Abstract
Treatment for acute myeloid leukemia (AML) typically involves intensive chemotherapy (IC); however, there is an unmet need for approximately 50% of AML patients who are deemed unfit or ineligible for IC. The purpose of this study was to evaluate, from a Canadian perspective, the economic impact of venetoclax in combination with azacitidine (Ven+Aza) for the treatment of patients with newly diagnosed AML who are 75 years or older or who have comorbidities that preclude using IC. A lifetime partitioned survival model was developed to assess the cost-effectiveness of Ven+Aza compared with Aza. Health states included event-free survival, progressive/relapsed disease, and death. Efficacy parameters were based on the VIALE-A trial. Analyses were conducted from Ministry of Health (MoH) and societal perspectives. Over a lifetime horizon, Ven+Aza was associated with a gain of 1.65 quality-adjusted life years (QALYs) compared with Aza. From an MoH perspective, Ven+Aza and Aza were associated with total costs of $204,305 and $82,333, respectively, resulting in an incremental cost–utility ratio of $73,841/QALY. Results were similar from a societal perspective. This economic evaluation demonstrates that, in comparison with Aza, Ven+Aza is a cost-effective strategy for the treatment of patients with newly diagnosed AML who are deemed unfit for IC.
1. Introduction
Acute myeloid leukemia (AML), which is characterized by an abnormal proliferation of immature myeloid cells with secondary hematopoietic insufficiency that infiltrate bone marrow, blood, and other tissues, is the most common form of acute leukemia in adults [1,2,3]. Worldwide, leukemia is one of the most common forms of cancer, with the eleventh highest incidence [4]. The most recent statistics from the Canadian Cancer Society reported that 1090 Canadians were newly diagnosed with AML in 2016 [5]. Previously thought to be an incurable disease, the prognosis of AML patients began to change with the introduction of new therapies starting in the 1960s; however, while these options have significantly impacted the lives of younger individuals, older patients are still at a disadvantage with limited treatment options [3,6]. Approximately 40% of AML patients under the age of 60 years, who receive intensive chemotherapy (IC) and are candidates for transplant, will be cured of their disease, whereas this estimate is between 5% and 15% for those who are older [3,6]. Additionally, the median survival of older AML patients ranges between 5 and 10 months with the current standard of care [3]. Given that AML already has a substantial impact on healthcare resource utilization, its increasing incidence in Canada should raise concerns [7]. New drugs that improve survival, response rates, duration of response, and quality of life (QoL), thereby reducing transfusions and hospitalizations, could substantially reduce the economic burden caused by AML [8,9].
The standard of care of AML treatment is intensive chemotherapy (IC) [10]. However, patients older than 65 (and particularly >75) years of age have a higher prevalence of unfavourable cytogenetics and more comorbidities, making them ineligible for IC due to the high toxicity of these regimens [11,12,13]. Indeed, estimates suggest that nearly 50% of AML patients may be ineligible or unsuitable candidates to receive standard IC during the initial induction phase [14,15,16,17]. Treatment alternatives for “unfit or ineligible” patients are limited to low-intensity treatment, best supportive care (BSC), or clinical trials with investigational drugs. Hypomethylating agents (HMAs), namely azacitidine (Aza) and decitabine, have become the standard of care for treating older or IC-ineligible AML patients [16,18,19]; however, Aza is approved for use in Canada only for AML patients with 20–30% blasts [18]. Low-dose cytarabine (LDAC) has been a primary option for older or IC-ineligible AML patients for many years; however, its place in AML therapy has shifted due to its relatively modest complete response (CR) and survival rates, the evolution of newer generation agents, and improved genetic profiling [16,19,20,21,22,23,24]. Given the limited treatment options and modest survival improvements of current therapies for IC-ineligible AML patients, there remains an unmet medical need for the development of more effective and safe therapies that can also improve patients’ QoL.
Venetoclax in combination with Aza (Ven+Aza) is indicated for the treatment of patients with newly diagnosed AML who are 75 years or older or who have comorbidities that preclude use of IC. The efficacy and safety of venetoclax in combination with HMAs have been assessed in three studies of patients with newly diagnosed AML who were ineligible for IC [25,26,27]. The VIALE-A study (NCT02993523) was a phase 3, randomized, double-blind, placebo-controlled, multicenter study comparing the efficacy and safety of Ven+Aza versus placebo (Pbo+Aza) among treatment-naïve patients with confirmed AML who were ineligible for IC due to medical comorbidities or age ≥ 75 years [26]. The results of this study concluded that the median overall survival (mOS: 14.7 months with Ven+Aza versus 9.6 months with Aza; hazard ratio [HR] for death: 0.66, 95% confidence interval [CI]: 0.52–0.85, p < 0.001), complete remission rate, transfusion-independence, duration of remission, and time-to-deterioration in quality of life were more favourable in patients randomized to Ven+Aza compared with Pbo+Aza. The safety profile of Ven+Aza was consistent with known side effect profiles of both agents, with adverse events (AEs) consistent with expectations for an older AML population. The most recently updated National Comprehensive Cancer Network (NCCN) guidelines recommend Ven+Aza as a category 1 (uniform NCCN consensus based on high-level evidence) preferred intervention for AML patients aged ≥60 years and ineligible for IC, with or without actionable mutations [28].
