Impact of Diabetes on Short-Term and Long-Term Outcomes of Ampullary Adenocarcinoma Patients after Curative Pancreatoduodenectomy
Abstract
:1. Background
2. Methods
2.1. Patients and Study Design
2.2. Covariates and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Short-Term Safety Outcomes
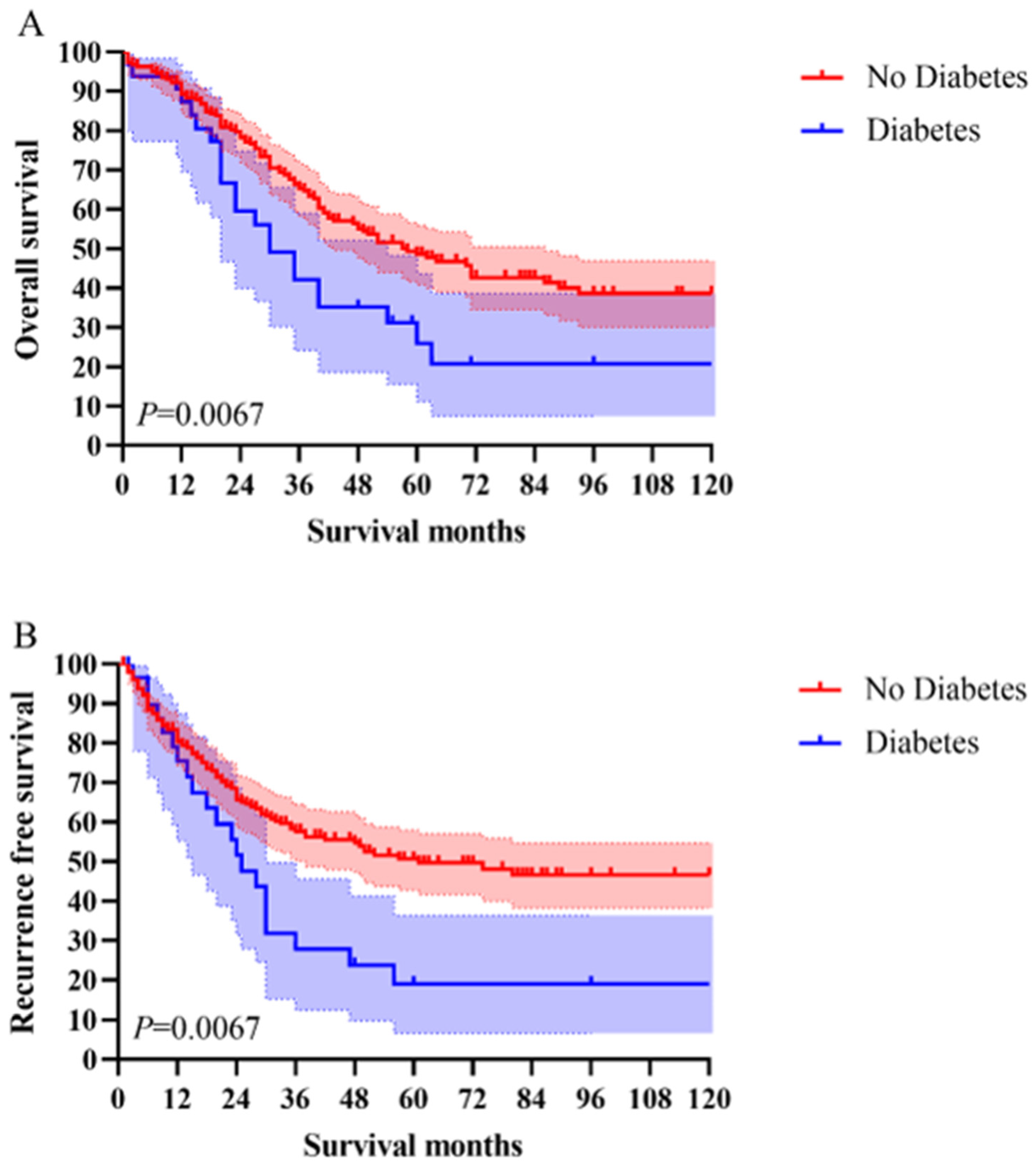
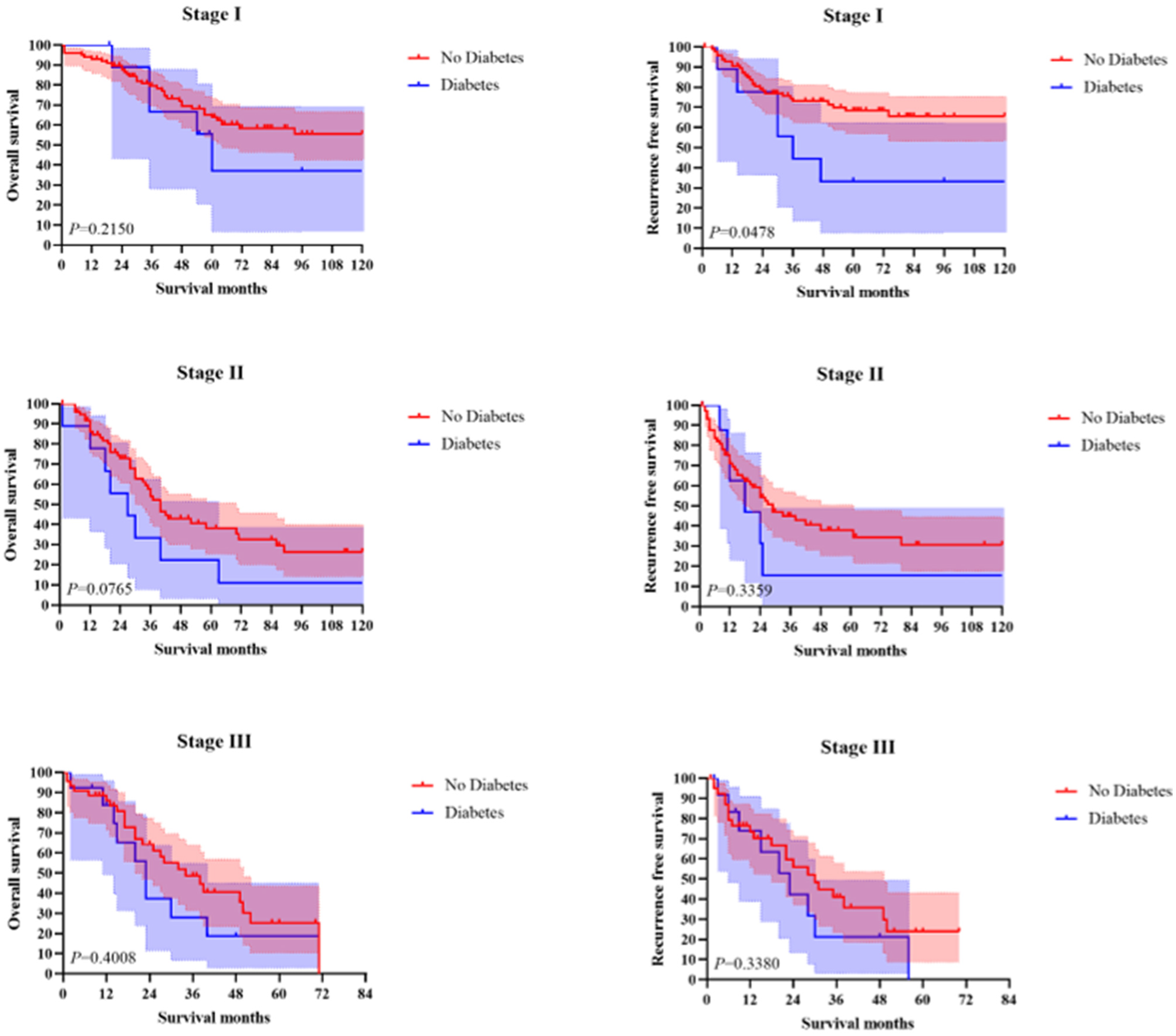
3.3. Long-Term Survival Analysis
3.3.1. Patterns of Treatment Failure
3.3.2. Survival Analysis
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, L.C.; Arruda, R.M.; Botelho, P.F.R.; Taveira, L.N.; Giardina, K.M.; de Oliveira, M.A.; Dias, J.; Oliveira, C.Z.; Fava, G.; Guimarães, D.P. Cap-assisted endoscopy increases ampulla of Vater visualization in high-risk patients. BMC Gastroenterol. 2020, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Wolk, S.; Aust, D.E.; Meier, F.; Saeger, H.D.; Ehehalt, F.; Weitz, J.; Welsch, T.; Distler, M. The pathohistological subtype strongly predicts survival in patients with ampullary carcinoma. Sci. Rep. 2019, 9, 12676. [Google Scholar] [CrossRef]
- Ishihara, S.; Horiguchi, A.; Miyakawa, S.; Endo, I.; Miyazaki, M.; Takada, T. Biliary tract cancer registry in Japan from 2008 to 2013. J. Hepato-Biliary-Pancreat. Sci. 2016, 23, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Ohtsuka, M.; Miyakawa, S.; Nagino, M.; Yamamoto, M.; Kokudo, N.; Sano, K.; Endo, I.; Unno, M.; Chijiiwa, K.; et al. Classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3(rd) English edition. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, C.; Li, Z.; Wang, T.; Zhao, L.; Niu, P.; Guo, C.; Chen, Y.; Che, X.; Zhao, D. Development and Validation of a New Lymph Node Ratio-Based Staging System for Ampullary Carcinoma After Curative Pancreaticoduodenectomy. Front. Oncol. 2021, 11, 811595. [Google Scholar] [CrossRef] [PubMed]
- Koontongkaew, S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J. Cancer 2013, 4, 66–83. [Google Scholar] [CrossRef]
