Specific Bile Microorganisms Caused by Intra-Abdominal Abscess on Pancreaticoduodenectomy Patients: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Antimicrobial Prophylaxis
2.2. Bile Cultures and Microbiology
2.3. Intra-Abdominal Abscess & Mortality
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costi, R.; De Pastena, M.; Malleo, G.; Marchegiani, G.; Butturini, G.; Violi, V.; Salvia, R.; Bassi, C. Poor Results of Pancreatoduodenectomy in High-Risk Patients with Endoscopic Stent and Bile Colonization are Associated with E. coli, Diabetes and Advanced Age. J. Gastrointest. Surg. 2016, 20, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Young, P.Y.; Khadaroo, R.G. Surgical site infections. Surg. Clin. N. Am. 2014, 94, 1245–1264. [Google Scholar] [CrossRef] [PubMed]
- Akashi, M.; Nagakawa, Y.; Hosokawa, Y.; Takishita, C.; Osakabe, H.; Nishino, H.; Katsumata, K.; Akagi, Y.; Itoi, T.; Tsuchida, A. Preoperative cholangitis is associated with increased surgical site infection following pancreaticoduodenectomy. J. Hepatobiliary Pancreat. Sci. 2020, 27, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-del Castillo, C.; Morales-Oyarvide, V.; McGrath, D.; Wargo, J.A.; Ferrone, C.R.; Thayer, S.P.; Lillemoe, K.D.; Warshaw, A.L. Evolution of the Whipple procedure at the Massachusetts General Hospital. Surgery 2012, 152, S56–S63. [Google Scholar] [CrossRef]
- Behrman, S.W.; Zarzaur, B.L. Intra-abdominal sepsis following pancreatic resection: Incidence, risk factors, diagnosis, microbiology, management, and outcome. Am. Surg. 2008, 74, 572–578, discussion 578–579. [Google Scholar] [CrossRef]
- El-Haddad, H.M.; Sabry, A.A.; Shehata, G.M. Endoscopic versus percutaneous biliary drainage for resectable pancreatic head cancer with hyperbilirubinemia and impact on pancreaticoduodenectomy: A randomized controlled study. Int. J. Surg. 2021, 93, 106043. [Google Scholar] [CrossRef]
- De Groen, P.C.; Gores, G.J.; LaRusso, N.F.; Gunderson, L.L.; Nagorney, D.M. Biliary tract cancers. N. Engl. J. Med. 1999, 341, 1368–1378. [Google Scholar] [CrossRef]
- Pisters, P.W.; Hudec, W.A.; Hess, K.R.; Lee, J.E.; Vauthey, J.N.; Lahoti, S.; Raijman, I.; Evans, D.B. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann. Surg. 2001, 234, 47–55. [Google Scholar] [CrossRef]
- Van der Gaag, N.A.; Rauws, E.A.; Van Eijck, C.H.; Bruno, M.J.; Van der Harst, E.; Kubben, F.J.; Gerritsen, J.J.; Greve, J.W.; Gerhards, M.F.; De Hingh, I.H.; et al. Preoperative biliary drainage for cancer of the head of the pancreas. N. Engl. J. Med. 2010, 362, 129–137. [Google Scholar] [CrossRef]
- Sato, N.; Kimura, T.; Kenjo, A.; Kofunato, Y.; Okada, R.; Ishigame, T.; Watanabe, J.; Marubashi, S. Early intra-abdominal infection following pancreaticoduodenectomy:associated factors and clinical impact on surgical outcome. Fukushima J. Med. Sci. 2020, 66, 124–132. [Google Scholar] [CrossRef]
- Wu, J.M.; Ho, T.W.; Yen, H.H.; Wu, C.H.; Kuo, T.C.; Yang, C.Y.; Tien, Y.W. Endoscopic Retrograde Biliary Drainage Causes Intra-Abdominal Abscess in Pancreaticoduodenectomy Patients: An Important but Neglected Risk Factor. Ann. Surg. Oncol. 2019, 26, 1086–1092. [Google Scholar] [CrossRef]
- Agha, R.; Abdall-Razak, A.; Crossley, E.; Dowlut, N.; Iosifidis, C.; Mathew, G. STROCSS Group STROCSS 2019 Guideline: Strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019, 72, 156–165. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Scheufele, F.; Aichinger, L.; Jager, C.; Demir, I.E.; Schorn, S.; Sargut, M.; Erkan, M.; Kleeff, J.; Friess, H.; Ceyhan, G.O. Effect of preoperative biliary drainage on bacterial flora in bile of patients with periampullary cancer. Br. J. Surg. 2017, 104, e182–e188. [Google Scholar] [CrossRef]
- Turnidge, J.; Paterson, D.L. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 2007, 20, 391–408, table of contents. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- DeOliveira, M.L.; Winter, J.M.; Schafer, M.; Cunningham, S.C.; Cameron, J.L.; Yeo, C.J.; Clavien, P.A. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann. Surg. 2006, 244, 931–937, discussion 937–939. [Google Scholar] [CrossRef]
- Povoski, S.P.; Karpeh, M.S.; Conlon, K.C.; Blumgart, L.H.; Brennan, M.F. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann. Surg. 1999, 230, 131–142. [Google Scholar] [CrossRef]
