Peripheral Blood Cell Ratios as Prognostic Indicators in a Neoadjuvant Chemotherapy-Treated Breast Cancer Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Pathology
2.3. Ratio Calculations
2.4. Ethics Approval and Consent to Participate
2.5. Statistical Analysis
2.6. Multivariate Analysis and Modeling
3. Results
3.1. Patient Demographics
3.2. Systemic Inflammatory Marker Ratios
3.3. Correlating Clinicopathological Details with Systemic Inflammatory Marker Ratios
3.4. Selected Cut-Off Values for Immunocyte Cell Ratios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azab, B.; Bhatt, V.R.; Phookan, J.; Murukutla, S.; Kohn, N.; Terjanian, T.; Widmann, W.D. Usefulness of the Neutrophil-to-Lymphocyte Ratio in Predicting Short- and Long-Term Mortality in Breast Cancer Patients. Ann. Surg. Oncol. 2012, 19, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Azab, B.; Shah, N.; Radbel, J.; Tan, P.; Bhatt, V.; Vonfrolio, S.; Habeshy, A.; Picon, A.; Bloom, S. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med. Oncol. 2013, 30, 432. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Cha, Y.J.; Yoon, C.; Kim, D.; Lee, J.; Park, S.; Cha, C.; Kim, J.Y.; Ahn, S.G.; Park, H.S.; et al. Prognostic value of neutrophil-to-lymphocyte ratio in human epidermal growth factor receptor 2-negative breast cancer patients who received neoadjuvant chemotherapy. Sci. Rep. 2020, 10, 13078. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, K.; Xiao, X.; Nie, Y.; Qu, S.; Gong, C.; Su, F.; Song, E. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: A retrospective study. BMC Cancer 2016, 16, 320. [Google Scholar] [CrossRef]
- Cho, U.; Park, H.S.; Im, S.Y.; Yoo, C.Y.; Jung, J.H.; Suh, Y.J.; Choi, H.J. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS ONE 2018, 13, e0200936. [Google Scholar] [CrossRef]
