front. Endocrinol. 2022, 13, 929091. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Piccioni, D.; Kato, S.; Boichard, A.; Wang, H.-Y.; Frampton, G.; Lippman, S.M.; Connelly, C.; Fabrizio, D.; Miller, V.; et al. Prevalence of PDL1 Amplification and Preliminary Response to Immune Checkpoint Blockade in Solid Tumors. JAMA Oncol. 2018, 4, 1237–1244. [Google Scholar] [CrossRef]
- Adam, P.; Kircher, S.; Sbiera, I.; Koehler, V.F.; Berg, E.; Knösel, T.; Sandner, B.; Fenske, W.K.; Bläker, H.; Smaxwil, C.; et al. FGF-Receptors and PD-L1 in Anaplastic and Poorly Differentiated Thyroid Cancer: Evaluation of the Preclinical Rationale. Front. Endocrinol. 2021, 12, 712107. [Google Scholar] [CrossRef]
- Emancipator, K. Keytruda and PD-L1: A Real-World Example of Co-development of a Drug with a Predictive Biomarker. AAPS J. 2020, 23, 5. [Google Scholar] [CrossRef]
- Fancello, L.; Gandini, S.; Pelicci, P.G.; Mazzarella, L. Tumor mutational burden quantification from targeted gene panels: Major advancements and challenges. J. Immunother. Cancer 2019, 7, 183. [Google Scholar] [CrossRef]
- Giordano, T.J. Genomic Hallmarks of Thyroid Neoplasia. Annu. Rev. Pathol. 2018, 13, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.-C.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-K.; Song, Y.S.; Lee, E.K.; Hwang, J.; Kim, H.H.; Jung, G.; Kim, Y.A.; Kim, S.-J.; Cho, S.W.; Won, J.-K.; et al. Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat. Commun. 2019, 10, 2764. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Singh, A.; Ham, J.; Po, J.W.; Niles, N.; Roberts, T.; Lee, C.S. The Genomic Landscape of Thyroid Cancer Tumourigenesis and Implications for Immunotherapy. Cells 2021, 10, 1082. [Google Scholar] [CrossRef]
- Rocha, M.L.; Schmid, K.W.; Czapiewski, P. The prevalence of DNA microsatellite instability in anaplastic thyroid carcinoma—Systematic review and discussion of current therapeutic options. Contemp. Oncol. 2021, 25, 213–223. [Google Scholar] [CrossRef]
- Lee, H.-O.; Hong, Y.; Etlioglu, H.E.; Cho, Y.B.; Pomella, V.; Van den Bosch, B.; Vanhecke, J.; Verbandt, S.; Hong, H.; Min, J.-W.; et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat. Genet. 2020, 52, 594–603. [Google Scholar] [CrossRef]
- Langouo Fontsa, M.; Padonou, F.; Willard-Gallo, K. Biomarkers and immunotherapy: Where are we? Curr. Opin. Oncol. 2022, 34, 579–586. [Google Scholar] [CrossRef]
- Cohen, R.; Rousseau, B.; Vidal, J.; Colle, R.; Diaz, L.A.; André, T. Immune Checkpoint Inhibition in Colorectal Cancer: Microsatellite Instability and Beyond. Target. Oncol. 2020, 15, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kannan, B.; Lim, B.Y.; Li, Z.; Lim, A.H.; Loh, J.W.; Ko, T.K.; Ng, C.C.-Y.; Chan, J.Y. The Multi-Dimensional Biomarker Landscape in Cancer Immunotherapy. Int. J. Mol. Sci. 2022, 23, 7839. [Google Scholar] [CrossRef] [PubMed]
- Lemery, S.; Keegan, P.; Pazdur, R. First FDA Approval Agnostic of Cancer Site—When a Biomarker Defines the Indication. N. Engl. J. Med. 2017, 377, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C.; Ain, K.B.; Asa, S.L.; Bible, K.C.; Brierley, J.D.; Burman, K.D.; Kebebew, E.; Lee, N.Y.; Nikiforov, Y.E.; Rosenthal, M.S.; et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2012, 22, 1104–1139. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Fallahi, P.; Ferrari, S.M.; Ruffilli, I.; Santini, F.; Minuto, M.; Galleri, D.; Miccoli, P. New targeted therapies for thyroid cancer. Curr. Genom. 2011, 12, 626–631. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Bocci, G.; Di Desidero, T.; Elia, G.; Ruffilli, I.; Ragusa, F.; Orlandi, P.; Paparo, S.R.; Patrizio, A.; Piaggi, S.; et al. Lenvatinib exhibits antineoplastic activity in anaplastic thyroid cancer in vitro and in vivo. Oncol. Rep. 2018, 39, 2225–2234. [Google Scholar] [CrossRef]