- Maskarinec, G.; Shvetsov, Y.B.; Conroy, S.M.; Haiman, C.A.; Setiawan, V.W.; Le Marchand, L. Type 2 diabetes as a predictor of survival among breast cancer patients: The multiethnic cohort. Breast Cancer Res. Treat. 2019, 173, 637–645. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Zhang, L.; Velasco-Gonzalez, C.; Yi, Y.; Pareti, L.A.; Trapido, E.J.; Chen, V.W.; Wu, X.C. Impact of diabetes and modifiable risk factors on pancreatic cancer survival in a population-based study after adjusting for clinical factors. Cancer Causes Control. CCC 2022, 33, 37–48. [Google Scholar] [CrossRef]
- Jing, C.; Wang, Z.; Fu, X. Effect of diabetes mellitus on survival in patients with gallbladder Cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 689. [Google Scholar] [CrossRef]
- Akhavan, S.; Ghahghaei-Nezamabadi, A.; Modaresgilani, M.; Mousavi, A.S.; Sepidarkish, M.; Tehranian, A.; Rezayof, E. Impact of diabetes mellitus on epithelial ovarian cancer survival. BMC Cancer 2018, 18, 1246. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Ma, X.; Deng, H.Y.; Zha, P.; Zhou, J.; Wang, R.L.; Jiang, R. Diabetes mellitus and survival of esophageal cancer patients after esophagectomy: A systematic review and meta-analysis. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2020, 33, doz098. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Meng, Y.; Lu, M.; Fan, W.; Huang, J.; Li, J.; Zhu, Z. Impact of type 2 diabetes mellitus on short-term and long-term outcomes of patients with esophageal squamous cell cancer undergoing resection: A propensity score analysis. Cancer Commun. 2018, 38, 14. [Google Scholar] [CrossRef] [PubMed]
- Amshoff, Y.; Maskarinec, G.; Shvetsov, Y.B.; Raquinio, P.H.; Grandinetti, A.; Setiawan, V.W.; Haiman, C.A.; Le Marchand, L. Type 2 diabetes and colorectal cancer survival: The multiethnic cohort. Int. J. Cancer 2018, 143, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Zhang, X.; Babic, A.; Morales-Oyarvide, V.; Zhang, Y.; Smith-Warner, S.A.; Wu, K.; Wang, M.; Wolpin, B.M.; Meyerhardt, J.A.; et al. Preexisting Type 2 Diabetes and Survival among Patients with Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2021, 30, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Bi, G.; Yao, G.; Bian, Y.; Xue, L.; Zhang, Y.; Lu, T.; Fan, H. The Effect of Diabetes Mellitus on Prognosis of Patients with Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Ann. Thorac. Cardiovasc. Surg. Off. J. Assoc. Thorac. Cardiovasc. Surg. Asia 2020, 26, 1–12. [Google Scholar] [CrossRef]
- Deng, H.Y.; Zheng, X.; Zha, P.; Peng, L.; Huang, K.L.; Qiu, X.M. Diabetes mellitus and survival of non-small cell lung cancer patients after surgery: A comprehensive systematic review and meta-analysis. Thorac. Cancer 2019, 10, 571–578. [Google Scholar] [CrossRef]
- Slavchev, S.; Kornovski, Y.; Yordanov, A.; Ivanova, Y.; Kostov, S.; Slavcheva, S. Survival in Advanced Epithelial Ovarian Cancer Associated with Cardiovascular Comorbidities and Type 2 Diabetes Mellitus. Curr. Oncol. 2021, 28, 3668–3682. [Google Scholar] [CrossRef]
- Bitterman, D.S.; Winter, K.A.; Hong, T.S.; Fuchs, C.S.; Regine, W.F.; Abrams, R.A.; Safran, H.; Hoffman, J.P.; Benson, A.B., III; Kasunic, T.; et al. Impact of Diabetes and Insulin Use on Prognosis in Patients With Resected Pancreatic Cancer: An Ancillary Analysis of NRG Oncology RTOG 9704. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 201–211. [Google Scholar] [CrossRef]
- Qiang, J.K.; Sutradhar, R.; Giannakeas, V.; Bhatia, D.; Singh, S.; Lipscombe, L.L. Impact of diabetes on colorectal cancer stage and mortality risk: A population-based cohort study. Diabetologia 2020, 63, 944–953. [Google Scholar] [CrossRef]
- Birch, R.J.; Downing, A.; Finan, P.J.; Howell, S.; Ajjan, R.A.; Morris, E.J.A. Improving outcome prediction in individuals with colorectal cancer and diabetes by accurate assessment of vascular complications: Implications for clinical practice. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021, 47, 999–1004. [Google Scholar] [CrossRef]