- Choi, H.; Noh, H.; Cho, I.-J.; Lim, S.-T.; Han, A. Changes in neutrophil to lymphocyte ratio (NLR) during neoadjuvant treatment correlated with patients’ survival. Breast Cancer 2020, 27, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B (Methodol.) 1972, 34, 187–202. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Cullinane, C.; Creavin, B.; Leary DP, O.; Sullivan MJ, O.; Kelly, L.; Redmond, H.P.; Corrigan, M.A. Can the Neutrophil to Lymphocyte Ratio Predict Complete Pathological Response to Neoadjuvant Breast Cancer Treatment? Systematic Review and Meta- Analysis. Clin. Breast Cancer 2020, 20, e675–e681. [Google Scholar] [CrossRef]
- Gago-Dominguez, M.; Matabuena, M.; Redondo, C.M.; Patel, S.P.; Carracedo, A.; Ponte, S.M.; Martínez, M.E.; Castelao, J.E. Neutrophil to lymphocyte ratio and breast cancer risk: Analysis by subtype and potential interactions. Sci. Rep. 2020, 10, 13203. [Google Scholar] [CrossRef]
- Galon, J.; Pagès, F.; Marincola, F.M.; Angell, H.K.; Thurin, M.; Lugli, A.; Zlobec, I.; Berger, A.; Bifulco, C.; Botti, G.; et al. Cancer classification using the Immunoscore: A worldwide task force. J. Transl. Med. 2012, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Grassadonia, A.; Graziano, V.; Iezzi, L.; Vici, P.; Barba, M.; Pizzuti, L.; Cicero, G.; Krasniqi, E.; Mazzotta, M.; Marinelli, D.; et al. Prognostic Relevance of Neutrophil to Lymphocyte Ratio (NLR) in Luminal Breast Cancer: A Retrospective Analysis in the Neoadjuvant Setting. Cells 2021, 10, 1685. [Google Scholar] [CrossRef] [PubMed]
- Graziano, V.; Grassadonia, A.; Iezzi, L.; Vici, P.; Pizzuti, L.; Barba, M.; Quinzii, A.; Camplese, A.; Marino, P.D.; Peri, M.; et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast 2019, 44, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Grenader, T.; Plotkin, Y.; Geffen, D.B. The Preoperative Neutrophil/Lymphocyte Ratio Does not Correlate with the 21-Gene Recurrence Score in Estrogen Receptor-Positive Breast Cancer Patients. Oncol. Res. Treat. 2015, 38, 24–27. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Jia, W.; Wu, J.; Jia, H.; Yang, Y.; Zhang, X.; Chen, K.; Su, F. The Peripheral Blood Neutrophil-To-Lymphocyte Ratio Is Superior to the Lymphocyte-To-Monocyte Ratio for Predicting the Long-Term Survival of Triple-Negative Breast Cancer Patients. PLoS ONE 2015, 10, e0143061. [Google Scholar] [CrossRef]