- Okamoto, K.; Kodama, K.; Takase, K.; Sugi, N.H.; Yamamoto, Y.; Iwata, M.; Tsuruoka, A. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013, 340, 97–103. [Google Scholar] [CrossRef]
- Tohyama, O.; Matsui, J.; Kodama, K.; Hata-Sugi, N.; Kimura, T.; Okamoto, K.; Minoshima, Y.; Iwata, M.; Funahashi, Y. Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J. Thyroid Res. 2014, 2014, 638747. [Google Scholar] [CrossRef]
- Santarpia, L.; El-Naggar, A.K.; Cote, G.J.; Myers, J.N.; Sherman, S.I. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2008, 93, 278–284. [Google Scholar] [CrossRef]
- Ratajczak, M.; Gaweł, D.; Godlewska, M. Novel Inhibitor-Based Therapies for Thyroid Cancer—An Update. Int. J. Mol. Sci. 2021, 22, 11829. [Google Scholar] [CrossRef]
- Shinohara, M.; Chung, Y.J.; Saji, M.; Ringel, M.D. AKT in thyroid tumorigenesis and progression. Endocrinology 2007, 148, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Mazumdar, A.; Dash, R.; Sarkar, D.; Fisher, P.B.; Mandal, M. ZD6474, a dual tyrosine kinase inhibitor of EGFR and VEGFR-2, inhibits MAPK/ERK and AKT/PI3-K and induces apoptosis in breast cancer cells. Cancer Biol. Ther. 2010, 9, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Di Desidero, T.; Antonelli, A.; Orlandi, P.; Ferrari, S.M.; Fioravanti, A.; Alì, G.; Fontanini, G.; Basolo, F.; Francia, G.; Bocci, G. Synergistic efficacy of irinotecan and sunitinib combination in preclinical models of anaplastic thyroid cancer. Cancer Lett. 2017, 411, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-J.; Au, A.Y.M.; Foukakis, T.; Barbaro, M.; Kiss, N.; Clifton-Bligh, R.; Staaf, J.; Borg, A.; Delbridge, L.; Robinson, B.G.; et al. Array-CGH identifies cyclin D1 and UBCH10 amplicons in anaplastic thyroid carcinoma. Endocr. Relat. Cancer 2008, 15, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, S.M.; Masoudi, H.; Niblock, P.; Turbin, D.; Rajput, A.; Hay, J.; Bugis, S.; Filipenko, D.; Huntsman, D.; Gilks, B. Anaplastic thyroid carcinoma: Expression profile of targets for therapy offers new insights for disease treatment. Ann. Surg. Oncol. 2007, 14, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhou, Z.; Chen, Z.; Xu, G.; Chen, Y. Fibroblast Growth Factor Receptors (FGFRs): Structures and Small Molecule Inhibitors. Cells 2019, 8, 614. [Google Scholar] [CrossRef]
- Yamazaki, H.; Yokose, T.; Hayashi, H.; Iwasaki, H.; Osanai, S.; Suganuma, N.; Nakayama, H.; Masudo, K.; Rino, Y.; Masuda, M. Expression of fibroblast growth factor receptor 4 and clinical response to lenvatinib in patients with anaplastic thyroid carcinoma: A pilot study. Eur. J. Clin. Pharmacol. 2020, 76, 703–709. [Google Scholar] [CrossRef]
