The Association between Survivorship Care Plans and Patient-Reported Satisfaction and Confidence with Follow-Up Cancer Care Provided by Primary Care Providers
Abstract
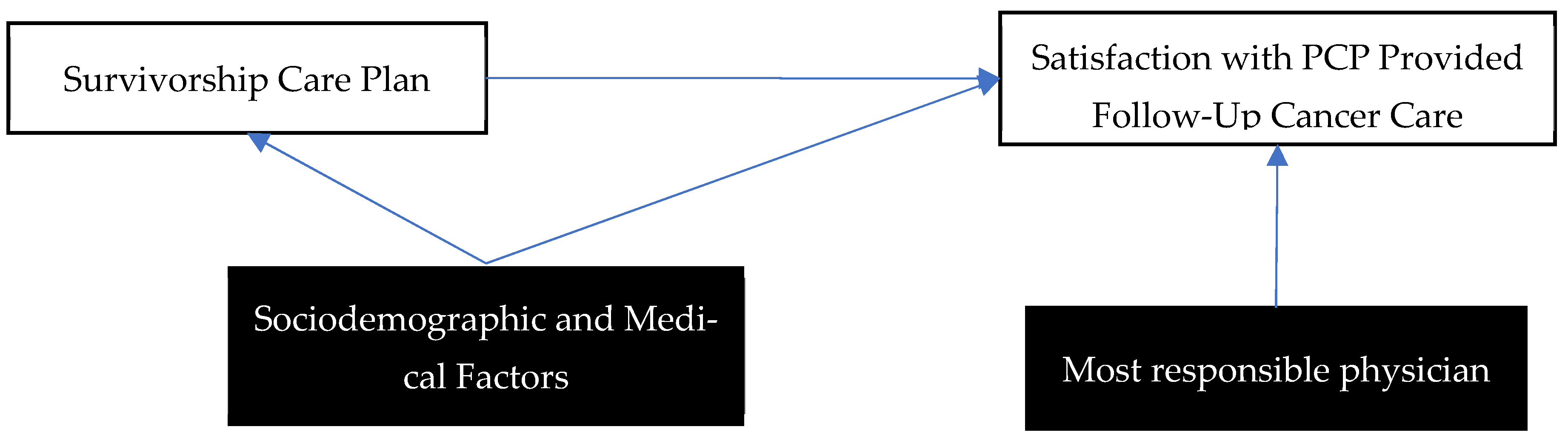
1. Introduction
2. Methods
2.1. Sociodemographic and Medical Variables
2.2. Survivorship Care Outcome Variables
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics: A 2020 Special Report on Lung Cancer; Canadian Cancer Statistics Advisory Committee: Toronto, ON, Canada, 2020. [Google Scholar]
- Truant, T.L.; Fitch, M.I.; O’Leary, C.; Stewart, J. Global perspectives on cancer survivorship: From lost in transition to leading into the future. Can. Oncol. Nurs. J. 2017, 27, 287–294. [Google Scholar] [PubMed]
- Sussman, J.; Beglaryan, H.; Payne, A. Follow-Up Model of Care for Cancer Survivors Recommendations. 2019, 19. Available online: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/58736 (accessed on 1 May 2022).
- Hewitt, M.; Ganz, P.A. From Cancer Patient to Cancer Survivor—Lost in Transition; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Sussman, J.; Beglaryan, H.; Payne, A. Follow-Up Model of Care for Cancer Survivors: Recommendations for the Delivery of Follow-Up Care for Cancer Survivors in Ontario. 2019. Available online: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/FollowUpModelsOfCareRecommendations.pdf (accessed on 1 May 2022).