- Mougalian, S.S.; Soulos, P.R.; Killelea, B.K.; Lannin, D.R.; Abu-Khalaf, M.M.; DiGiovanna, M.P.; Sanft, T.B.; Pusztai, L.; Gross, C.P.; Chagpar, A.B. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer 2015, 121, 2544–2552. [Google Scholar] [CrossRef]
- Noh, H.; Eomm, M.; Han, A. Usefulness of Pretreatment Neutrophil to Lymphocyte Ratio in Predicting Disease-Specific Survival in Breast Cancer Patients. J. Breast Cancer 2013, 16, 55–59. [Google Scholar] [CrossRef]
- Okuturlar, Y.; Gunaldi, M.; Tiken, E.E.; Oztosun, B.; Inan, Y.O.; Ercan, T.; Tuna, S.; Kaya, A.O.; Harmankaya, O.; Kumbasar, A. Utility of Peripheral Blood Parameters in Predicting Breast Cancer Risk. Asian Pac. J. Cancer Prev. 2015, 16, 2409–2412. [Google Scholar] [CrossRef]
- Pistelli, M.; Lisa, M.D.; Ballatore, Z.; Caramanti, M.; Pagliacci, A.; Battelli, N.; Ridolfi, F.; Santoni, M.; Maccaroni, E.; Bracci, R.; et al. Pre-treatment neutrophil to lymphocyte ratio may be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer 2015, 15, 195. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.H.; Ahn, S.H.; Kim, S.O.; Kim, J.E.; Ahn, J.-H.; Jung, K.H.; Kim, S.-B.; Ko, B.S.; Lee, J.W.; Son, B.H.; et al. Comparison of metabolic changes after neoadjuvant endocrine and chemotherapy in ER-positive, HER2-negative breast cancer. Sci. Rep. 2021, 11, 10510. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W. Clinical Prediction Models, A Practical Approach to Development, Validation, and Updating. Stat. Biol. Health 2019, 18, 53–99. [Google Scholar] [CrossRef]
- Ulas, A.; Avci, N.; Kos, T.; Cubukcu, E.; Olmez, O.F.; Bulut, N.; Degirmenci, M. Are neutrophil/lymphocyte ratio and platelet/lymphocyte ratio associated with prognosis in patients with HER2-positive early breast cancer receiving adjuvant trastuzumab? J. BUON 2015, 20, 714–722. [Google Scholar] [PubMed]
- Wang, L.; Si, H.; Wang, J.; Feng, L.; Zhai, W.; Dong, S.; Yu, Z. Blood cell parameters as prognostic predictors of disease development for patients with advanced non-small cell lung cancer. Oncol. Lett. 2020, 20, 1101–1110. [Google Scholar] [CrossRef]