- Ott, P.A.; Hodi, F.S.; Buchbinder, E.I. Inhibition of Immune Checkpoints and Vascular Endothelial Growth Factor as Combination Therapy for Metastatic Melanoma: An Overview of Rationale, Preclinical Evidence, and Initial Clinical Data. Front. Oncol. 2015, 5, 202. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, J.; Du, Q.; Hu, M.; Wei, Y.; Wang, M.; Li, H.; Li, Q. Multi-Omics Analysis of the Anti-tumor Synergistic Mechanism and Potential Application of Immune Checkpoint Blockade Combined With Lenvatinib. Front. Cell Dev. Biol. 2021, 9, 730240. [Google Scholar] [CrossRef]
- Kato, Y.; Tabata, K.; Kimura, T.; Yachie-Kinoshita, A.; Ozawa, Y.; Yamada, K.; Ito, J.; Tachino, S.; Hori, Y.; Matsuki, M.; et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS ONE 2019, 14, e0212513. [Google Scholar] [CrossRef]
- Grünwald, V.; Powles, T.; Choueiri, T.K.; Hutson, T.E.; Porta, C.; Eto, M.; Sternberg, C.N.; Rha, S.Y.; He, C.S.; Dutcus, C.E.; et al. Lenvatinib plus everolimus or pembrolizumab versus sunitinib in advanced renal cell carcinoma: Study design and rationale. Future Oncol. 2019, 15, 929–941. [Google Scholar] [CrossRef]
- Gunda, V.; Gigliotti, B.; Ashry, T.; Ndishabandi, D.; McCarthy, M.; Zhou, Z.; Amin, S.; Lee, K.E.; Stork, T.; Wirth, L.; et al. Anti-PD-1/PD-L1 therapy augments lenvatinib’s efficacy by favorably altering the immune microenvironment of murine anaplastic thyroid cancer. Int. J. Cancer 2019, 144, 2266–2278. [Google Scholar] [CrossRef]
- Kudo, M. Targeted and immune therapies for hepatocellular carcinoma: Predictions for 2019 and beyond. World J. Gastroenterol. 2019, 25, 789–807. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.H.; Barletta, J.A.; Nehs, M.; Uppaluri, R.; Alexander, E.K.; Haddad, R.I.; Hanna, G.J.; Margalit, D.N.; Tishler, R.B.; Schoenfeld, J.D.; et al. A phase II study of nivolumab (N) plus ipilimumab (I) in radioidine refractory differentiated thyroid cancer (RAIR DTC) with exploratory cohorts in anaplastic (ATC) and medullary thyroid cancer (MTC). J. Clin. Oncol. 2020, 38, 6513. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Varga, A.; Brose, M.S.; Aggarwal, R.R.; Lin, C.-C.; Prawira, A.; de Braud, F.; Tamura, K.; Doi, T.; Piha-Paul, S.A.; et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer 2019, 19, 196. [Google Scholar] [CrossRef]
- Nabhan, F.; Kander, E.; Shen, R.; Agrawal, A.; Sukrithan, V.; Zhou, Y.; Goyal, A.; Roll, K.; Shah, M.; Konda, B. Pembrolizumab in a Patient with Treatment-Naïve Unresectable BRAF-Mutation Negative Anaplastic Thyroid Cancer. Case Rep. Endocrinol. 2021, 2021, 5521649. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Ferrarotto, R.; Garden, A.S.; Ahmed, S.; Busaidy, N.L.; Dadu, R.; Williams, M.D.; Skinner, H.; Gunn, G.B.; Grosu, H.; et al. Neoadjuvant BRAF- and Immune-Directed Therapy for Anaplastic Thyroid Carcinoma. Thyroid Off. J. Am. Thyroid Assoc. 2018, 28, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Spalart, V.; Legius, B.; Segers, K.; Coolen, J.; Maes, B.; Decoster, L. Dramatic Response to First Line Single Agent Pembrolizumab in Anaplastic Thyroid Carcinoma. Case Rep. Endocrinol. 2019, 2019, 9095753. [Google Scholar] [CrossRef]
