Abstract
Objective: To assess the value of using the prognostic nutritional index (PNI) and serum albumin/globulin ratio (AGR) in predicting the overall survival (OS) of patients with penile cancer (PC) undergoing penectomy. Materials and methods: A retrospective analysis of 123 patients who were admitted to our hospital due to PC from April 2010 to September 2021 and who underwent penectomy were included in the study. The optimal cut-off value of the PNI and AGR was determined by receiver operating characteristic curve analysis. Kaplan–Meier analysis and the Cox proportional hazard model were used to evaluate the correlation between the PNI, AGR, and OS in patients with PC. Results: A total of 16 of the 123 patients died during the follow-up period, and the median follow-up time was 58.0 months. The best cut-off values of the PNI and AGR were set to 49.03 (95% confidence interval 0.705–0.888, Youden index = 0.517, sensitivity = 57.9%, specificity = 93.7%, p < 0.001) and 1.28 (95% confidence interval 0.610–0.860, Youden index = 0.404, sensitivity = 84.1%, specificity = 56.2%, p = 0.003). The Kaplan–Meier analysis showed that the OS of the patients in the high PNI group and the high AGR group was significantly higher than that of the patients in the low PNI group and the low AGR group (p < 0.001). The univariable analysis showed that the aCCI, the clinical N stage, the pathological stage, and the PNI, AGR, SII, and PLR are all predictors of OS in patients with PC (p < 0.05). The multivariable analysis showed that the PNI (risk rate [HR] = 0.091; 95% CI: 0.010–0.853; p = 0.036) and the AGR (risk rate [HR] = 0.171; 95% CI: 0.043–0.680; p = 0.012) are independent prognostic factors for predicting OS in patients with PC undergoing penectomy. Conclusions: Both the PNI score and the serum AGR are independent prognostic factors for predicting OS in patients with PC undergoing penectomy.
1. Introduction
Penile cancer (PC) is a malignant tumor that originates from the head of the penis, the coronal sulcus, the inner plate mucosa of the foreskin, or the skin of the penis. Due to demographic differences in many countries, including ethnic groups, religions, and sanitary conditions, the incidence of PC in different parts of the world varies widely. The incidence of PC has been reported to be as high as 19:100,000 men in some underdeveloped Asian, African, and Latin American countries, while the incidence in developed regions, such as Europe and the United States, is only about 0.1–0.9:100,000 men [1]. PC has become a rare men’s malignancy in China, accompanying the improvement of China’s medical and health level. Although both patients and doctors do not hope to perform a penectomy for PC patients, due to the fact that the penis is a sign of maleness, partial or radical penectomy is still the best choice for the treatment of late stage or deep infiltrating tumors in the head of the penis [2]. The prognostic assessments of PC are mainly based on histopathology and clinical staging, and there is a lack of effective clinical biomarkers [3]. Therefore, identifying novel clinical prognostic parameters to assist in predicting the clinical outcome of patients with PC after penectomy is critical.
Some commonly used inflammatory nutritional biomarkers have been found, such as the albumin/globulin ratio (AGR), the prognostic nutritional index (PNI), the systemic immune-inflammation index (SII), the neutrophil–lymphocyte ratio (NLR), and the platelet–lymphocyte ratio (PLR), which can predict the prognosis of cancer patients [4,5,6]. To the best of our knowledge, there are no relevant studies evaluating the inflammatory nutritional biomarkers as predictive markers for the prognosis of patients receiving penectomy. Therefore, we conducted this study.
2. Patients and Methods
2.1. Patients
We retrospectively analyzed the clinical data of 123 patients who underwent penectomy (partial/total penectomy) in our hospital from April 2010 to September 2021. Our study was approved by the Institutional Review Board (IRB) of The Third Xiangya Hospital of Central South University (Grant No. 21154). All the patients were diagnosed with PC through clinical laboratory testing, imaging examinations, and the biopsy of penile masses before surgery. The exclusion criteria included: (1) incomplete clinical data or follow-up data; (2) patients suffering from diseases that affect systemic nutrition or immune status, such as autoimmune diseases; (3) patients with blood infections or blood diseases, such as leukemia; (4) patients taking any immunosuppressive drugs or any nutritional supplements before surgery; and (5) patients with other tumor diseases or with lymph node or distant metastasis before surgery.