- Hill, R.E.; Wakefield, C.E.; Cohn, R.J.; Fardell, J.E.; Brierley, M.E.; Kothe, E.; Jacobsen, P.B.; Hetherington, K.; Mercieca-Bebber, R. Survivorship Care Plans in Cancer: A Meta-Analysis and Systematic Review of Care Plan Outcomes. Oncologist 2020, 25, e351–e372. [Google Scholar] [CrossRef]
- Jacobsen, P.B.; DeRosa, A.P.; Henderson, T.O.; Mayer, D.K.; Moskowitz, C.S.; Paskett, E.D.; Rowland, J.H. Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery. J. Clin. Oncol. 2018, 36, 2088–2100. [Google Scholar] [CrossRef]
- Høeg, B.L.; Bidstrup, P.E.; Karlsen, R.V.; Friberg, A.S.; Albieri, V.; Dalton, S.O.; Saltbaek, L.; Andersen, K.K.; Horsboel, T.A.; Johansen, C. Follow-up strategies following completion of primary cancer treatment in adult cancer survivors. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Brothers, B.M.; Easley, A.; Salani, R.; Andersen, B.L. Do survivorship care plans impact patients’ evaluations of care? A randomized evaluation with gynecologic oncology patients. Gynecol. Oncol. 2013, 129, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Maly, R.C.; Liang, L.J.; Liu, Y.; Griggs, J.J.; Ganz, P.A. Randomized controlled trial of survivorship care plans among low-income, predominantly latina breast cancer survivors. J. Clin. Oncol. 2017, 35, 1814–1821. [Google Scholar] [CrossRef]
- Boekhout, A.H.; Maunsell, E.; Pond, G.R.; Julian, J.A.; Coyle, D.; Levine, M.N.; Grunfeld, E. A survivorship care plan for breast cancer survivors: Extended results of a randomized clinical trial. J. Cancer Surviv. 2015, 9, 683–691. [Google Scholar] [CrossRef]
- Mayer, D.K.; Birken, S.A.; Check, D.K.; Chen, R.C. Summing it up: An integrative review of studies of cancer survivorship care plans (2006–2013). Cancer 2015, 121, 978–996. [Google Scholar] [CrossRef]
- Hershman, D.L.; Greenlee, H.; Awad, D.; Kalinsky, K.; Maurer, M.; Kranwinkel, G.; Brafman, L.; Jayasena, R.; Tsai, W.Y.; Neugut, A.I.; et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res. Treat. 2013, 138, 795–806. [Google Scholar] [CrossRef]
- Jefford, M.; Gough, K.; Drosdowsky, A.; Russell, L.; Aranda, S.; Butow, P.; Phipps-Nelson, J.; Young, J.; Krishnasamy, M.; Ugalde, A.; et al. A Randomized Controlled Trial of a Nurse-Led Supportive Care Package (Survivor Care) for Survivors of Colorectal Cancer. Oncologist 2016, 21, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Nicolaije, K.A.H.; Ezendam, N.P.M.; Vos, M.C.; Pijnenborg, J.M.A.; Boll, D.; Boss, E.A.; Hermans, R.H.M.; Engelhart, K.C.M.; Haartsen, J.E.; Pijlman, B.M.; et al. Impact of an automatically generated cancer survivorship care plan on patient-reported outcomes in routine clinical practice: Longitudinal outcomes of a pragmatic, cluster randomized trial. J. Clin. Oncol. 2015, 33, 3550–3559. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Stricker, C.T.; Panzer, S.L.L.; Arvey, S.A.; Baker, K.S.; Casillas, J.; Ganz, P.A.; McCabe, M.S.; Nekhlyudov, L.; Overholser, L.; et al. Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: Results of a multicenter trial. J. Oncol. Pract. 2015, 11, e222–e229. [Google Scholar] [CrossRef] [PubMed]
- Sprague, B.L.; Dittus, K.L.; Pace, C.M.; Dulko, D.; Pollack, L.A.; Hawkins, N.A.; Geller, B.M. Patient Satisfaction With Breast and Colorectal Cancer Survivorship Care Plans. Clin. J. Oncol. Nurs. 2013, 17, 266–272. [Google Scholar] [CrossRef]