- Higashiyama, T.; Sugino, K.; Hara, H.; Ito, K.-I.; Nakashima, N.; Onoda, N.; Tori, M.; Katoh, H.; Kiyota, N.; Ota, I.; et al. Phase II study of the efficacy and safety of lenvatinib for anaplastic thyroid cancer (HOPE). Eur. J. Cancer 2022, 173, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Brose, M.S.; Sherman, E.J.; Licitra, L.; Schlumberger, M.; Sherman, S.I.; Bible, K.C.; Robinson, B.; Rodien, P.; Godbert, Y.; et al. Open-Label, Single-Arm, Multicenter, Phase II Trial of Lenvatinib for the Treatment of Patients with Anaplastic Thyroid Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kiyota, N.; Yamazaki, T.; Chayahara, N.; Nakano, K.; Inagaki, L.; Toda, K.; Enokida, T.; Minami, H.; Imamura, Y.; et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019, 15, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Kiyota, N.; Yamazaki, T.; Chayahara, N.; Nakano, K.; Inagaki, L.; Toda, K.; Enokida, T.; Minami, H.; Imamura, Y.; et al. Lenvatinib for Anaplastic Thyroid Cancer. Front. Oncol. 2017, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Toda, K.; Fujiwara, Y.U.; Wang, X.; Ohmoto, A.; Urasaki, T.; Hayashi, N.; Sato, Y.; Nakano, K.; Yunokawa, M.; et al. Neutrophil-to-Lymphocyte Ratio as a Prognostic Marker for Anaplastic Thyroid Cancer Treated with Lenvatinib. In Vivo 2020, 34, 2859–2864. [Google Scholar] [CrossRef]
- Ishihara, S.; Onoda, N.; Noda, S.; Tauchi, Y.; Morisaki, T.; Asano, Y.; Kashiwagi, S.; Takashima, T.; Ohira, M. Treatment of anaplastic thyroid cancer with tyrosine kinase inhibitors targeted on the tumor vasculature: Initial experience in clinical practice. Endocr. J. 2021, 68, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Toda, S.; Murayama, D.; Kato, S.; Matsui, A. Relationship between adverse events associated with lenvatinib treatment for thyroid cancer and patient prognosis. Mol. Clin. Oncol. 2021, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Dadu, R.; Ferrarotto, R.; Busaidy, N.L.; Habra, M.A.; Zafereo, M.; Gross, N.; Hess, K.R.; Gule-Monroe, M.; Williams, M.D.; et al. Real-World Experience with Targeted Therapy for the Treatment of Anaplastic Thyroid Carcinoma. Thyroid Off. J. Am. Thyroid Assoc. 2018, 28, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ahn, J.; Song, D.E.; Yoon, J.H.; Kang, H.-C.; Lim, D.J.; Kim, W.G.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; et al. Real-world experience of lenvatinib in patients with advanced anaplastic thyroid cancer. Endocrine 2021, 71, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jung, H.A.; Shim, J.H.; Park, W.-Y.; Kim, T.H.; Lee, S.-H.; Kim, S.W.; Ahn, M.-J.; Park, K.; Chung, J.H. Multimodal treatments and outcomes for anaplastic thyroid cancer before and after tyrosine kinase inhibitor therapy: A real-world experience. Eur. J. Endocrinol. 2021, 184, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Sparano, C.; Godbert, Y.; Attard, M.; Do Cao, C.; Zerdoud, S.; Roudaut, N.; Joly, C.; Berdelou, A.; Hadoux, J.; Lamartina, L.; et al. Limited efficacy of lenvatinib in heavily pretreated anaplastic thyroid cancer: A French overview. Endocr. Relat. Cancer 2021, 28, 15–26. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, J.; Zheng, X.; Gao, M. Efficacy and Safety of Lenvatinib in Anaplastic Thyroid Carcinoma: A Meta-Analysis. Front. Endocrinol. 2022, 13, 920857. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.-P.H.; Henry, S.; Zanetta, S.; Kaminsky, M.-C.; Michoux, N.; Rommel, D.; Schmitz, S.; Bompas, E.; Dillies, A.-F.; Faivre, S.; et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.P.; Ma, B.B.Y.; King, A.D.; Mo, F.; Chan, S.L.; Kam, M.K.M.; Loong, H.H.; Ahuja, A.T.; Zee, B.C.Y.; Chan, A.T.C. Hemorrhagic complications in a phase II study of sunitinib in patients of nasopharyngeal carcinoma who has previously received high-dose radiation. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Blevins, D.P.; Dadu, R.; Hu, M.; Baik, C.; Balachandran, D.; Ross, W.; Gunn, B.; Cabanillas, M.E. Aerodigestive fistula formation as a rare side effect of antiangiogenic tyrosine kinase inhibitor therapy for thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2014, 24, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Berdelou, A.; Borget, I.; Godbert, Y.; Nguyen, T.; Garcia, M.-E.; Chougnet, C.N.; Ferru, A.; Buffet, C.; Chabre, O.; Huillard, O.; et al. Lenvatinib for the Treatment of Radioiodine-Refractory Thyroid Cancer in Real-Life Practice. Thyroid Off. J. Am. Thyroid Assoc. 2018, 28, 72–78. [Google Scholar] [CrossRef]
- Savvides, P.; Nagaiah, G.; Lavertu, P.; Fu, P.; Wright, J.J.; Chapman, R.; Wasman, J.; Dowlati, A.; Remick, S.C. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid Off. J. Am. Thyroid Assoc. 2013, 23, 600–604. [Google Scholar] [CrossRef]
- Bible, K.C.; Suman, V.J.; Menefee, M.E.; Smallridge, R.C.; Molina, J.R.; Maples, W.J.; Karlin, N.J.; Traynor, A.M.; Kumar, P.; Goh, B.C.; et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2012, 97, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Dadu, R.; Gule-Monroe, M.; Busaidy, N.L.; Ferrarotto, R.; Habra, M.A.; Zafereo, M.; Williams, M.D.; Gunn, G.B.; Grosu, H.; et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J. Immunother. Cancer 2018, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Dierks, C.; Ruf, J.; Seufert, J.; Kreissl, M.; Klein, C.; Spitzweg, C.; Kroiss, M.; Thomusch, O.; Lorenz, K.; Zielke, A.; et al. Phase II ATLEP trial: Final results for lenvatinib/pembrolizumab in metastasized anaplastic and poorly differentiated thyroid carcinoma. Ann. Oncol. 2022, 33 (Suppl. S7), S750–S757. [Google Scholar] [CrossRef]
- Shih, S.-R.; Chen, K.-H.; Lin, K.-Y.; Yang, P.-C.; Chen, K.-Y.; Wang, C.-W.; Chen, C.-N.; Lin, C.-F.; Lin, C.-C. Immunotherapy in anaplastic thyroid cancer: Case series. J. Formos. Med. Assoc. Taiwan Yi Zhi 2022, 121, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Luongo, C.; Porcelli, T.; Sessa, F.; De Stefano, M.A.; Scavuzzo, F.; Damiano, V.; Klain, M.; Bellevicine, C.; Matano, E.; Troncone, G.; et al. Combination of Lenvatinib and Pembrolizumab as Salvage Treatment for Paucicellular Variant of Anaplastic Thyroid Cancer: A Case Report. Curr. Oncol. Tor. Ont. 2021, 28, 5401–5407. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Taylor, M.H.; Evans, T.R.J.; Okusaka, T.; Glen, H.; Lubiniecki, G.M.; Dutcus, C.; Smith, A.D.; Okpara, C.E.; Hussein, Z.; et al. Lenvatinib dose, efficacy, and safety in the treatment of multiple malignancies. Expert Rev. Anticancer Ther. 2022, 22, 383–400. [Google Scholar] [CrossRef] [PubMed]
| Study | ICI | Nb of Patient Included | Median Age | Previous Systemic Treatment (%) | ORR | Median PFS (Months) | Median OS (Months) | One Year Survival Rate |
|---|---|---|---|---|---|---|---|---|
| Hatashima 2022 [12] | Pembrolizumab (12 patients). Nivolumab (1 patient) | 13 | 70 | 23% | 16% | 1.9 | 4.4 | 38% |
| Capdevila 2020 [20] | Spartalizumab | 42 | 62 | 40% | 19% | 1.7 | 5.9 | 40% |
| Lorch 2020 [65] | Ipilimumab + Nivolumab | 10 | 65 | NA | 30% | NA | NA | NA |