2.2. Data Collection
All the patients included in our study underwent routine blood work and liver and kidney function assessments one week before surgery. The definitions of the prognostic nutritional index (PNI), the albumin/globulin ratio (AGR), the systemic immune-inflammation index (SII), the neutrophil–lymphocyte ratio (NLR), and the platelet–lymphocyte ratio (PLR) were shown as follows: PNI = 1 × serum albumin (g/L) + 5 × lymphocyte count (109/L); AGR = serum albumin/(total serum protein-serum albumin); SII = platelet × neutrophil/lymphocyte count; NLR = neutrophil/lymphocyte count; and PLR = platelet/lymphocyte count. The collected clinical pathological data included the age of the patient, the scope of penile resection, necrosis, clinical T and N grades, and pathological types. The clinical staging of the tumors was determined according to the Tumor, Lymph Node, and Metastasis (TNM) staging system of the Joint Committee on Cancer of the United States (7th Edition) [7]. The data presented in this study are available in the Supplementary Material.
2.3. Follow-Up
Follow-up information was obtained by searching for outpatient review information and telephone follow-up with the patients. The follow-up time was defined as the date of diagnosis of PC and penectomy to 31 September 2021. Follow-up occurred every four months for the first two years after surgery and every six months from the third year after surgery onward. The follow-up included a physical examination, functional assessment, blood biochemical test, and a thorough examination of patients to determine any possibility of recurrence or metastasis.
2.4. Statistical Analysis
All the data were processed using SPSS v25.0 statistical software. The highest value of the Youden index was calculated using the receiver operating characteristic (ROC) curve as the best cut-off value of the PNI, AGR, SII, PLR, and NLR and grouped separately. When the measurement data conformed to a normal distribution, mean ± standard deviation was used. The measurement data that did not conform to a normal distribution were expressed as the median (range). The Kaplan–Meier survival analysis and log-rank test were used to compare the effects of different groups on overall survival (OS) in patients with PC undergoing penectomy. The variables with significant differences in the univariable analysis (p < 0.05) were included in the Cox proportional hazards regression model for multivariable survival analysis, and the hazard ratio (HR) and 95% confidence interval (CI) were estimated. Differences with a p < 0.05 were considered to be statistically significant.
3. Results
3.1. Patients’ Information
A total of 123 patients with PC who underwent penectomy were included in this study, of which 101 (82.1%) underwent partial penectomy and 22 (17.9%) underwent total penectomy. The median follow-up time of the 123 patients was 58 months. The median follow-up time of the 107 patients who survived was 61 months. The age of the patients ranged from 29 to 90 years old, with a median age of 59 years. The preoperative comorbidities of the patients were quantified by the age-adjusted Charlson comorbidity index (aCCI) [8]. The median value of the aCCI was 3.00. There were 94 cases (76.4%) with T < 2 in the clinical stage (Tis 12 cases, Ta 45 cases, and T1 37 cases) and 29 cases (23.6%) with T ≥ 2 (T2 27 cases, T3 1 case, and T4 1 case). In the clinical stage, there were 115 cases (93.5%) with n = 0 and 8 cases (6.5%) with n > 0 (They are the inguinal lymphadenopathy found during clinical palpation, which should be inflammatory enlargement.). Pathological classification showed that there were 12 cases (9.8%) of carcinoma in situ and 111 cases (90.2%) of squamous cell carcinoma. The median values of the preoperative PNI, AGR, SII, PLR, and NLR were 49.30, 1.51, 464.53, 118.52, and 2.33, respectively. Table 1 summarizes the clinicopathological characteristics of the patients in our study.

Table 1.
Characteristics of the 123 patients.