- Zenan, H.; Zixiong, L.; Zhicheng, Y.; Mei, H.; Xiongbin, Y.; Tiantian, W.; Min, D.; Renbin, L.; Changchang, J. Clinical prognostic evaluation of immunocytes in different molecular subtypes of breast cancer. J. Cell. Physiol. 2019, 234, 20584–20602. [Google Scholar] [CrossRef]
| Characteristic | N | Overall N = 353 1 | Luminal A N = 108 1 | Luminal B N = 122 1 | HER2-Positive N = 80 1 | Triple Negative N = 43 1 | p-Value 2 |
|---|---|---|---|---|---|---|---|
| Overall survival, months (range) | 353 | 45.0 (25.0, 67.0) | 32.5 (14.0, 48.0) | 49.0 (33.0, 72.8) | 52.0 (27.5, 72.0) | 51.0 (34.0, 67.0) | <0.001 |
| Disease-free survival months (range) | 353 | 40.0 (18.0, 65.0) | 28.5 (12.0, 44.0) | 45.5 (27.8, 71.0) | 46.5 (13.8, 65.8) | 51.0 (27.5, 67.0) | <0.001 |
| Recurrence | 353 | 0.041 | |||||
| No | 288.0 (81.6%) | 83.0 (28.8%) | 107.0 (37.2%) | 60.0 (20.8%) | 38.0 (13.2%) | ||
| Yes | 65.0 (18.4%) | 25.0 (38.5%) | 15.0 (23.1%) | 20.0 (30.8%) | 5.0 (7.7%) | ||
| Age | 353 | 55.0 (46.0, 68.0) | 47.5 (41.0, 56.2) | 62.0 (51.0, 72.0) | 62.0 (53.8, 71.0) | 46.0 (40.5, 55.5) | <0.001 |
| Tumor size, in mm (range) | 200 | 22.0 (12.0, 30.2) | NA | 22.0 (15.0, 31.2) | 21.5 (9.2, 30.0) | NA | 0.31 |
| Tumor grade (percent) | 353 | ||||||
| 0 | 14.0 (4.0%) | 3.0 (21.4%) | 4.0 (28.6%) | 6.0 (42.9%) | 1.0 (7.1%) | ||
| 1 | 7.0 (2.0%) | 7.0 (100.0%) | 0.0 (0.0%) | 0.0 (0.0%) | 0.0 (0.0%) | ||
| 2 | 136.0 (38.5%) | 65.0 (47.8%) | 53.0 (39.0%) | 11.0 (8.1%) | 7.0 (5.1%) | ||
| 3 | 196.0 (55.5%) | 33.0 (16.8%) | 65.0 (33.2%) | 63.0 (32.1%) | 35.0 (17.9%) | ||
| Adjuvant chemotherapy | 338 | ||||||
| Yes | 137.0 (40.5%) | 0.0 (0.0%) | 82.0 (59.9%) | 55.0 (40.1%) | 0.0 (0.0%) | ||
| No | 36.0 (10.65%) | 0.0 (0.0%) | 23.0 (63.9%) | 13.0 (36.1%) | 0.0 (0.0%) | ||
| Unknown | 165.0 (48.8%) | 108.0 (65.5%) | 9.0 (5.5%) | 5.0 (3.0%) | 43.0 (26.1%) | ||
| Metastasis | 353 | ||||||
| No | 190.0 (53.8%) | 0.0 (0.0%) | 118.0 (62.1%) | 72.0 (37.9%) | 0.0 (0.0%) | ||
| Yes | 12.0 (3.4%) | 0.0 (0.0%) | 4.0 (33.3%) | 8.0 (66.7%) | 0.0 (0.0%) | ||