| Study References | Methodology | Nb of Patient Included | Median Age (Years) | Previous Systemic Treatment Rate | ORR | Median PFS (Months) | Median OS (Months) | One-Year Survival Rate |
|---|---|---|---|---|---|---|---|---|
| Huang 2022 [81] | Meta-analysis | 176 | NA | NA | 15% | 3.1 | 3.2 | 18.9% |
| Higashiyama 2022 [70] | Prospective | 42 | 73 | 60% | 12% | NA | 5.0 | 11.9% |
| Tahara 2017 [73] | Prospective | 17 | 65 | 59% | 24 | 7.4 | 10.6 | NA |
| Wirth 2021 [71] | Prospective | 34 | NA | 70% | 3% | 2.6 | 3.2 | 28% |
| Takahashi 2019 [72] | Prospective | 17 | 65 | NA | 24% | 7.4 | 10.6 | NA |
| Fukuda 2020 [74] | Retrospective | 13 | 68 | 69% | 23% | 3.8 | 10.2 | NA |
| Ishihara 2021 [75] | Retrospective | 10 | 69 | 50% | 30% | NA | 4.7 | 15% |
| Iwasaki 2021 [76] | Retrospective | 32 | 77 | NA | 19% | NA | 3.2 | NA |
| Iyer 2018 [77] | Retrospective | 10 | 67 | NA | 30% | 2.6 | 3.9 | NA |
| Kim 2020 [78] | Retrospective | 14 | 66 | NA | 29% | 5.7 | 6.7 | NA |
| Park 2021 [79] | Retrospective | 11 | NA | NA | 27% | NA | NA | NA |
| Sparano 2021 [80] | Retrospective | 15 | 67 | 93% | 0% | NA | 2.7 | NA |
| Yamazaki 2020 [58] | Retrospective | 20 | 74 | NA | 10% | NA | NA | NA |
| References | Methodology | Nb of Patient Included | Median Age (Years) | Previous Systemic Treatment | ORR | Median PFS (Months) | Median OS (Months) | One Year Survival Rate |
|---|---|---|---|---|---|---|---|---|
| Dierks 2021 [13] | Retrospective | 6 | NA | 83% | 66% | 17.7 | 18.5 | 50% |
| Dierks 2022 [89] | Prospective | 29 | NA | NA | 52% | 10 | 11 | NA |
| Iyer 2018 [88] | Retrospective | 5 | 60 | 60% | 60% | 8.3 | 8.3 | 40% |
| References | Age (Years) | Previous Systemic Treatment | Follow Up | Best Response | Duration of Treatment Response (Months) | OS (Months) |
|---|---|---|---|---|---|---|
| Shih 2022 [90] | 71 | No | 1 months | PD | 1 | 1 |
| Shih 2022 [90] | 58 | Yes | 2.7 months | PR | 2.7 | 2.7 |
| McCrary 2022 [18] | 54 | Yes | NA | PR | NA | NA |
| McCrary 2022 [18] | 59 | No | 3 months | PR | NA | NA |
| Luongo 2021 [91] | 54 | Yes | 18 months | PR | 18 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudin, L.; Morvan, J.-B.; Thariat, J.; Métivier, D.; Marcy, P.-Y.; Delarbre, D. Rationale Efficacy and Safety Evidence of Lenvatinib and Pembrolizumab Association in Anaplastic Thyroid Carcinoma. Curr. Oncol. 2022, 29, 7718-7731. https://doi.org/10.3390/curroncol29100610
Boudin L, Morvan J-B, Thariat J, Métivier D, Marcy P-Y, Delarbre D. Rationale Efficacy and Safety Evidence of Lenvatinib and Pembrolizumab Association in Anaplastic Thyroid Carcinoma. Current Oncology. 2022; 29(10):7718-7731. https://doi.org/10.3390/curroncol29100610
Chicago/Turabian StyleBoudin, Laurys, Jean-Baptiste Morvan, Juliette Thariat, Denis Métivier, Pierre-Yves Marcy, and David Delarbre. 2022. "Rationale Efficacy and Safety Evidence of Lenvatinib and Pembrolizumab Association in Anaplastic Thyroid Carcinoma" Current Oncology 29, no. 10: 7718-7731. https://doi.org/10.3390/curroncol29100610
APA StyleBoudin, L., Morvan, J.-B., Thariat, J., Métivier, D., Marcy, P.-Y., & Delarbre, D. (2022). Rationale Efficacy and Safety Evidence of Lenvatinib and Pembrolizumab Association in Anaplastic Thyroid Carcinoma. Current Oncology, 29(10), 7718-7731. https://doi.org/10.3390/curroncol29100610