In order to inform Canadian healthcare decision makers, we conducted an analysis of the costs and effectiveness of treating newly diagnosed AML patients ineligible for IC with Ven+Aza compared with Aza alone, which is the standard of care in Canada.
2. Materials and Methods
2.1. Model Structure
A three-state partitioned survival model (PSM) was developed to assess, over a lifetime horizon, the economic impact of Ven+Aza versus Aza monotherapy and comprised three mutually exclusive health states: (i) event-free survival (EFS), (ii) progressive/relapsed disease (PD/RL), and (iii) death (Figure 1). EFS was defined as the time from the date of treatment initiation to the date of first documented progression or relapse from complete remission/complete remission with incomplete blood count recovery (CR/CRi), or treatment failure or death due to any cause. All patients began in EFS at the model start. The proportion of patients in the EFS health state of the model was set to be equal to the EFS curve of each treatment. Within EFS, a proportion of time was assumed in CR/CRi, which was estimated by applying the CR/CRi rate to the EFS curve. The remaining time was assumed not in CR/CRi. The PD/RL state included living patients who progressed or relapsed. The proportion of patients in the PD/RL health state was set to be equal to the difference between the proportion of living patients, which was based on the OS curve, and the proportion of EFS patients. During each cycle, patients were redistributed among the three health states, with death being the absorbing state. A 28-day model cycle was used for estimating the proportion of patients in each health state over time, according to the duration of treatment cycles.
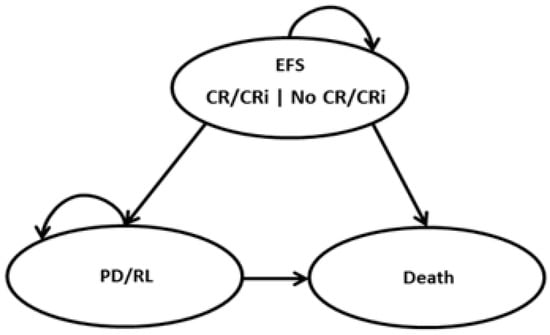
Figure 1.
Partitioned survival model structure. EFS: Event-free survival, CR/CRi: complete remission/complete remission with incomplete blood count recovery, and PD/RL: progressive/relapsed disease.
2.2. Efficacy and Safety Data
Efficacy and safety inputs were extracted from the VIALE-A trial and included OS, EFS, and CR/CRi, which were derived from individual patient-level data (IPD) [26]. In the base-case analysis, the efficacy inputs for OS and EFS for Ven+Aza were predicted using parametric survival models. Fit of parametric survival models chosen were evaluated using several criteria, including the Akaike and Bayesian information criteria, visual inspection, examination of the log-cumulative hazard plots, testing the proportional hazard assumptions, clinical input, and external validation (Table 1) [29]. The parametric survival models were used to inform OS and EFS until year 5. Since Kaplan–Meier (KM) curves plateau at the end of the study period, especially in the Ven+Aza KM curves, a cure assumption was applied to long-term survivors in the EFS with CR/CRi health state, which was supported by clinical expert opinion. Specifically, it was deemed appropriate to consider “cured” patients who are still in the EFS with CR/CRi health state after 5 years, which was more conservative than other estimates noted in other cost-effectiveness AML models [30]. Afterwards, these patients were considered cured and were assumed to follow survival of the general population, which was modelled using the general population mortality based on the 2019 Canadian life tables [31]. Adverse events (AEs) with an incidence rate ≥ 5% in at least one treatment arm in the VIALE-A trial were considered [26].

Table 1.
Model efficacy inputs.
2.3. Utilities
The pooled patient-level data of the EuroQol Group-5 Dimension-5 Level Instrument (EQ-5D-5L) from the VIALE-A and VIALE-C trials (venetoclax in combination with LDAC) were used to derive utilities as the populations studied in both trials were similar (AML patients aged 75 years or older or who have comorbidities that preclude use of IC) [36,37]. EQ-5D-5L scores were estimated based on individual dimension scores and adjusted using Canada preference-weights (Table 1) [32]. Disutility values associated with AEs were extracted from the literature [34]. AEs not reported in the literature were assumed to be equal to those under the same AE category or the average disutility of all AEs. Patients receiving subsequent hematopoietic stem cell transplantation (HSCT) were assumed to have additional HSCT disutility for a one-year period following HSCT [35]. The subsequent HSCT rates for Ven+Aza and Aza were obtained from the VIALE-A trial [26]. These estimates were validated from the expert opinion of a Canadian haemato-oncologist.