| Unknown | 151.0 (42.8%) | 108.0 (71.5%) | 0.0 (0.0%) | 0.0 (0.0%) | 43.0 (28.5%) |
| Characteristic | N | Overall, N = 353 1 | Luminal A N = 108 1 | Luminal B N = 122 1 | HER2-Positive N = 80 1 | Triple Negative N = 43 1 | p-Value 2 |
|---|---|---|---|---|---|---|---|
| Total WCC | 352 | 7.4 (6.0, 8.7) | 6.6 (5.7, 8.6) | 7.6 (6.0, 8.7) | 7.7 (6.5, 8.8) | 7.6 (6.4, 8.9) | 0.082 |
| Red blood cell count (RBC) % | 353 | 4.5 (4.2, 4.7) | 4.5 (4.2, 4.7) | 4.5 (4.2, 4.8) | 4.4 (4.2, 4.7) | 4.5 (4.2, 4.7) | 0.72 |
| Hemoglobin | 353 | 13.4 (12.6, 14.1) | 13.4 (12.7, 14.0) | 13.4 (12.6, 14.3) | 13.2 (12.5, 13.9) | 13.4 (12.5, 14.0) | 0.59 |
| Hematocrit | 353 | 0.4 (0.4, 0.4) | 0.4 (0.4, 0.4) | 0.4 (0.4, 0.4) | 0.4 (0.4, 0.4) | 0.4 (0.4, 0.4) | 0.45 |
| Neutrophils #, 103/μL | 353 | 4.7 (3.5, 5.9) | 4.0 (3.2, 5.7) | 4.9 (3.6, 5.9) | 4.8 (3.9, 5.6) | 5.2 (4.0, 6.0) | 0.025 |
| Lymphocytes | 353 | 1.8 (1.4, 2.3) | 1.7 (1.4, 2.1) | 1.9 (1.4, 2.4) | 2.0 (1.6, 2.5) | 1.6 (1.3, 2.0) | 0.089 |
| Monocytes #, 103/μL | 353 | 0.4 (0.3, 0.5) | 0.4 (0.3, 0.5) | 0.4 (0.3, 0.4) | 0.4 (0.3, 0.5) | 0.4 (0.3, 0.5) | 0.79 |
| Eosinophils #, 103/μL | 353 | 0.2 (0.1, 0.2) | 0.2 (0.1, 0.3) | 0.1 (0.1, 0.2) | 0.2 (0.1, 0.3) | 0.1 (0.1, 0.2) | 0.023 |
| Basophils #, 103/μL | 353 | 0.52 | |||||
| 0–0.09 | 211.0 (59.8%) | 65.0 (30.8%) | 72.0 (34.1%) | 44.0 (20.9%) | 30.0 (14.2%) | ||
| 0.1–0.59 | 141.0 (39.9.0%) | 42.0 (29.8%) | 50.0 (35.5%) | 36.0 (25.5%) | 13.0 (9.2%) | ||
| >0.6 | 1.0 (0.3%) | 1.0 (100.0%) | 0.0 (0.0%) | 0.0 (0.0%) | 0.0 (0.0%) |
| Characteristic | N | Overall N = 353 1 | Luminal A N = 108 1 | Luminal B N = 122 1 | Her2 Positive N = 80 1 | Triple Negative N = 43 1 | p-Value 2 |
|---|---|---|---|---|---|---|---|
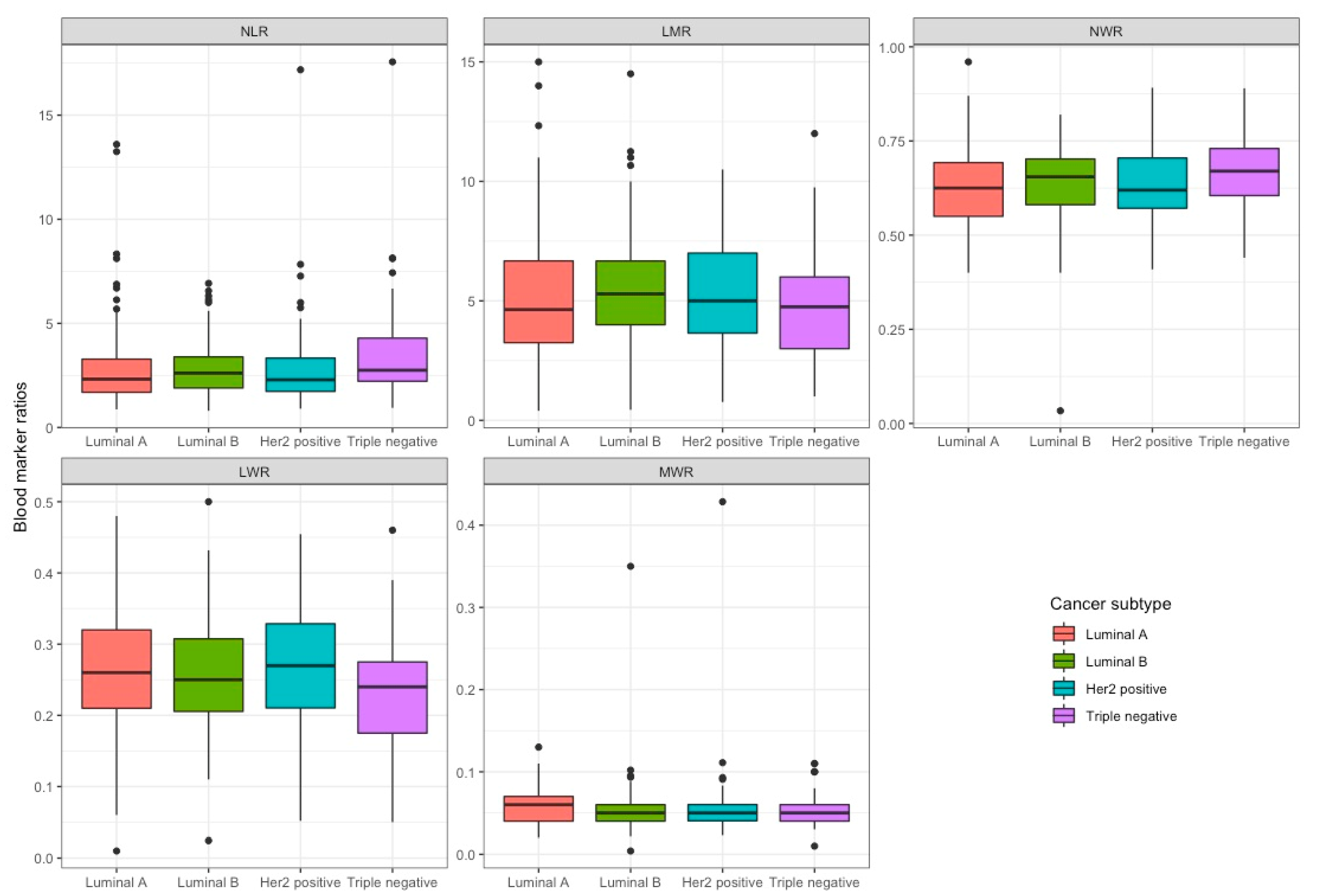
| NLR | 353 | 2.5 (1.8, 3.5) | 2.3 (1.7, 3.3) | 2.6 (1.9, 3.4) | 2.3 (1.7, 3.3) | 2.8 (2.2, 4.3) | 0.092 |
| LMR | 353 | 5.0 (3.5, 6.7) | 4.6 (3.2, 6.7) | 5.3 (4.0, 6.7) | 5.0 (3.7, 7.0) | 4.8 (3.0, 6.0) | 0.19 |
| NWR | 353 | 0.6 (0.6, 0.7) | 0.6 (0.6, 0.7) | 0.7 (0.6, 0.7) | 0.6 (0.6, 0.7) | 0.7 (0.6, 0.7) | 0.070 |