- DiMartino, L.D.; Birken, S.A.; Mayer, D.K. The Relationship Between Cancer Survivors’ Socioeconomic Status and Reports of Follow-up Care Discussions with Providers. J. Cancer Educ. 2017, 32, 749–755. [Google Scholar] [CrossRef]
- Maly, R.C.; Liu, Y.; Diamant, A.L.; Thind, A. The impact of primary care physicians on follow-up care of underserved breast cancer survivors. J. Am. Board Fam. Med. 2013, 26, 629–636. [Google Scholar] [CrossRef]
- Booth, C.M.; Li, G.; Zhang-Salomons, J.; Mackillop, W.J. The impact of socioeconomic status on stage of cancer at diagnosis and survival. Cancer 2010, 116, 4160–4167. [Google Scholar] [CrossRef]
- Ward, E.; Jemal, A.; Cokkinides, V.; Singh, G.K.; Cardinez, C.; Ghafoor, A.; Thun, M. Cancer Disparities by Race/Ethnicity and Socioeconomic Status. CA. Cancer J. Clin. 2004, 54, 78–93. [Google Scholar] [CrossRef]
- Weaver, K.E.; Geiger, A.M.; Lu, L.; Case, L.D. Rural-urban disparities in health status among US cancer survivors. Cancer 2013, 119, 1050–1057. [Google Scholar] [CrossRef]
- Newman, L.A.; Griffith, K.A.; Jatoi, I.; Simon, M.S.; Crowe, J.P.; Colditz, G.A. Meta-Analysis of Survival in African American and White American Patients With Breast Cancer: Ethnicity Compared With Socioeconomic Status. J. Clin. Oncol. 2006, 24, 1342–1349. [Google Scholar] [CrossRef]
- Byers, T.E.; Wolf, H.J.; Bauer, K.R.; Bolick-Aldrich, S.; Chen, V.W.; Finch, J.L.; Fulton, J.P.; Schymura, M.J.; Shen, T.; Van Heest, S.; et al. The impact of socioeconomic status on survival after cancer in the United States. Cancer 2008, 113, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Kumachev, A.; Trudeau, M.E.; Chan, K.K.W. Associations among socioeconomic status, patterns of care, and outcomes in breast cancer patients in a universal health care system: Ontario’s experience. Cancer 2016, 122, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Clegg, L.X.; Reichman, M.E.; Miller, B.A.; Hankey, B.F.; Singh, G.K.; Lin, Y.D.; Goodman, M.T.; Lynch, C.F.; Schwartz, S.M.; Chen, V.W.; et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control 2009, 20, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Fitch, M.; Zomer, S.; Lockwood, G.; Louzado, C.; Shaw Moxam, R.; Rahal, R.; Green, E. Experiences of adult cancer survivors in transitions. Support. Care Cancer 2019, 27, 2977–2986. [Google Scholar] [CrossRef]
- Fitch, M.I. Supportive care framework. Can. Oncol. Nurs. J. 2008, 18, 6–24. [Google Scholar] [CrossRef]
- Schmidt, C.O.; Kohlmann, T. When to use the odds ratio or the relative risk? Int. J. Public Health 2008, 53, 165–167. [Google Scholar] [CrossRef]
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Projected estimates of cancer in Canada in 2020. Cmaj 2020, 192, E199–E205. [Google Scholar] [CrossRef]
- Guirguis, S.; Fitch, M.; Maganti, M.; Gupta, A.A.; D’Agostino, N.; Korenblum, C.; Jones, J.M. Biopsychosocial Factors Associated with Supportive Care Needs in Canadian Adolescent and Young Adult Cancer Survivors. J. Clin. Med. 2021, 10, 2628. [Google Scholar] [CrossRef]
- McLeod, C.B.; Lavis, J.N.; Mustard, C.A.; Stoddart, G.L. Income Inequality, Household Income, and Health Status in Canada: A Prospective Cohort Study. Am. J. Public Health 2003, 93, 1287–1293. [Google Scholar] [CrossRef]
- Maddison, A.R.; Asada, Y.; Urquhart, R. Inequity in access to cancer care: A review of the Canadian literature. Cancer Causes Control 2011, 22, 359–366. [Google Scholar] [CrossRef]
- Williamson, T.J.; Stanton, A.L. Adjustment to Life as a Cancer Survivor. In Handbook of Cancer Survivorship; Feuerstein, M., Nekhlyudov, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 29–48. [Google Scholar] [CrossRef]