2.4. Cost Data
From a Canadian Ministry of Health (MoH) perspective, the model considered the following cost components: initial treatment costs (including drug and administration), subsequent HSCT costs, subsequent treatment costs (including drug and administration), AE costs associated with initial treatments, medical costs associated with health states (i.e., hospitalization, blood transfusion, and other monitoring costs), and terminal care costs. From a societal perspective, costs associated with productivity loss were also considered. These estimates were validated from the expert opinion of a Canadian haemato-oncologist. The resource use specific to Ven+Aza and Aza alone were obtained from the overall population in the VIALE-A trial [26]. The costs and resource use that were not available in the VIALE-A trial were obtained from various Canadian sources, including the literature, public databases, and a Canadian haemato-oncologist, to the extent feasible (Table 2). For detailed cost inputs, assumptions, and sources, refer to the Supplementary Materials. All costs estimated before 2020 were inflated to 2020 Canadian dollars using the Consumer Price Index (CPI) [38].

Table 2.
Model cost inputs (Canadian dollars).
2.5. Incremental Cost–Utility Analysis
The effectiveness outcome was the average quality-adjusted life years (QALYs). The incremental QALYs were calculated as the difference in the average QALYs over the time horizon between the two treatments. The incremental cost–utility ratios (ICURs) were calculated by dividing the difference in total costs between Ven+Aza and Aza by the difference in QALYs between both treatment arms. Analyses were conducted from both a Canadian Ministry of Health (MoH) and a societal perspective. The cost-effectiveness of Ven+Aza versus Aza was assessed at a willingness-to-pay (WTP) threshold of $100,000/QALY.
2.6. Sensitivity Analyses
Both deterministic and probabilistic analyses were performed for this economic evaluation to assess the impact of the variation of each parameter on the base-case results. The robustness of the base-case results was assessed through deterministic sensitivity analyses (DSA). This was performed by varying each single variable individually within the lower and upper bounds of all key parameters. For this analysis, model parameters were varied using standard errors, standard deviations, or 95% confidence intervals (CIs), when available, and a range of ±25% for other parameters. A probabilistic sensitivity analysis (PSA) was also performed to assess the overall impact of uncertainty associated with study parameters. Simultaneous variations in all key parameters were performed using Monte Carlo simulations. A total of 5000 Monte Carlo simulations were performed using appropriate distributions (beta distribution bounded by 0 and 1 for probabilities and utility values, and gamma distribution for disutilities, treatment duration, and cost parameters). Results of the PSA were presented as cost-effectiveness acceptability curves and the probability of being cost-effective at a threshold of $100,000/QALY was estimated [57].
3. Results
3.1. Base-Case Analysis
Ven+Aza was associated with an average of 2.53 QALYs, compared with an average of 0.87 QALY for Aza, for a QALY gain of 1.65 (Table 3). From an MoH perspective, Ven+Aza and Aza were associated with total costs of $204,305 and $82,333 (difference of $121,973), respectively, which resulted in an ICUR of $73,841/QALY. From a societal perspective, Ven+Aza and Aza were associated with total costs of $177,510 and $77,955 (difference of $99,554), respectively. As a result, the ICUR of Ven+Aza versus Aza over a 5-year time horizon from this perspective was $60,269/QALY.

Table 3.
Cost–utility results.
3.2. Sensitivity Analysis
According to the DSA, the ICURs of Ven+Aza compared with Aza varied between $54,896/QALY and $92,659/QALY from an MoH perspective and between $41,324/QALY and $79,087/QALY from a societal perspective. The parameters with the greatest impact on the base-case ICURs from both MoH and societal perspectives were: (i) the median treatment duration of Ven+Aza, (ii) the treatment cost of Ven+Aza, (iii) the 10-year time horizon, and (iv) the consideration of long-term extrapolation applied to all patients alive after 5 years (Figure 2).
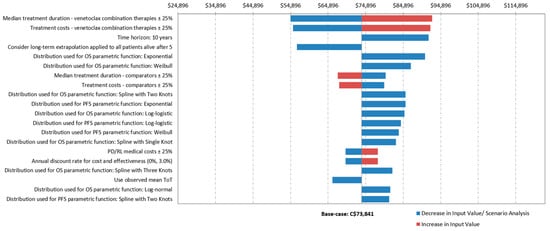
Figure 2.
Results of one-way sensitivity analyses from a Ministry of Health perspective. $CA: Canadian dollars.
According to a WTP threshold of $100,000/QALY, Ven+Aza was a cost-effective alternative compared with Aza in 98.8% of the Monte Carlo simulations from an MoH perspective (Figure 3a,b). From a societal perspective, Ven+Aza was cost-effective in 99.7% of Monte Carlo simulations.