| LWR | 353 | 0.3 (0.2, 0.3) | 0.3 (0.2, 0.3) | 0.3 (0.2, 0.3) | 0.3 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.15 |
| MWR | 353 | 0.1 (0.0, 0.1) | 0.1 (0.0, 0.1) | 0.1 (0.0, 0.1) | 0.1 (0.0, 0.1) | 0.1 (0.0, 0.1) | 0.21 |
| p-Value (Correlation) | |||||
|---|---|---|---|---|---|
| NLR | LMR | NWR | LWR | MWR | |
| Luminal A (n = 108) | |||||
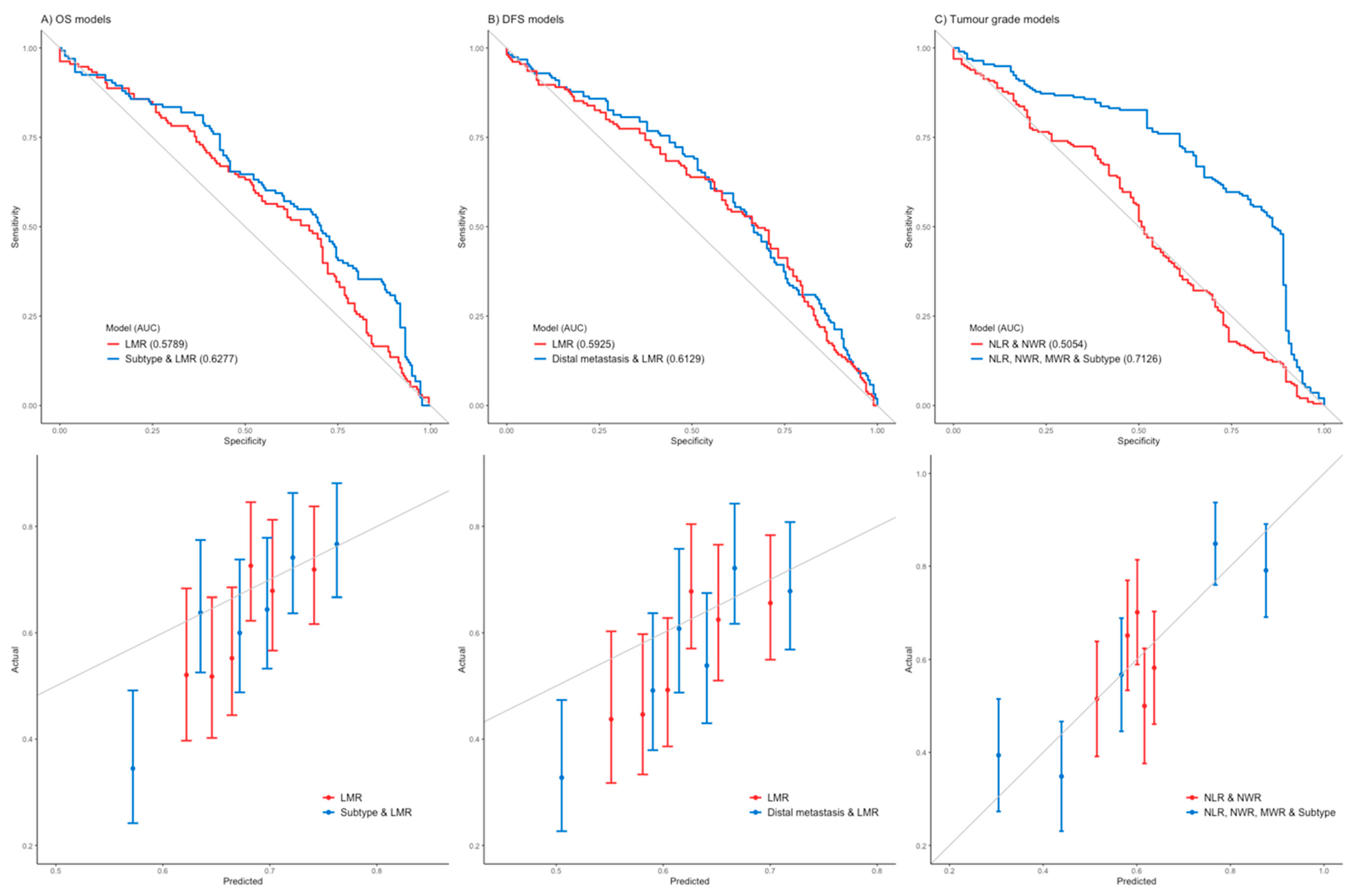
| OS * | 0.599 (0.05) | 0.232 (0.12) | 0.710 (0.04) | 0.829 (−0.02) | 0.010 (−0.25) |
| DFS * | 0.918 (0.01) | 0.250 (0.11) | 0.946 (0.01) | 0.874 (0.02) | 0.018 (−0.23) |
| Tumor grade ** | 0.494 | 0.339 | 0.495 | 0.219 | 0.788 |
| Luminal B (n = 122) | |||||
| OS * | 0.167 (−0.13) | 0.018 (0.21) | 0.575 (−0.05) | 0.114 (0.14) | 0.014 (−0.22) |
| DFS * | 0.169 (−0.13) | 0.005 (0.25) | 0.487 (−0.06) | 0.088 (0.15) | 0.009 (−0.24) |
| Tumor grade ** | 0.155 | 0.265 | 0.100 | 0.557 | 0.204 |
| HER2-positive (n = 80) | |||||
| OS * | 0.018 (−0.26) | 0.086 (0.19) | 0.005 (−0.31) | 0.006 (0.30) | 0.774 (0.03) |
| DFS * | 0.006 (−0.30) | 0.223 (0.14) | 0.001 (−0.36) | 0.003 (0.32) | 0.442 (0.09) |
| Tumor grade ** | 0.978 | 0.212 | 0.482 | 0.277 | 0.725 |
| Triple negative (n = 43) | |||||
| OS * | 0.575 (−0.09) | 0.442 (0.12) | 0.757 (−0.05) | 0.567 (−0.09) | 0.108 (−0.25) |
| DFS * | 0.463 (−0.11) | 0.316 (0.16) | 0.646 (−0.07) | 0.702 (−0.06) | 0.083 (−0.27) |
| Tumor grade ** | 0.992 | 0.260 | 0.961 | 0.821 | 0.089 |
| Combined cohort (n = 353) | |||||
| OS * | 0.138 (−0.08) | 0.002 (0.16) | 0.327 (−0.05) | 0.143 (0.08) | 0.005 (−0.15) |
| DFS * | 0.038 (−0.11) | 0.002 (0.16) | 0.125 (−0.08) | 0.053 (0.10) | 0.015 (−0.13) |
| Tumor grade ** | 0.224 | 0.364 | 0.029 | 0.068 | 0.480 |
| Cut Off * (Sensitivity, Specificity) | |||||
|---|---|---|---|---|---|
| NLR | LMR | NWR | LWR | MWR | |
| Luminal A | <1.53 (80.0%, 12.5%) | <7.17 (81.7%, 20.8%) | <0.54 (80.0%, 16.7%) | <0.20 (81.7%, 18.8%) | <0.035 (85.0%, 18.8%) |
| Luminal B | <1.96 (80.6%, 32.6%) | <5.69 (80.6%, 46.5%) | <0.60 (80.6%, 36.0%) | <0.30 (80.6%, 31.4%) | <0.040 (80.6%, 20.9%) |