- Yang, I.P.; Tsai, H.L.; Huang, C.W.; Lu, C.Y.; Miao, Z.F.; Chang, S.F.; Juo, S.H.; Wang, J.Y. High blood sugar levels significantly impact the prognosis of colorectal cancer patients through down-regulation of microRNA-16 by targeting Myb and VEGFR2. Oncotarget 2016, 7, 18837–18850. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tao, S.F.; Zheng, Y.X. Prognostic significance of vascular endothelial growth factor expression and microvessel density in carcinoma of ampulla of Vater. Hepatogastroenterology 2006, 53, 45–50. [Google Scholar] [PubMed]
- Zheng, Y.; Wu, C.; Yang, J.; Zhao, Y.; Jia, H.; Xue, M.; Xu, D.; Yang, F.; Fu, D.; Wang, C.; et al. Insulin-like growth factor 1-induced enolase 2 deacetylation by HDAC3 promotes metastasis of pancreatic cancer. Signal Transduct. Target. Ther. 2020, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Law, P.T.Y.; Wong, C.K.; Chan, J.C.N.; Chan, P.K.S. Exendin-4 Exhibits Enhanced Anti-tumor Effects in Diabetic Mice. Sci. Rep. 2017, 7, 1791. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, G.; von Haefen, C.; Wollersheim, T.; Spies, C. Severe perioperative hyperglycemia attenuates postoperative monocytic function, basophil count and T cell activation. Minerva Anestesiol. 2017, 83, 921–929. [Google Scholar] [CrossRef]
- Yachida, S.; Wood, L.D.; Suzuki, M.; Takai, E.; Totoki, Y.; Kato, M.; Luchini, C.; Arai, Y.; Nakamura, H.; Hama, N.; et al. Genomic Sequencing Identifies ELF3 as a Driver of Ampullary Carcinoma. Cancer Cell. 2016, 29, 229–240. [Google Scholar] [CrossRef]
- van Belle, E.; Cosenza, A.; Baptista, S.B.; Vincent, F.; Henderson, J.; Santos, L.; Ramos, R.; Pouillot, C.; Calé, R.; Cuisset, T.; et al. Usefulness of Routine Fractional Flow Reserve for Clinical Management of Coronary 325 Artery Disease in Patients With Diabetes. JAMA Cardiol. 2020, 5, 272–281. [Google Scholar] [CrossRef]
| Characteristic | Total | Diabetes | No Diabetes | p-Value | |||
|---|---|---|---|---|---|---|---|
| n = 266 (100.00%) | n = 32 (100.00%) | n = 234 (100.00%) | |||||
| Sex | 0.44 | ||||||
| Male | 145 | 54.51% | 20 | 62.50% | 125 | 53.42% | |
| Female | 121 | 45.49% | 12 | 37.50% | 109 | 46.58% | |
| Age | 0.08 | ||||||
| ≤50 | 70 | 26.32% | 4 | 12.50% | 66 | 28.21% | |
| >50 | 196 | 73.68% | 28 | 87.50% | 168 | 71.79% | |