3.2. The Best Cut-Off Values of PNI, AGR, SII, and PLR before Treatment
The cut-off values for the PNI, AGR, SII, and PLR were determined by the ROC curve, and the optimal cut-off values were set at 49.03, 1.28, 636.99, and 121.95, respectively. The area under the curve (AUC) of the PNI was 0.797 (95% CI: 0.705–0.888, Youden index = 0.517, sensitivity = 57.9%, specificity = 93.7%, p < 0.001). The AUC of the AGR was 0.735 (95% Cl: 0.610–0.860, Youden index = 0.404, sensitivity = 84.1%, specificity = 56.2%, p = 0.003). The AUC of the SII was 0.657 (95% Cl: 0.505–0.808, Youden index = 0.326, sensitivity = 62.5%, specificity = 70.1%, p = 0.044). The AUC of the PLR was 0.654 (95% Cl: 0.521–0.787, Youden index = 0.320, sensitivity = 75.0%, specificity = 57.0%, p = 0.047) (Figure 1).
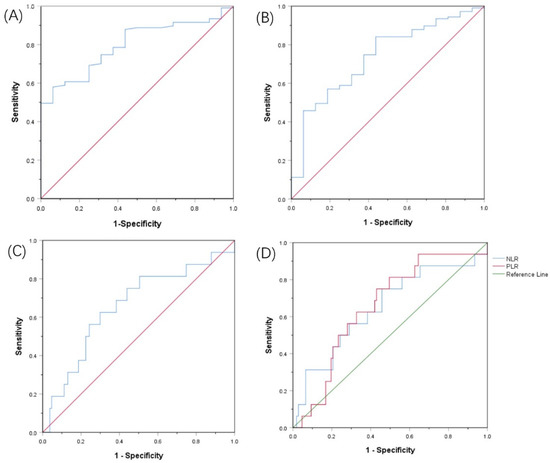
Figure 1.
Receiver operating characteristic curves for pretreatment: (A) PNI, (B) AGR, (C) SII, (D) RLR and NLR based on OS.
3.3. Comparison of Clinical Data of Patients in Different Groups
The optimal cut-off values of the PNI, AGR, SII, and PLR were used as the standard, and the patients below the cut-off value were defined as the low-level group, while those higher than or equal to the cut-off value were defined as the high-level group. Because the maximum area under the curve when NLR = 2.34 is 0.648 (95%Cl 0.493–0.803, Youden index = 0.292, sensitivity = 75.0%, specificity = 54.2%, p = 0.057), p > 0.05 has no statistical significance. Therefore, it is not used as a standard for grouping. The clinical N stage of the patients in the low AGR group was worse than that in the high AGR group (p = 0.039, Table 2). In addition, compared with the low SII group, the high SII group retained less penile tissue. (p = 0.027, Table 2).

Table 2.
Comparison of different PNI/AGR/SII/PLR groups.
3.4. Kaplan–Meier Survival Analysis
The Kaplan–Meier survival analysis showed that the median OS of the patients with a PNI < 49.03 was 100.4 months, which was lower than the median OS of the patients with a PNI ≥ 49.03 of 135.8 months (p < 0.001). The median OS of the patients with an AGR ≥ 1.28 before surgery was 128.2 months, which was significantly higher than the median OS of 75.7 months for the patients with an AGR <1.28 (p < 0.001). The median OS of the patients with an SII ≥ 636.99 before surgery was 10.5 months, which was significantly lower than the median OS of the patients with an SII < 636.99 of 128.0 months (p = 0.010). The median OS of the patients with a PLR ≥ 121.95 before surgery was 104.2 months, which was significantly lower than the median OS of the patients with a PLR < 121.95 of 129.9 months (p = 0.012) (Figure 2).