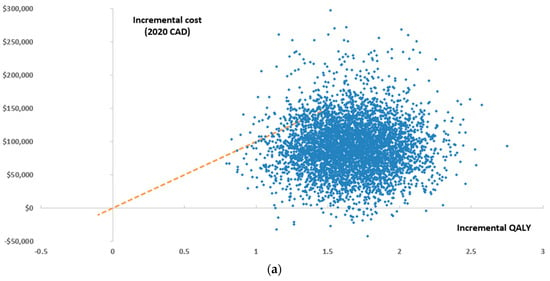
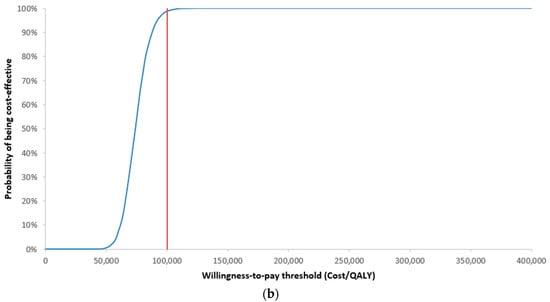
Figure 3.
Results of the probabilistic sensitivity analysis from an MoH perspective presented as a (a) scatter plot and (b) cost-effectiveness acceptability curve, QALY: quality-adjusted life year.
4. Discussion
The objective of this study was to assess, from a Canadian perspective, the economic impact of Ven+Aza versus Aza alone for the treatment of patients with newly diagnosed AML who are 75 years or older or who have comorbidities that preclude the use of IC. Consequently, a PSM model was used, with health states including EFS, CR/CRi, and PD/RL, to model disease progression and evaluate treatment efficacy over the model time horizon. Efficacy data were obtained from the VIALE-A clinical trial. Utilities were obtained from clinical trials and the literature. Costs and resource use that were not available in the clinical trial were obtained from various Canadian sources, including the literature, public databases, and input of a Canadian clinical expert, to the extent feasible. Analyses were conducted from both a Canadian MoH and societal perspective by including costs associated with drug acquisition and administration, subsequent HSCT, subsequent treatments, medical costs, costs associated with AEs, follow-up, terminal care, and productivity loss. According to the probabilistic analysis from the MoH perspective, Ven+Aza is associated with an ICUR of $73,841/QALY compared with Aza.
A study was conducted from a US third-party payer perspective, using similar assumptions. The ICUR for VEN+AZA vs. AZA was estimated to be higher than the Canadian ICUR (US$96,579 per QALY gained), which may be due to the higher costs incurred in the US (drug acquisition costs, medical costs, etc.) compared with Canadian costs [58]. Another US study using a different approach also assessed the cost-effectiveness of Ven+Aza in unfit patients with previously untreated AML [59]. Results determined a QALY gain of 0.61 and incremental cost of $159,595, resulting in an ICUR of $260,343/QALY. However, this study differs in several respects from our Canadian analysis. First, US costs, including drug costs, are higher compared with Canada. Dose intensity was also not taken into consideration for drug costs calculations. Furthermore, our study was populated with individual patient-level data (IPD) as well as specific VIALE-A quality of life utility data (adjusted for Canada), which was not available in their study. In addition, our study also differentiated the higher utility value for patients in the EFS health state with CR/CRi, which was not considered in the US study. Based on these limitations, we contend that our Canadian analysis is more robust and reflective of the clinical reality.
As with any economic evaluation, there are inherent limitations to this cost–utility analysis. Although the lifetime horizon allowed the model to assess the cost-effectiveness of Ven+Aza over the lifespan of patients, several assumptions had to be made. Importantly, because the median follow-up in the VIALE-A trial was only 20.5 months for the data used in this analysis, it was necessary to project the KM curve for EFS and OS, as well as the median treatment duration, beyond the end of the follow-up period by fitting parametric survival distributions to the data from the trial; however, AML patients ineligible for IC are often older and have poor prognoses, with a 5-year OS rate of less than 10% [16]. Therefore, data extrapolation may not be aligned with the real-life survival of this patient population. These projections are associated with substantial uncertainty that may have a material impact on cost-effectiveness. In addition, the cure assumption included within the model is associated with some limitations. After year 5 of the model simulation, those who remained in the EFS with the CR/CRi health state were considered cured, though there is uncertainty around the treatment duration of patients in EFS with CR/CRi, specifically for those who are older and ineligible for IC. It is known that although some patients are in remission, treatment will be given continuously throughout the patient’s lifetime. However, in line with expert opinion, the cure assumption was still included within the model. That said, when “cure” is not considered, there is a difference of only $1696/QALY compared with the base-case analysis, demonstrating the minimal impact of this assumption on overall cost-effectiveness. Lastly, several inputs were based on clinical expert opinion, including the proportion of patients using subsequent treatments, the frequency of healthcare resource utilization per health state, as well as the proportion of AEs managed on an inpatient basis. The validation of the cure assumption within the model, established at 5 years, was also validated by clinical expert opinion. While management of the disease and health care resource utilization may vary across the country, these assumptions may have a material impact on the cost-effectiveness estimates.