- Hinnen, C.; Pool, G.; Holwerda, N.; Sprangers, M.; Sanderman, R.; Hagedoorn, M. Lower levels of trust in one’s physician is associated with more distress over time in more anxiously attached individuals with cancer. Gen. Hosp. Psychiatry 2014, 36, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Hillen, M.A.; de Haes, H.C.J.M.; Smets, E.M.A. Cancer patients’ trust in their physician—A review. Psychooncology. 2011, 20, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Rushton, M.; Morash, R.; Larocque, G.; Liska, C.; Stoica, L.; DeGrasse, C.; Segal, R. Wellness beyond cancer program: Building an effective survivorship program. Curr. Oncol. 2015, 22, e419–e434. [Google Scholar] [CrossRef] [PubMed]
| No SCP Received 1 | SCP Received | |||||
|---|---|---|---|---|---|---|
| n | Proportion | n | Proportion | Chi-Square Residuals | p-Value | |
| N | 5108 | 4862 | ||||
| Cancer Type | <0.001 | |||||
| Blood | 186 | 55.03 | 152 | 44.97 | −1.33 | |
| Breast | 1700 | 55.27 | 1376 | 44.73 | −5.15 * | |
| Colorectal | 772 | 44.86 | 949 | 55.14 | 6.11 * | |
| Lymphoma | 357 | 59.70 | 241 | 40.30 | −4.16 * | |
| Melanoma | 496 | 58.63 | 350 | 41.37 | −4.36 * | |
| Prostate | 904 | 43.86 | 1157 | 56.14 | 7.88 * | |
| Other | 272 | 58.75 | 191 | 41.25 | −3.21* | |
| Metastasis | 0.022 | |||||
| No Mets | 3902 | 50.67 | 3799 | 49.33 | 2.61 | |
| Primary Mets | 298 | 54.58 | 248 | 45.42 | −1.57 | |
| Secondary Mets | 215 | 50.59 | 210 | 49.41 | 0.31 | |
| Unsure | 465 | 55.53 | 370 | 44.47 | −2.55 | |
| Age Groups | 0.004 | |||||
| AYA 2 | 171 | 59.58 | 116 | 40.42 | −2.88 * | |
| Adults | 4919 | 50.96 | 4733 | 49.04 | 2.88 * | |
| Marital Status | <0.001 | |||||
| Divorced/Separated/Widowed | 926 | 52.32 | 844 | 47.68 | −0.99 | |
| Married/Partnered | 3703 | 50.37 | 3649 | 49.63 | 2.99 * | |
| Single | 429 | 57.35 | 319 | 42.65 | −3.48 * | |
| Education | <0.001 | |||||
| Graduate Degree | 562 | 61.09 | 358 | 38.91 | −6.28 * | |
| ≤Highschool | 2561 | 46.82 | 2909 | 53.18 | 9.89 * | |
| Undergraduate or College | 1866 | 55.77 | 1480 | 44.23 | −6.46 * | |
| Sex 3 | <0.001 | |||||
| Female | 2928 | 54.98 | 2398 | 45.02 | −8.05 * | |
| Male | 2156 | 46.88 | 2443 | 53.12 | 8.05 * | |
| Rural/Urban | 0.003 | |||||
| Rural | 1688 | 49.14 | 1747 | 50.86 | 3.02 * | |
| Urban | 3346 | 52.33 | 3048 | 47.67 | −3.02 * | |
| Income Level | <0.001 | |||||
| High | 1483 | 55.73 | 1178 | 44.27 | −5.11 * | |
| Middle | 1962 | 49.05 | 2038 | 50.95 | 4.78 * | |
| Low | 636 | 51.62 | 596 | 48.38 | 0.06 | |
| Employment | <0.001 | |||||
| Employed 4 | 1934 | 55.24 | 1567 | 44.76 | −5.86 * | |
| Unpaid 5 | 290 | 48.60 | 3076 | 51.40 | 6.48 * | |
| Unemployed | 155 | 56.57 | 119 | 43.43 | −1.80 | |
| Immigration Status | 0.825 | |||||
| Yes | 824 | 50.99 | 792 | 49.01 | −0.22 | |
| No | 4231 | 51.29 | 4018 | 48.71 | 0.22 | |
| No SCP Received 1 | SCP Received | ||||
|---|---|---|---|---|---|
| n | Proportion | n | Proportion | p-Value | |
| Primary Care Provider | p < 0.001 | ||||
| General Practitioner | 1442 | 44.80 | 1777 | 55.20 | |
| Oncologist | 1078 | 50.00 | 1078 | 50.00 | |
| Both | 2294 | 54.40 | 1923 | 45.60 | |
| PCP involvement | p < 0.001 | ||||
| Not involved | 1986 | 62.32 | 1201 | 37.68 | |
| Involved | 2840 | 45.05 | 3464 | 54.95 | |
| No General Practitioner | 135 | 58.95 | 94 | 41.05 | |
| Understanding needs | p < 0.001 | ||||