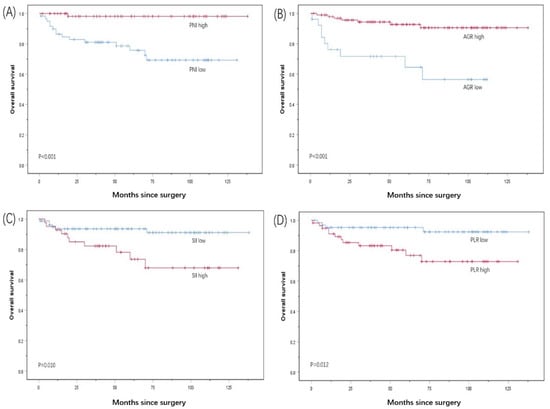
Figure 2.
Kaplan–Meier survival curves for OS according to (A) PNI, (B) AGR, (C) Sll, and (D) PLR in PC patients.
3.5. Univariable and Multivariable Analysis
The results of the univariable analysis showed that the age-adjusted Charlson comorbidity index (aCCI), clinical N staging, pathological classification, and the preoperative PNI, AGR, SII, PLR, and OS of patients with PC were statistically correlated (all p < 0.05, Table 3). The multivariable analysis revealed that the clinical N stage (p = 0.033; Table 3), pathological type (p = 0.045; Table 3), preoperative PNI (risk rate [HR] = 0.091; 95% CI: 0.010–0.853; p = 0.036; Table 3), and AGR (risk rate [HR] = 0.171; 95% CI: 0.043–0.680; p = 0.012; Table 3) were all independent prognostic factors. Thus, we plotted new ROC curves combining all the factors that were statistically different in the multivariate analysis (Figure 3). The AUC of the PNI group was 0.758 (95% CI: 0.655–0.862, p = 0.001). The AUC of the AGR group was 0.702 (95% CI: 0.549–0.854, p = 0.009). The AUC of the clinical N stage was 0.430 (95% CI: 0.267–0.592, p = 0.365). The AUC of the pathological type was 0.380 (95% CI: 0.223–0.537, p = 0.123). p > 0.05 has no statistical significance. Therefore, we finally found that the PNI and AGR are better independent prognostic factors for OS in patients with PC treated with penectomy.

Table 3.
Univariate and multivariate analysis of factors.
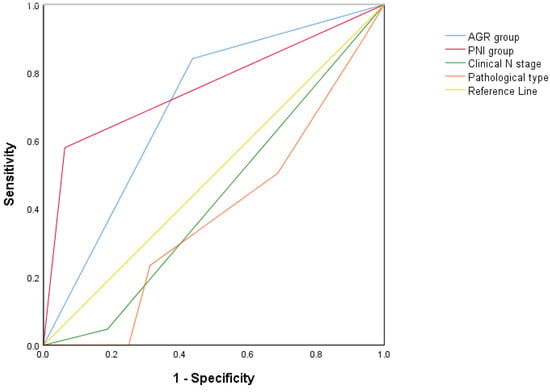
Figure 3.
Receiver operating characteristic curves for all the independent factors of multivariable analysis.
4. Discussion
PC is a relatively rare men’s malignant tumor, which can seriously affect the quality of life of men. In the process of the diagnosis and treatment of penile cancer, clinicians need to choose scientific and effective diagnosis and treatment methods, which not only consider the overall survival rate of patients, but also avoid overtreatment, such as unnecessary penectomy and inguinal lymphadenectomy. There is still a lack of specific prognostic biomarkers for PC; so, it is very important to explore reliable clinical markers for the diagnosis and treatment of PC. In this study, we evaluated the prognostic value of the PNI score and serum AGR in predicting OS in patients with PC. Both the PNI score and the serum AGR can reflect the systemic nutrition and inflammation status of tumor patients, and both are non-invasive biomarkers.