5. Conclusions
AML is predominantly a disease of the elderly and is associated with poor survival. Patients older than 65 years of age have a higher prevalence of unfavourable cytogenetics and more comorbid medical conditions that make them ineligible for IC due to the high toxicity of these regimens. Indeed, less-fit, IC-ineligible AML patients who receive low-intensity treatment or BSC have an extremely poor prognosis, with a median 1-year survival rate of 15–20%. For IC-ineligible patients, achieving a CR or CRi is important but rarely achieved; this outcome provides longer survival and reduced symptom burden, including transfusion independence and improved QoL. Additionally, less intensive therapies for patients ineligible for IC do not provide durable responses or deeper remissions (approximately 18% achieve CR with HMAs or LDAC). Considering the favourable efficacy and safety profiles seen in the VIALE-A study and recommendations from recently updated key AML guidelines, Ven+Aza demonstrates a clear and indisputable therapeutic value. The current economic model predicted that Ven+Aza would offer marked benefits to patients with newly diagnosed AML who are ineligible for IC in terms of LYs and QALYs in comparison with Aza alone. Specifically, the results suggested that venetoclax was a highly effective treatment with good economic value. With an assumed per-cycle price of approximately $13,000 for Ven+Aza, the ICUR varied between $54,896/QALY and $92,659/QALY from an MoH perspective, which is quite acceptable for a safe and effective oncologic treatment that fulfils an unmet clinical need for patients with this fatal condition.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol29100592/s1, Table S1: Proportion of Patients Receiving Subsequent Treatments by First-Line Treatment; Table S2: Administration Cost Parameters for Oral Therapies; Table S3: Administration Cost Parameters for IV Therapies; Table S4: Administration Costs per Treatment; Table S5: Unit Costs of Hospitalization and Blood Transfusion; Table S6: Health Care Resource Utilization by Health State; Table S7: Grade 3/4 AEs ≥ 5% for Each Treatment; Table S8: Follow-up Costs; Table S9: Follow-up and Monitoring Frequency by Health State; Table S10: Follow-up and Monitoring Costs by Health State; Table S11: Costs of Terminal Care; Table S12: Indirect Cost Parameters; Table S13: Employment Rate. References [60,61,62] are citied in the Supplementary Materials.
Author Contributions
K.M. and K.G. were involved in the study conception and design, modelling, analysis and interpretation of the data, and drafting the manuscript. K.M., J.L. and Y.A. were involved in the study conception and design, revision of the manuscript, and study supervision. X.C. and C.N.B. were involved in the study conception and design, modelling, and revision of the manuscript. A.C.S. was involved in the study conception and design and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AbbVie Corporation. The design, study conduct, and financial support for the study were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
The authors thank Christopher Vannabouathong for revising the manuscript. Christopher Vannabouathong received funding from PeriPharm Inc.
Conflicts of Interest
J.L. is a partner at PeriPharm Inc., a company that has served as a consultant to AbbVie Corporation and has received funding from AbbVie Corporation. K.M. and K.G. are employees of PeriPharm Inc. Y.A. and C.N.B. are employees of AbbVie Corporation. X.C. is an employee at Analysis Group Inc. and has received funding from AbbVie Corporation. Employees of AbbVie may hold equity. A.C.S. has received personal fees from AbbVie Corporation during the conduct of the study.
References
- Alibhai, S.M.H.; Leach, M.; Minden, M.D.; Brandwein, J. Outcomes and quality of care in acute myeloid leukemia over 40 years. Cancer 2009, 115, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Deschler, B.; Lübbert, M. Acute myeloid leukemia: Epidemiology and etiology. Cancer 2006, 107, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- GLOBOCAN. Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 19 October 2020).
- Canadian Cancer Society. Acute Myelogenous Leukemia Statistics. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/leukemia-acute-myelogenous-aml/statistics/?region=on (accessed on 26 October 2020).