| Disagree | 584 | 71.05 | 238 | 28.95 | |
| Neutral | 955 | 66.32 | 485 | 33.68 | |
| Agree | 3001 | 44.49 | 3744 | 55.51 | |
| Knows where to find supports and services | p < 0.001 | ||||
| Disagree | 460 | 73.72 | 164 | 26.28 | |
| Neutral | 909 | 61.63 | 566 | 38.37 | |
| Agree | 2974 | 45.63 | 3543 | 54.37 | |
| Able to refer me directly to supports and services | p < 0.001 | ||||
| Disagree | 439 | 73.53 | 158 | 26.47 | |
| Neutral | 832 | 60.51 | 543 | 39.49 | |
| Agree | 3059 | 46.33 | 3543 | 53.67 | |
| Confidence in ability to provide follow-up care | p < 0.001 | ||||
| Disagree | 871 | 70.41 | 366 | 29.59 | |
| Neutral | 814 | 62.23 | 494 | 37.77 | |
| Agree | 2915 | 44.54 | 3629 | 55.46 | |
| Received an SCP | ||||||
|---|---|---|---|---|---|---|
| Unadjusted Model | Adjusted Model | |||||
| Risk Ratio | 95% CIs | p-Value | Risk Ratio | 95% CIs | p-Value | |
| Clinician responsible for follow-up care | ||||||
| Oncologist | (ref.) | (ref.) | ||||
| Both | 1.47 | (1.34, 1.61) | p < 0.001 | 1.49 | (1.33, 1.68) | p < 0.001 |
| General Practitioner | 1.19 | (1.08, 1.32) | p < 0.001 | 1.35 | (1.18, 1.54) | p = 0.001 |
| PCP Involvement | ||||||
| Not involved | (ref) | (ref) | ||||
| Involved | 2.02 | (1.85, 2.20) | p < 0.001 | 1.96 | (1.76, 2.18) | p < 0.001 |
| PCP understands needs | ||||||
| Neutral | (ref) | (ref) | ||||
| Disagree | 0.78 | (0.64, 0.94) | p = 0.009 | 1.03 | (0.82, 1.30) | p = 0.844 |
| Agree | 2.45 | (2.17, 2.76) | p < 0.001 | 2.17 | (1.87, 2.53) | p < 0.001 |
| PCP knows where to find supports and services | ||||||
| Neutral | (ref.) | (ref.) | ||||
| Disagree | 0.55 | (0.44, 0.68) | p < 0.001 | 0.71 | (0.55, 0.92) | p = 0.009 |
| Agree | 1.90 | (1.69, 2.13) | p < 0.001 | 1.74 | (1.50, 2.01) | p < 0.001 |
| PCP is able to refer me directly to supports and services | ||||||
| Neutral | (ref.) | (ref.) | ||||
| Disagree | 0.53 | (0.43, 0.65) | p < 0.001 | 0.65 | (0.50, 0.85) | p = 0.001 |
| Agree | 1.76 | (1.56, 1.99) | p < 0.001 | 1.53 | (1.32, 1.78) | p < 0.001 |
| Confidence ability for PCP to provide follow-up care | ||||||
| Neutral | (ref.) | (ref.) | ||||
| Disagree | 0.67 | (0.57, 0.80) | p < 0.001 | 0.78 | (0.63, 0.95) | p = 0.016 |
| Agree | 2.03 | (1.79, 2.29) | p < 0.001 | 1.83 | (1.56, 2.14) | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, A.K.; Mutsaers, B.; Lebel, S. The Association between Survivorship Care Plans and Patient-Reported Satisfaction and Confidence with Follow-Up Cancer Care Provided by Primary Care Providers. Curr. Oncol. 2022, 29, 7343-7354. https://doi.org/10.3390/curroncol29100577
Chu AK, Mutsaers B, Lebel S. The Association between Survivorship Care Plans and Patient-Reported Satisfaction and Confidence with Follow-Up Cancer Care Provided by Primary Care Providers. Current Oncology. 2022; 29(10):7343-7354. https://doi.org/10.3390/curroncol29100577
Chicago/Turabian StyleChu, Alanna K., Brittany Mutsaers, and Sophie Lebel. 2022. "The Association between Survivorship Care Plans and Patient-Reported Satisfaction and Confidence with Follow-Up Cancer Care Provided by Primary Care Providers" Current Oncology 29, no. 10: 7343-7354. https://doi.org/10.3390/curroncol29100577
APA StyleChu, A. K., Mutsaers, B., & Lebel, S. (2022). The Association between Survivorship Care Plans and Patient-Reported Satisfaction and Confidence with Follow-Up Cancer Care Provided by Primary Care Providers. Current Oncology, 29(10), 7343-7354. https://doi.org/10.3390/curroncol29100577