The latest research shows that systemic malnutrition and inflammation are closely related to the poor prognosis of various malignant tumors [9,10]. Thus far, a variety of proteins have been found in human plasma, among which albumin accounts for more than half [11]. Serum albumin (ALB), a central water-soluble protein produced by the liver, maintains the osmotic pressure of the capillaries and eliminates free radicals in the blood. It is also commonly used in clinical practice to assess the nutritional status of patients [12]. Malnutrition can lead to delayed postoperative healing and decreased collagen synthesis, thereby damaging the body’s immune system [13]. At the same time, malnutrition can lead to increased mortality after surgery and can reduce the resistance of patients to malignant tumors [14]. A study on ALB and cancer found that the serum ALB level of cancer subjects was significantly lower than that of non-cancer subjects [15]. ALB is also closely related to the prognosis of a variety of cancers and can be used as a predictor of the recurrence, metastasis, and death of a variety of malignant tumors [16,17,18]. In a study of a physical examination crowd, the correlation between low levels of ALB and tumor morbidity and mortality was also found [19]. In addition to being a nutritional marker, ALB is also an inflammatory response index [20]. Various pro-inflammatory cytokines can regulate the synthesis of albumin in the hepatocytes as part of the tumor inflammatory response and, at the same time, can promote tumor growth and metastasis, destroy the host’s immune response, and promote drug resistance [21]. Numerous studies have confirmed that markers of chronic inflammation play a key role in cancer recurrence, metastasis, and progression and are closely related to the OS of a variety of malignant tumors [22,23,24].
The PNI index is calculated from the ALB and lymphocyte count and can reflect the body’s nutritional and inflammatory state to a certain extent. Lymphocytes also play a very important role in the immune surveillance of malignant tumors. They can inhibit the proliferation and metastasis of malignant tumors by promoting the production of cytokines and the apoptosis of cytotoxic cells [25]. At present, researchers have studied the role of the PNI index in the prognosis of various malignant tumors. The total tumor recurrence rate and OS of patients in the higher PNI score group were higher than those in the lower PNI score group. The PNI index has been found to be an independent prognostic factor for a variety of malignant tumors, but none of these reports assessed the prognosis of PC [26,27,28].
Serum AGR is calculated by the ratio of ALB to serum globulin and can reflect the patient’s nutritional and inflammatory state. Serum globulin is calculated by subtracting the ALB from the total serum protein. Therefore, it contains a variety of pro-inflammatory proteins, such as C-reactive protein (CRP), immunoglobulin (Igs), and complement components. Studies have shown that elevated levels of CRP before surgery can lead to poor cancer prognosis [29]. Studies of patients with lung cancer have also found that a higher serum globulin level was associated with a lower survival rate [30]. Additionally, studies of patients with colorectal cancer have found that high complement and IgA levels are also poor prognosis indicators [31]. Serum AGR has also been confirmed as an independent prognostic factor for a variety of malignant tumors [16,32,33].
Squamous cell carcinoma is the most common pathological type of PC, accounting for about 95% of patients with PC. The pathological type of all the patients in our study was squamous cell carcinoma. Recently, some researchers found that the PNI index and serum AGR level are significantly related to the OS of patients with esophageal cancer [34,35], oral cancer [36,37], and laryngeal cancer [38,39]. The researchers conducted a multivariate analysis of the PNI index and serum AGR level with the patients’ comorbidities, age, and other factors and found that both the PNI and the AGR were independent predictors of postoperative OS, which is consistent with our research conclusions.
5. Conclusions
In this study, we found that both the PNI score and the serum AGR can be used as independent prognostic factors for predicting OS in patients with PC undergoing penectomy. Higher PNI scores and higher AGR levels are associated with longer OS for patients with PC. We confirmed the importance of preoperative nutritional support and inflammation control for the prognosis of patients with PC undergoing penectomy. Additionally, we found that poor clinical staging and higher levels of PLR and SII are related to the poor prognosis of patients with PC. The PLR and SII are also commonly used clinical nutrition and inflammation markers, once again corroborating the results of this study. However, there are limitations to our study. On the one hand, our retrospective study was with a small sample size of 123 patients. Therefore, our results require further confirmation by a multi-institutional, prospective study with a large number of patients to provide a better conclusion. On the other hand, our research lacks the measurement of specific inflammatory markers such as CRP and cytokine levels. Therefore, whether the preoperative PNI score and AGR are valid as prognosis indicators for patients with PC requires further investigation and verification.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol29100596/s1, the original data.