| Jaundice | 0.15 | ||||||
| No | 68 | 25.56% | 12 | 37.50% | 56 | 23.93% | |
| Yes | 198 | 74.44% | 20 | 62.50% | 178 | 76.07% | |
| Intraoperative transfusion | 0.87 | ||||||
| No | 124 | 46.62% | 14 | 43.75% | 110 | 47.01% | |
| Yes | 142 | 53.38% | 18 | 56.25% | 124 | 52.99% | |
| Tumor size | 0.34 | ||||||
| ≤2 cm | 125 | 46.99% | 12 | 37.50% | 113 | 48.29% | |
| >2 cm | 141 | 53.01% | 20 | 62.50% | 121 | 51.71% | |
| Differentiation | 0.37 | ||||||
| Poor | 104 | 39.10% | 16 | 50.00% | 88 | 37.61% | |
| Moderate | 112 | 42.11% | 13 | 40.63% | 99 | 42.31% | |
| Well | 49 | 18.42% | 3 | 9.38% | 46 | 19.66% | |
| Regional nodes examined | 1.00 | ||||||
| ≤11 | 131 | 49.25% | 16 | 50.00% | 115 | 49.15% | |
| >12 | 135 | 50.75% | 16 | 50.00% | 119 | 50.85% | |
| T stage | 0.19 | ||||||
| T1 | 29 | 10.90% | 2 | 6.25% | 27 | 11.54% | |
| T2 | 104 | 39.10% | 9 | 28.13% | 95 | 40.60% | |
| T3 | 133 | 50.00% | 21 | 65.63% | 112 | 47.86% | |
| Lymph node metastasis | 0.29 | ||||||
| No | 176 | 66.17% | 18 | 56.25% | 158 | 67.52% | |
| Yes | 90 | 33.83% | 14 | 43.75% | 76 | 32.48% | |
| TNM stage | 0.05 | ||||||
| I | 112 | 42.11% | 10 | 31.25% | 102 | 43.59% | |
| II | 96 | 36.09% | 9 | 28.13% | 87 | 37.18% | |
| III | 58 | 21.80% | 13 | 40.63% | 45 | 19.23% | |
| Blood vessel invasion | 0.34 | ||||||
| No | 197 | 74.06% | 21 | 65.63% | 176 | 75.21% | |
| Yes | 69 | 25.94% | 11 | 34.38% | 58 | 24.79% | |
| Adjuvant treatment | 0.91 | ||||||
| No | 134 | 50.38% | 15 | 46.88% | 119 | 50.85% | |
| Yes | 93 | 34.96% | 12 | 37.50% | 81 | 34.62% | |
| Unknown | 39 | 14.66% | 5 | 15.63% | 34 | 14.53% | |
| Safety | Total | Diabetes | No Diabetes | p-Value | |||
|---|---|---|---|---|---|---|---|
| n = 266 (100.00%) | n = 32 (100.00%) | n = 234 (100.00%) | |||||
| Surgical time | 0.07 | ||||||
| ≤6 h | 183 | 68.80% | 17 | 53.13% | 166 | 70.94% | |
| >6 h | 83 | 31.20% | 15 | 46.88% | 68 | 29.06% | |
| Blood transfusion | 0.87 | ||||||
| No | 124 | 46.62% | 14 | 43.75% | 110 | 47.01% | |
| Yes | 142 | 53.38% | 18 | 56.25% | 124 | 52.99% | |
| Postoperative complications | 0.74 | ||||||