- Eisfeld, A.-K.; Kohlschmidt, J.; Mrózek, K.; Blachly, J.S.; Walker, C.J.; Nicolet, D.; Orwick, S.; Maharry, S.; Carroll, A.J.; Stone, R.M.; et al. Mutation patterns identify adult patients with de novo acute myeloid leukemia aged 60 years or older who respond favorably to standard chemotherapy: An analysis of Alliance studies. Leukemia 2018, 32, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Shysh, A.C.; Nguyen, L.T.; Guo, M.; Vaska, M.; Naugler, C.; Rashid-Kolvear, F. The incidence of acute myeloid leukemia in Calgary, Alberta, Canada: A retrospective cohort study. BMC Public Heal. 2017, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bosshard, R.; O’Reilly, K.; Ralston, S.; Chadda, S.; Cork, D. Systematic reviews of economic burden and health-related quality of life in patients with acute myeloid leukemia. Cancer Treat. Rev. 2018, 69, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.D.; Skikne, B.S.; Kucmin-Bemelmans, M.I.; Alleman, M.C.; Hensen, M.M. Overall Economic Burden of Total Treatment Costs in Acute Myeloid Leukemia throughout the Course of the Disease. Blood 2012, 120, 3614. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Appelbaum, F.R.; Gundacker, H.; Head, D.R.; Slovak, M.L.; Willman, C.L.; Godwin, J.E.; Anderson, J.E.; Petersdorf, S.H. Age and acute myeloid leukemia. Blood 2006, 107, 3481–3485. [Google Scholar] [CrossRef]
- Leith, C.P.; Kopecky, K.J.; Godwin, J.; McConnell, T.; Slovak, M.L.; Chen, I.-M.; Head, D.R.; Appelbaum, F.R.; Willman, C.L. Acute Myeloid Leukemia in the Elderly: Assessment of Multidrug Resistance (MDR1) and Cytogenetics Distinguishes Biologic Subgroups with Remarkably Distinct Responses to Standard Chemotherapy. A Southwest Oncology Group Study. Blood 1997, 89, 3323–3329. [Google Scholar] [CrossRef]
- Tallman, M.S.; Altman, J.K.; Appelbaum, F.R.; Bhatt, V.R.; Bixby, D.; de Lima, M.; Fathi, A.T.; Fiorella, M.; Foran, J.M.; Gojo, I.; et al. NCCN Clinical Practice Guidelines in Oncology (NCCN): Acute Myeloid Leukemia Version 3.2020; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2020. [Google Scholar]
- Cashen, A.F.; Schiller, G.J.; O’Donnell, M.R.; DiPersio, J.F. Multicenter, Phase II Study of Decitabine for the First-Line Treatment of Older Patients with Acute Myeloid Leukemia. J. Clin. Oncol. 2010, 28, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Deschler, B.; De Witte, T.; Mertelsmann, R.; Lübbert, M. Treatment decision-making for older patients with high-risk myelodysplastic syndrome or acute myeloid leukemia: Problems and approaches. Haematologica 2006, 91, 1513–1522. [Google Scholar] [PubMed]
- Griffiths, E.A.; Carraway, H.E.; Chandhok, N.S.; Prebet, T. Advances in non-intensive chemotherapy treatment options for adults diagnosed with acute myeloid leukemia. Leuk. Res. 2020, 91, 106339. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Doucette, K.; Norsworthy, K. Recent drug approvals for acute myeloid leukemia. J. Hematol. Oncol. 2019, 12, 1–20. [Google Scholar] [CrossRef]
- Celgene Inc. Product Monograph: VIDAZA® (Azacitidine for Injection). Available online: https://media.celgene.com/content/uploads/sites/23/Vidaza-Product_Monograph_-_English_Version.pdf (accessed on 26 October 2020).
- Bashir, Y.; Geelani, S.; Bashir, N.; Mir, S.A.; Mushtaq, M.; Jan, M.A.; Rasool, J. Role of low dose cytarabine in elderly patients with acute myeloid leukemia: An experience. S. Asian J. Cancer 2015, 4, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Castaigne, S.; Tilly, H.; Sigaux, F.; Daniel, M.T.; Degos, L. Treatment of Leukemia with Low Dose Ara-C: A Study of 159 Cases. Haematol. Blood Transfus. 1985, 29, 56–59. [Google Scholar] [CrossRef]
- Palmieri, R.; Paterno, G.; De Bellis, E.; Mercante, L.; Buzzatti, E.; Esposito, F.; Del Principe, M.I.; Maurillo, L.; Buccisano, F.; Venditti, A. Therapeutic Choice in Older Patients with Acute Myeloid Leukemia: A Matter of Fitness. Cancers 2020, 12, 120. [Google Scholar] [CrossRef]
- Walter, R.B.; Estey, E.H. Management of older or unfit patients with acute myeloid leukemia. Leukemia 2014, 29, 770–775. [Google Scholar] [CrossRef]
- Brandwein, J.M.; Zhu, N.; Kumar, R.; Leber, B.; Sabloff, M.; Sandhu, I.; Kassis, J.; Olney, H.J.; Elemary, M.; Schuh, A.C. Treatment of older patients with acute myeloid leukemia (AML): Revised Canadian consensus guidelines. Am. J. Blood Res. 2017, 7, 30–40. [Google Scholar]
- The Leukemia/Bone Marrow Transplant Program of BC. Healthcare Professionals Cancer Management Guidelines: Acute Myeloid Leukemia (AML). Available online: http://www.leukemiabmtprogram.com/healthcare_professionals/cancer_management_guidelines/AML.html (accessed on 2 November 2020).