Author Contributions
W.-J.S. and N.-C.L. initiated the research question and supervised all aspects of the study; J.G. and Z.-P.X. contributed to data acquisition; J.-Y.L. supervised the statistical procedures of the manuscript; W.-J.S. and N.-C.L. wrote the first version of the manuscript; Z.L. and L.-Y.H. reviewed this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Institutional Review Board (IRB) of The Third Xiangya Hospital of Central South University (Grant No. 21154).
Informed Consent Statement
Patient consent was waived due to our study is a retrospective study, and the research data were obtained by consulting the medical records of patients.
Data Availability Statement
The data presented in this study are available in the Supplementary Material.
Acknowledgments
All work was completed at the Department of Urology, Central South University, The Third Xiangya Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mobilio, G.; Ficarra, V. Genital treatment of penile carcinoma. Curr. Opin. Urol. 2001, 11, 299–304. [Google Scholar]
- Mulherkar, R.; Hasan, S.; Wegner, R.E.; Verma, V.; Glaser, S.M.; Kalash, R.; Beriwal, S.; Horne, Z.D. National patterns of care for early-stage penile cancers in the United States: How is radiation and brachytherapy utilized?—ScienceDirect. Brachytherapy 2019, 18, 503–509. [Google Scholar]
- Marchioni, M.; Berardinelli, F.; de Nunzio, C.; Spiess, P.; Porpiglia, F.; Schips, L.; Cindolo, L. New insight in penile cancer. Minerva Urol. E Nefrol. 2018, 70, 559–569. [Google Scholar]
- Van Berckelaer, C.; Van Geyt, M.; Linders, S.; Rypens, C.; Trinh, X.B.; Tjalma, W.A.A.; Van Laere, S.; Colpaert, C.; Dirix, L.; Van Dam, P.A. A high neutrophil- lymphocyte ratio and platelet-lymphocyte ratio are associated with a worse outcome in inflammatory breast cancer. Breast 2020, 53, 212–220. [Google Scholar]
- Kawata, A.; Taguchi, A.; Baba, S.; Miyamoto, Y.; Tanikawa, M.; Sone, K.; Tsuruga, T.; Mori, M.; Oda, K.; Kawana, K.; et al. A low preoperative albumin-to- globulin ratio is a negative prognostic factor in patients with surgically treated cervical cancer. Int. J. Clin. Oncol. 2021, 26, 980–985. [Google Scholar]
- Huang, X.; Hu, H.; Zhang, W.; Shao, Z. Prognostic value of prognostic nutritional index and systemic immune-inflammation index in patients with osteosarcoma. J. Cell. Physiol. 2019, 234, 18408–18414. [Google Scholar]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Wei, C.K.; Wu, C.C.; Su, Y.C.; Yu, C.H.; Lee, C.C. Adjusted Age-Adjusted Charlson Comorbidity Index Score as a Risk Measure of Perioperative Mortality before Cancer Surgery. PLoS ONE 2016, 11, e0148076. [Google Scholar]
- Huang, J.; Yuan, Y.; Wang, Y.; Chen, Y.; Kong, W.; Xue, W.; Chen, H.; Zhang, J.; Huang, Y. Preoperative prognostic nutritional index is a significant predictor of survival in patients with localized upper tract urothelial carcinoma after radical nephroureterectomy. Urol. Oncol. 2017, 35, 671.e1–671.e9. [Google Scholar]
- Song, W.; Tian, C.; Kai, W.; Zhang, R.; Zou, S. Preoperative platelet lymphocyte ratio as independent predictors of prognosis in pancreatic cancer: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0178762. [Google Scholar]