| No | 155 | 58.27% | 20 | 62.50% | 135 | 57.69% | |
| Yes | 111 | 41.73% | 12 | 37.50% | 99 | 42.31% | |
| Postoperative bleeding | 0.51 | ||||||
| No | 242 | 90.98% | 28 | 87.50% | 214 | 91.45% | |
| Yes | 24 | 9.02% | 4 | 12.50% | 20 | 8.55% | |
| Infection | 1.00 | ||||||
| No | 226 | 84.96% | 27 | 84.38% | 199 | 85.04% | |
| Yes | 40 | 15.04% | 5 | 15.63% | 35 | 14.96% | |
| Gastroparesis | 0.55 | ||||||
| No | 237 | 89.10% | 30 | 93.75% | 207 | 88.46% | |
| Yes | 29 | 10.90% | 2 | 6.25% | 27 | 11.54% | |
| Fistula | 0.41 | ||||||
| No | 225 | 84.59% | 25 | 78.13% | 200 | 85.47% | |
| Yes | 41 | 15.41% | 7 | 21.88% | 34 | 14.53% | |
| Gastroenteric anastomotic fistula | 3 | 1.13% | 2 | 6.25% | 1 | 0.43% | |
| Pancreatic fistula | 15 | 5.65% | 2 | 6.25% | 13 | 5.56% | |
| Biliary fistula | 23 | 8.65% | 3 | 9.38% | 20 | 8.55% | |
| Recurrence | Total | Diabetes | No Diabetes | p-Value | |||
|---|---|---|---|---|---|---|---|
| n = 266 (100.00%) | n = 32 (100.00%) | n = 234 (100.00%) | |||||
| Recurrence | 0.02 | ||||||
| No | 146 | 54.89% | 11 | 34.38% | 135 | 57.69% | |
| Yes | 120 | 45.11% | 21 | 65.63% | 99 | 42.31% | |
| Locoregional recurrence | 1.00 | ||||||
| No | 211 | 79.32% | 25 | 78.13% | 186 | 79.49% | |
| Yes | 55 | 20.68% | 7 | 21.88% | 48 | 20.51% | |
| Systemic recurrence | <0.01 | ||||||
| No | 195 | 73.31% | 16 | 50.00% | 179 | 76.50% | |
| Yes | 71 | 26.69% | 16 | 50.00% | 55 | 23.50% | |
| Liver metastasis | 0.03 | ||||||
| No | 209 | 78.57% | 20 | 62.50% | 189 | 80.77% | |
| Yes | 57 | 21.43% | 12 | 37.50% | 45 | 19.23% | |
| Lung/Bone/Other metastasis | 1.00 | ||||||
| No | 260 | 97.74% | 32 | 100.00% | 228 | 97.44% | |
| Yes | 6 | 2.26% | 0 | 0.00% | 6 | 2.56% | |
| Peritoneal seeding | 0.11 | ||||||
| No | 256 | 96.24% | 29 | 90.63% | 227 | 97.01% | |
| Yes | 10 | 3.76% | 3 | 9.38% | 7 | 2.99% | |
| Outcomes | Diabetes | No Diabetes | p-Value |
|---|---|---|---|
| n = 32 (12.03%) | n = 234 (87.97%) | ||
| Median overall survival time, months | 28.5 | 34 | |
| 5-year survival rates/% | 26.00% | 47.10% | |