- Pollyea, D.A.; Pratz, K.W.; Jonas, B.A.; Letai, A.; Pullarkat, V.A.; Wei, A.; Konopleva, M.Y.; Recher, C.; Frankfurt, O.; David Rizzieri, D.; et al. Venetoclax in combination with hypomethylating agents induces rapid, deep, and durable responses in patients with AML ineligible for intensive therapy. In Proceedings of the American Society of Hematology (ASH)–60th Annual Meeting, San Diego, CA, USA, 1–4 December 2018. [Google Scholar]
- Dinardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Dinardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2018, 133, 7–17. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Acute Myeloid Leukemia (Version 3.2021); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Technical Support Document 14. Survival Analysis for Economic Evaluations Alongside Clinical Trials-Extrapolation with Patient-Level Data. Available online: http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf (accessed on 5 November 2020).
- Tremblay, G.; Dolph, M.; Patel, S.; Brandt, P.; Forsythe, A. Cost-effectiveness analysis for midostaurin versus standard of care in acute myeloid leukemia in the United Kingdom. Cost Eff. Resour. Alloc. 2018, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Deaths and Mortality Rates, by Age Group (Table: 13-10-0710-01). Available online: https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1310071001 (accessed on 9 November 2020).
- Xie, F.; Pullenayegum, E.; Gaebel, K.; Bansback, N.; Bryan, S.; Ohinmaa, A.; Poissant, L.; Johnson, J.A.; Canadian EQ-5D-5L Valuation Study Group. A Time Trade-off-derived Value Set of the EQ-5D-5L for Canada. Med. Care 2016, 54, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; Dinardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 2020, 135, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Wehler, E.; Storm, M.; Kowal, S.; Campbell, C.; Boscoe, A. A health state utility model estimating the impact of ivosidenib on quality of life in patients with relapsed/refractory acute myeloid leukemia. In Proceedings of the 23rd Congress of the European Hematology Association, Stockholm, Sweden, 14–17 June 2018; Available online: https://investor.agios.com/static-files/25de7161-9a10-4d8c-8a03-8bd5e7d0a5d3 (accessed on 9 November 2020).
- Guadagnolo, B.A.; Punglia, R.S.; Kuntz, K.M.; Mauch, P.M.; Ng, A.K. Cost-Effectiveness Analysis of Computerized Tomography in the Routine Follow-Up of Patients After Primary Treatment for Hodgkin’s Disease. J. Clin. Oncol. 2006, 24, 4116–4122. [Google Scholar] [CrossRef]
- Pratz, K.W.; Panayiotidis, P.; Recher, C.; Wei, X.; Jonas, B.A.; Montesinos, P.; Ivanov, V.; Schuh, A.C.; Dinardo, C.D.; Novak, J.; et al. 589 Delays in Time to Deterioration of Health-Related Quality of Life Were Observed in Patients with Acute Myeloid Leukemia Receiving Venetoclax in Combination with Azacitidine or in Combination with Low-Dose Cytarabine. In Proceedings of the 62nd ASH Annual Meeting and Exposition (Virtual), San Diego, CA, USA, 5–8 December 2020. [Google Scholar]
- Pratz, K.; Lachaine, J.; Suh, H.; Li, X.; Schuh, A.; Chai, X.; Xie, J.; Yin, L.; Bui, C. POSB357 Health State Utilities for Patients with Acute Myeloid Leukemia Who Are Ineligible for Intensive Chemotherapy. Value Health 2022, 25, S230. [Google Scholar] [CrossRef]
- Statistics Canada. Consumer Price Index by product group, monthly, percentage change, not seasonally adjusted, Canada, provinces, Whitehorse, Yellowknife and Iqaluit (Table: 18-10-0004-13). Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000413 (accessed on 1 October 2020).
- IQVIA. Delta PA; IQVIA: Durham, NC, USA, 2020. [Google Scholar]
- Government of Canada. Dispensing Fee Policies in Public Drug Plans, 2017/18. Available online: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=1308&lang=en (accessed on 1 October 2020).
- Ministry of Health and Long Term Care of Ontario. Schedule of Benefits: Physician Services Under the Health Insurance Act (Effective April 1, 2020). Available online: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20200306.pdf (accessed on 1 October 2020).
- Cancer Care Ontario. Drug Formulary-Regimens. Available online: https://www.cancercareontario.ca/en/drugformulary/regimens?f%5B0%5D=field_type_of_cancer%3A626 (accessed on 1 October 2020).
- Government of Canada. Job Bank-Wage Report. Available online: https://www.jobbank.gc.ca/wagereport/location/geo9219 (accessed on 1 October 2020).