- Nanjappa, V.; Thomas, J.K.; Marimuthu, A.; Muthusamy, B.; Radhakrishnan, A.; Sharma, R.; Khan, A.A.; Balakrishnan, L.; Sahasrabuddhe, N.A.; Kumar, S.; et al. Plasma Proteome Database as a resource for proteomics research: 2014 update. Nucleic Acids Res. 2013, 42, D959–D965. [Google Scholar]
- Doweiko, J.P.; Nompleggi, D. Reviews: The Role of Albumin in Human Physiology and Pathophysiology, Part III: Albumin and Disease States. J. Parenter. Enter. Nutr. 1991, 15, 476–483. [Google Scholar]
- Yu, J.; Hong, B.; Park, J.Y.; Hwang, J.H.; Kim, Y.K. Impact of Prognostic Nutritional Index on Postoperative Pulmonary Complications in Radical Cystectomy: A Propensity Score-Matched Analysis. Ann. Surg. Oncol. 2021, 28, 1859–1869. [Google Scholar]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar]
- Guthrie, G.; Roxburgh, C.; Farhan-Alanie, O.; Horgan, P.; McMillan, D. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br. J. Cancer 2013, 109, 24–28. [Google Scholar]
- Wang, N.; Liu, J.Y.; Li, X.; Deng, M.H.; Long, Z.; Tang, J.; Yao, K.; Zhang, Y.C.; He, L.Y. Pretreatment serum albumin/globulin ratio as a prognostic biomarker in metastatic prostate cancer patients treated with maximal androgen blockade. Asian J. Androl. 2018, 21, 56–61. [Google Scholar]
- Masafumi, O.; Tomohiko, K.; Toshihiro, U.; Nobushige, T.; Tetsuo, S.; Masayuki, K.; Atsushi, K.; Satoshi, F. Prognostic role of the preoperative serum albumin: Globulin ratio after radical nephroureterectomy for upper tract urothelial carcinoma. Int. J. Urol. 2018, 25, 871–878. [Google Scholar]
- Gregg, J.R.; Cookson, M.S.; Phillips, S.; Salem, S.; Chang, S.S.; Clark, P.E.; Davis, R.; Stimson, C.J., Jr.; Aghazadeh, M.; Smith, J.A., Jr.; et al. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J. Urol. 2011, 185, 90–96. [Google Scholar]
- Suh, B.; Park, S.; Shin, D.W.; Yun, J.M.; Keam, B.; Yang, H.-K.; Ahn, E.; Lee, H.; Park, J.K.; Cho, B. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 2260–2266. [Google Scholar]
- Barbosa-Silva, M.C. Subjective and objective nutritional assessment methods: What do they really assess? Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 248–254. [Google Scholar]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumors, immune cells, and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar]
- Park, J.H.; Watt, D.G.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C. Colorectal cancer, systemic inflammation, and outcome: Staging the tumor and staging the host. Ann. Surg. 2016, 263, 326–336. [Google Scholar]
- Toiyama, Y.; Inoue, Y.; Saigusa, S.; Kawamura, M.; Kawamoto, A.; Okugawa, Y.; Koji, J.; Tanaka, K.; Mohri, Y.; Kusunoki, M. C-reactive protein as a predictor of recurrence in patients with rectal cancer undergoing chemoradiotherapy followed by surgery. Anticancer Res. 2013, 33, 5065–5074. [Google Scholar]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar]
- Wu, X.; Jiang, Y.; Ge, H.; Diao, P.F.; Wang, D.M.; Wang, Y.L.; Cheng, J. Predictive value of prognostic nutritional index in patients with oral squamous cell carcinoma. Oral Dis. 2020, 26, 903–911. [Google Scholar]
- Ucar, G.; Ergun, Y.; Acikgoz, Y.; Uncu, D. The prognostic value of the prognostic nutritional index in patients with metastatic colorectal cancer. Asia Pac. J. Clin. Oncol. 2020, 16, e179–e184. [Google Scholar]