| HER2-positive | <1.82 (80.0%, 34.5%) | <6.29 (80.6%, 46.5%) | <0.59 (80.0%, 41.8%) | <0.33 (80.0%, 32.7%) | <0.039 (80.0%, 18.2%) |
| Triple negative | <2.31 (83.3%, 35.5%) | <2.08 (83.3%, 6.5%) | <0.62 (83.3%, 35.5%) | <0.28 (83.3%, 29.0%) | <0.035 (83.3%, 9.7%) |
| Combined | <1.70 (80.5%, 20.9%) | <6.71 (82.0%, 27.3%) | <0.56 (80.5%, 19.1%) | <0.33 (80.5%, 21.4%) | <0.04 (81.2%, 18.6%) |
| Cut Off * (Sensitivity, Specificity) | |||||
|---|---|---|---|---|---|
| NLR | LMR | NWR | LWR | MWR | |
| Luminal A | <1.59 (80.0%, 14.0%) | <7.17 (80.0%, 18.6%) | <0.54 (81.5%, 18.6%) | <0.35 (80.0%, 11.6%) | <0.035 (80.0%, 11.6%) |
| Luminal B | <1.96 (81.0%, 33.8%) | <5.69 (81.0%, 48.8%) | <0.60 (81.0%, 37.5%) | <0.30 (81.0%, 32.5%) | <0.040 (81.0%, 21.3%) |
| HER2-positive | <1.77 (82.4%, 34.8%) | <7.38 (82.4%, 26.1%) | <0.58 (82.4%, 37.0%) | <0.33 (82.4%, 32.6%) | <0.039 (82.4%, 15.2%) |
| Triple negative | <2.31 (85.7%, 37.9%) | <6.59 (85.7%, 20.7%) | <0.61 (85.7%, 31.0%) | <0.28 (85.7%, 31.0%) | <0.035 (85.7%, 10.3%) |
| Combined | <1.79 (80.0%, 25.2%) | <6.71 (80.6%, 27.3%) | <0.57 (81.3%, 24.2%) | <0.32 (80.6%, 23.2%) | <0.04 (80.6%, 16.7%) |
| p-Value (Cutoff *; # of Low-Marker Values, and # of High-Marker Values) | |||||
|---|---|---|---|---|---|
| NLR | LMR | NWR | LWR | MWR | |
| Luminal A | 0.969 (1.79; 31 and 77) | 0.004 (5.29; 68 and 40) | 0.761 (0.56; 33 and 75) | 0.836 (0.30; 73 and 35) | 0.022 (0.06; 72 and 36) |
| Luminal B | 0.760 (1.79; 23 and 99) | 0.027 (5.29; 61 and 61) | 0.795 (0.56; 22 and 100) | 0.622 (0.30; 88 and 34) | 0.008 (0.06; 92 and 30) |
| HER2-positive | 0.013 (1.79; 22 and 58) | 0.111 (5.29; 42 and 38) | 0.380 (0.56; 14 and 66) | 0.043 (0.30; 51 and 29) | 0.541 (0.06; 60 and 20) |
| Triple negative | 0.169 (1.79; 5 and 38) | 0.498 (5.29; 25 and 18) | 0.430 (0.56; 3 and 40) | 0.503 (0.30; 36 and 7) | 0.330 (0.06; 33 and 10) |
| Combined | 0.419 (1.79; 81 and 272) | <0.001 (5.29; 196 and 157) | 0.833 (0.56; 72 and 281) | 0.349 (0.30; 248 and 105) | 0.001 (0.06; 257 and 96) |
| p-Value (Cutoff *; # of Low-Marker Values, and # of High-Marker Values) | |||||
|---|---|---|---|---|---|