| Follow-up period | 1~168 | 1~240 | |
| No. of deaths | 24 | 113 | |
| Univariate HR (95% CI) for OS | 1.698 [1.091–2.642] | 1 (Reference) | 0.019 |
| Multivariate HR (95% CI) for OS | 1.597 [1.005–2.537] | 1 (Reference) | 0.047 |
| Univariate HR (95% CI) for RFS | 1.897 [1.179–3.052] | 1 (Reference) | 0.008 |
| Multivariate HR (95% CI) for RFS | 1.768 [1.068–2.925] | 1 (Reference) | 0.027 |
| No. of patients with PC | 12 | 99 | |
| Univariate OR (95% CI) for PC | 0.818 [0.382–1.752] | 1 (Reference) | 0.605 |
| Multivariate OR (95% CI) for PC | 0.818 [0.382–1.752] | 1 (Reference) | 0.605 |
| No. of patients with recurrence | 21 | 99 | |
| Univariate OR (95% CI) for recurrence | 2.603 [1.200–5.246] | 1 (Reference) | 0.015 |
| Multivariate OR (95% CI) for recurrence | 2.384 [1.065–5.336] | 1 (Reference) | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Sun, C.; Fei, H.; Li, Z.; Guo, C.; Chen, Y.; Che, X.; Zhao, D. Impact of Diabetes on Short-Term and Long-Term Outcomes of Ampullary Adenocarcinoma Patients after Curative Pancreatoduodenectomy. Curr. Oncol. 2022, 29, 6724-6734. https://doi.org/10.3390/curroncol29100528
Zhang X, Sun C, Fei H, Li Z, Guo C, Chen Y, Che X, Zhao D. Impact of Diabetes on Short-Term and Long-Term Outcomes of Ampullary Adenocarcinoma Patients after Curative Pancreatoduodenectomy. Current Oncology. 2022; 29(10):6724-6734. https://doi.org/10.3390/curroncol29100528
Chicago/Turabian StyleZhang, Xiaojie, Chongyuan Sun, He Fei, Zefeng Li, Chunguang Guo, Yingtai Chen, Xu Che, and Dongbing Zhao. 2022. "Impact of Diabetes on Short-Term and Long-Term Outcomes of Ampullary Adenocarcinoma Patients after Curative Pancreatoduodenectomy" Current Oncology 29, no. 10: 6724-6734. https://doi.org/10.3390/curroncol29100528
APA StyleZhang, X., Sun, C., Fei, H., Li, Z., Guo, C., Chen, Y., Che, X., & Zhao, D. (2022). Impact of Diabetes on Short-Term and Long-Term Outcomes of Ampullary Adenocarcinoma Patients after Curative Pancreatoduodenectomy. Current Oncology, 29(10), 6724-6734. https://doi.org/10.3390/curroncol29100528