- Li, N.; Song, W.; Gao, J.; Xu, Z.; Long, Z.; Liu, J.; He, L. The prognostic nutritional index predicts the biochemical recurrence of patients treated with robot-assisted laparoscopic radical prostatectomy. Prostate 2022, 82, 221–226. [Google Scholar]
- Grimm, T.; Buchner, A.; Schneevoigt, B.; Kretschmer, A.; Apfelbeck, M.; Grabbert, M.; Jokisch, J.F.; Stief, C.G.; Karl, A. Impact of preoperative hemoglobin and CRP levels on cancer-specific survival in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder: Results of a single-center study. World J. Urol. 2016, 34, 703–708. [Google Scholar]
- Cohen, M.H.; Makuch, R.; Johnston-Early, A.; Ihde, D.C.; Bunn, P.A., Jr.; Fossieck, B.E.; Minna, J.D. Laboratory parameters as an alternative to performance status in prognostic stratification of patients with small cell lung cancer. Cancer Treat Rep. 1981, 65, 187–195. [Google Scholar]
- Codina Cazador, A.; Salva Lacombe, J.A.; Fernandez-Llamazares Rodriguez, J.; Ruiz Feliu, B.; Codina Barreras, A.; Moreno Aguado, V. Immunoglobulins and the complement system in colorectal cancer. Rev. Esp. Enferm. Apar. Dig. 1989, 75, 143–148. [Google Scholar]
- Chung, J.W.; Dong, J.P.; Chun, S.Y.; Choi, S.H.; Lee, J.N.; Kim, B.S.; Kim, H.T.; Kim, T.H.; Yoo, E.S.; Byun, S.S.; et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with non-metastatic renal cell carcinoma undergoing partial or radical nephrectomy. Sci. Rep. 2020, 10, 11999. [Google Scholar]
- Liu, J.; Dai, Y.; Zhou, F.; Long, Z.; Li, Y.; Liu, B.; Xie, D.; Tang, J.; Tan, J.; Yao, K.; et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 484.e1–484.e8. [Google Scholar]
- Noh, J.H.; Na, H.K.; Kim, Y.H.; Song, H.J.; Kim, H.R.; Choi, K.D.; Lee, G.H.; Jung, H.Y. Influence of Preoperative Nutritional Status on Patients Who Undergo Upfront Surgery for Esophageal Squamous Cell Carcinoma. Nutr. Cancer 2022, 74, 2910–2919. [Google Scholar]
- Li, X.H.; Gu, W.S.; Wang, X.P.; Lin, J.H.; Zheng, X.; Zhang, L.; Kang, T.; Zhang, Z.X.; Liu, W.L. Low Preoperative albumin-to-globulin ratio Predict Poor Survival and Negatively Correlated with Fibrinogen in Resectable Esophageal Squamous Cell Carcinoma. J. Cancer 2017, 8, 1833–1842. [Google Scholar]
- Tu, I.W.; Shannon, N.B.; Thankappan, K.; Balasubramanian, D.; Pillai, V.; Shetty, V.; Rangappa, V.; Chandrasekhar, N.H.; Kekatpure, V.; Kuriakose, M.A.; et al. Risk Stratification in Oral Cancer: A Novel Approach. Front. Oncol. 2022, 12, 836803. [Google Scholar] [CrossRef]
- Wang, Y.T.; Fang, K.H.; Hsu, C.M.; Chang, G.H.; Lai, C.H.; Lee, Y.C.; Tsai, M.S.; Huang, E.I.; Tsai, Y.T. Retrospective study on the potential of albumin/globulin ratio as a prognostic biomarker for oral cavity cancer patients. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 227–238. [Google Scholar]
- Fu, Y.; Chen, S.W.; Chen, S.Q.; Ou-Yang, D.; Liu, W.W.; Song, M.; Yang, A.K.; Zhang, Q. A Preoperative Nutritional Index for Predicting Cancer-Specific and Overall Survival in Chinese Patients with Laryngeal Cancer: A Retrospective Study. Medicine 2016, 95, e2962. [Google Scholar]
- Chen, W.Z.; Yu, S.T.; Xie, R.; Lv, Y.X.; Xu, D.B.; Yu, J.C. Preoperative albumin/globulin ratio has predictive value for patients with laryngeal squamous cell carcinoma. Oncotarget 2017, 8, 48240–48247. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).