| NLR | LMR | NWR | LWR | MWR | |
| Luminal A | 0.789 (1.79; 31 and 77) | 0.004 (5.29; 68 and 40) | 0.380 (0.62; 54 and 54) | 0.816 (0.30; 73 and 35) | 0.022 (0.06; 72 and 36) |
| Luminal B | 0.586 (1.79; 23 and 99) | 0.005 (5.29; 61 and 61) | 0.835 (0.62; 46 and 76) | 0.517 (0.30; 88 and 34) | 0.007 (0.06; 92 and 30) |
| HER2-positive | 0.035 (1.79; 22 and 58) | 0.202 (5.29; 42 and 38) | 0.021 (0.62; 40 and 40) | 0.071 (0.30; 51 and 29) | 0.250 (0.06; 60 and 20) |
| Triple negative | 0.224 (1.79; 5 and 38) | 0.401 (5.29; 25 and 18) | 0.939 (0.62; 16 and 27) | 0.570 (0.30; 36 and 7) | 0.250 (0.06; 33 and 10) |
| Combined | 0.447 (1.79; 81 and 272) | <0.001 (5.29; 196 and 157) | 0.218 (0.62; 156 and 197) | 0.456 (0.30; 248 and105) | 0.004 (0.06; 257 and 96) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalali, A.; Miresse, D.; Fahey, M.R.; Ni Mhaonaigh, N.; McGuire, A.; Bourke, E.; Kerin, M.J.; Brown, J.A.L. Peripheral Blood Cell Ratios as Prognostic Indicators in a Neoadjuvant Chemotherapy-Treated Breast Cancer Cohort. Curr. Oncol. 2022, 29, 7512-7523. https://doi.org/10.3390/curroncol29100591
Jalali A, Miresse D, Fahey MR, Ni Mhaonaigh N, McGuire A, Bourke E, Kerin MJ, Brown JAL. Peripheral Blood Cell Ratios as Prognostic Indicators in a Neoadjuvant Chemotherapy-Treated Breast Cancer Cohort. Current Oncology. 2022; 29(10):7512-7523. https://doi.org/10.3390/curroncol29100591
Chicago/Turabian StyleJalali, Amirhossein, David Miresse, Matthew R. Fahey, Niamh Ni Mhaonaigh, Andrew McGuire, Emer Bourke, Michael J. Kerin, and James A. L. Brown. 2022. "Peripheral Blood Cell Ratios as Prognostic Indicators in a Neoadjuvant Chemotherapy-Treated Breast Cancer Cohort" Current Oncology 29, no. 10: 7512-7523. https://doi.org/10.3390/curroncol29100591
APA StyleJalali, A., Miresse, D., Fahey, M. R., Ni Mhaonaigh, N., McGuire, A., Bourke, E., Kerin, M. J., & Brown, J. A. L. (2022). Peripheral Blood Cell Ratios as Prognostic Indicators in a Neoadjuvant Chemotherapy-Treated Breast Cancer Cohort. Current Oncology, 29(10), 7512-7523. https://doi.org/10.3390/curroncol29100591