- Pettigrew, M.; Kavan, P.; Surprenant, L.; Lim, H.J. Comparative net cost impact of the utilization of panitumumab versus cetuximab for the treatment of patients with metastatic colorectal cancer in Canada. J. Med. Econ. 2015, 19, 145–157. [Google Scholar] [CrossRef]
- Cancer Care Ontario. HYDR Regimen. Available online: https://www.cancercareontario.ca/en/drugformulary/regimens/monograph/51466 (accessed on 1 November 2020).
- Stahl, M.; DeVeaux, M.; Montesinos, P.; Itzykson, R.; Ritchie, E.K.; Sekeres, M.A.; Barnard, J.D.; Podoltsev, N.A.; Brunner, A.M.; Komrokji, R.S. Hypomethylating agents in relapsed and refractory AML: Outcomes and their predictors in a large international patient cohort. Blood Adv. 2018, 2, 923–932. [Google Scholar] [CrossRef]
- Ontario Ministry of Health and Long Term Care. Ontario Care Costing Analysis Tool. Available online: https://hsimi.ca/occp/occpreports/ (accessed on 1 October 2020).
- Interprovincial Health Insurance Agreements Coordinating Committee (IHIACC). Interprovincial Billing Rates for Designated High Cost Transplants Effective for Discharges on or after April 1, 2020. Available online: http://www.health.gov.on.ca/en/pro/programs/ohip/bulletins/na_86/high_cost.pdf (accessed on 1 October 2020).
- Canadian Institute for Health Information. Patient Cost Estimator. Available online: https://www.cihi.ca/en/patient-cost-estimator (accessed on 1 October 2020).
- Canadian Institute for Health Information. Care in Canadian ICUs. Available online: https://secure.cihi.ca/free_products/ICU_Report_EN.pdf (accessed on 1 October 2020).
- Lagerquist, O.; Poseluzny, D.; Werstiuk, G.; Slomp, J.; Nahirniak, S.; Maier, M.; Clarke, G. The cost of transfusing a unit of red blood cells: A costing model for Canadian hospital use. ISBT Sci. Ser. 2017, 12, 375–380. [Google Scholar] [CrossRef]
- Ministry of Health and Long Term Care of Ontario. Schedule of Benefits of Laboratory Services (Effective July 1, 2020). Available online: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/lab/lab_mn2020.pdf (accessed on 1 October 2020).
- Canadian Cancer Society. Follow-Up After Treatment for Acute Myelogenous Leukemia. Available online: https://www.cancer.ca/en/cancer-information/cancer-type/leukemia-acute-myelogenous-aml/treatment/follow-up/?region=on (accessed on 1 October 2020).
- De Oliveira, C.; Pataky, R.; Bremner, K.E.; Rangrej, J.; Chan, K.K.W.; Cheung, W.Y.; Hoch, J.S.; Peacock, S.; Krahn, M.D. Phase-specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer 2016, 809, 1–12. [Google Scholar] [CrossRef]
- Statistics Canada. Average Usual Hours and Wages by Selected Characteristics, Monthly, Unadjusted for Seasonality (Table: 14-10-0320-02). Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410032002 (accessed on 1 October 2020).
- Statistics Canada. Labour Force Characteristics by Age Group, Monthly, Seasonally Adjusted (Table: 14-10-0287-02). Available online: https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1410028702 (accessed on 1 October 2020).
- Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the Economic Evaluation of Health Technologies: Canada, 4th Edition. Available online: https://www.cadth.ca/guidelines-economic-evaluation-health-technologies-canada-4th-edition (accessed on 1 October 2020).
- Pratz, K.W.; Chai, X.; Xie, J.; Yin, L.; Nie, X.; Montez, M.; Iantuono, E.; Downs, L.; Ma, E. Cost Effectiveness Analysis of Venetoclax Plus Azacitidine Versus Azacitidine in Newly Diagnosed Adult Patients with Acute Myeloid Leukemia Who Are Ineligible for Intensive Chemotherapy from a United States Payer Perspective. Blood 2021, 138, 112. [Google Scholar] [CrossRef]
- Patel, K.K.; Zeidan, A.M.; Shallis, R.M.; Prebet, T.; Podoltsev, N.; Huntington, S.F. Cost-effectiveness of azacitidine and venetoclax in unfit patients with previously untreated acute myeloid leukemia. Blood Adv. 2021, 5, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Cancer Care Ontario. AZCTVENE Regimen. Available online: https://www.cancercareontario.ca/en/drugformulary/regimens/monograph/66881 (accessed on 25 November 2020).
- Cancer Care Ontario. CYTA(LD) Regimen. Available online: https://www.cancercareontario.ca/en/drugformulary/regimens/monograph/51351 (accessed on 1 November 2020).
- American Cancer Society. Stem Cell Transplant for Acute Myeloid Leukemia (AML). Available online: https://www.cancer.org/cancer/acute-myeloid-leukemia/treating/bone-marrow-stem-cell-transplant.html (accessed on 1 October 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).