- Karim, S.A.M.; Abdulla, K.S.; Abdulkarim, Q.H.; Rahim, F.H. The outcomes and complications of pancreaticoduodenectomy (Whipple procedure): Cross sectional study. Int. J. Surg. 2018, 52, 383–387. [Google Scholar] [CrossRef]
- Jagannath, P.; Dhir, V.; Shrikhande, S.; Shah, R.C.; Mullerpatan, P.; Mohandas, K.M. Effect of preoperative biliary stenting on immediate outcome after pancreaticoduodenectomy. Br. J. Surg. 2005, 92, 356–361. [Google Scholar] [CrossRef]
- Limongelli, P.; Pai, M.; Bansi, D.; Thiallinagram, A.; Tait, P.; Jackson, J.; Habib, N.A.; Williamson, R.C.; Jiao, L.R. Correlation between preoperative biliary drainage, bile duct contamination, and postoperative outcomes for pancreatic surgery. Surgery 2007, 142, 313–318. [Google Scholar] [CrossRef]
- Ng, Z.Q.; Suthananthan, A.E.; Rao, S. Effect of preoperative biliary stenting on post-operative infectious complications in pancreaticoduodenectomy. Ann. Hepatobiliary Pancreat. Surg. 2017, 21, 212–216. [Google Scholar] [CrossRef][Green Version]
- Wu, J.M.; Kuo, T.C.; Yang, C.Y.; Chiang, P.Y.; Jeng, Y.M.; Huang, P.H.; Tien, Y.W. Resolution of diabetes after pancreaticoduodenectomy in patients with and without pancreatic ductal cell adenocarcinoma. Ann. Surg. Oncol. 2013, 20, 242–249. [Google Scholar] [CrossRef]
- Cortes, A.; Sauvanet, A.; Bert, F.; Janny, S.; Sockeel, P.; Kianmanesh, R.; Ponsot, P.; Ruszniewski, P.; Belghiti, J. Effect of bile contamination on immediate outcomes after pancreaticoduodenectomy for tumor. J. Am. Coll. Surg. 2006, 202, 93–99. [Google Scholar] [CrossRef]
- Sahora, K.; Morales-Oyarvide, V.; Ferrone, C.; Fong, Z.V.; Warshaw, A.L.; Lillemoe, K.D.; Fernandez-del Castillo, C. Preoperative biliary drainage does not increase major complications in pancreaticoduodenectomy: A large single center experience from the Massachusetts General Hospital. J. Hepatobiliary Pancreat. Sci. 2016, 23, 181–187. [Google Scholar] [CrossRef]
- Fong, Z.V.; McMillan, M.T.; Marchegiani, G.; Sahora, K.; Malleo, G.; De Pastena, M.; Loehrer, A.P.; Lee, G.C.; Ferrone, C.R.; Chang, D.C.; et al. Discordance Between Perioperative Antibiotic Prophylaxis and Wound Infection Cultures in Patients Undergoing Pancreaticoduodenectomy. JAMA Surg. 2016, 151, 432–439. [Google Scholar] [CrossRef]
- Coppola, A.; La Vaccara, V.; Farolfi, T.; Fiore, M.; Cascone, C.; Ramella, S.; Spoto, S.; Ciccozzi, M.; Angeletti, S.; Coppola, R.; et al. Different Biliary Microbial Flora Influence Type of Complications after Pancreaticoduodenectomy: A Single Center Retrospective Analysis. J. Clin. Med. 2021, 10, 2180. [Google Scholar] [CrossRef]
- Heckler, M.; Mihaljevic, A.L.; Winter, D.; Zhou, Z.; Liu, B.; Tanaka, M.; Heger, U.; Michalski, C.W.; Buchler, M.W.; Hackert, T. Escherichia coli Bacterobilia Is Associated with Severe Postoperative Pancreatic Fistula After Pancreaticoduodenectomy. J. Gastrointest. Surg. 2020, 24, 1802–1808. [Google Scholar] [CrossRef]
- Gyoten, K.; Kato, H.; Hayasaki, A.; Fujii, T.; Iizawa, Y.; Murata, Y.; Tanemura, A.; Kuriyama, N.; Kishiwada, M.; Mizuno, S.; et al. Association between gastric Candida colonization and surgical site infections after high-level hepatobiliary pancreatic surgeries: The results of prospective observational study. Langenbecks Arch. Surg. 2021, 406, 109–119. [Google Scholar] [CrossRef]
- Kato, H.; Iizawa, Y.; Nakamura, K.; Gyoten, K.; Hayasaki, A.; Fujii, T.; Murata, Y.; Tanemura, A.; Kuriyama, N.; Azumi, Y.; et al. The Critical Role of Biliary Candidiasis in Development of Surgical Site Infections after Pancreatoduodenectomy: Results of Prospective Study Using a Selective Culture Medium for Candida Species. Biomed. Res. Int. 2018, 2018, 5939724. [Google Scholar] [CrossRef]
- Carlson, E. Enhancement by Candida albicans of Staphylococcus aureus, Serratia marcescens, and Streptococcus faecalis in the establishment of infection in mice. Infect. Immun. 1983, 39, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Haque, A.; Mukhopadhyay, G.; Narayan, R.P.; Prasad, R. Interactions between bacteria and Candida in the burn wound. Burns 2005, 31, 375–378. [Google Scholar] [CrossRef] [PubMed]
- De Pastena, M.; Paiella, S.; Azzini, A.M.; Marchegiani, G.; Malleo, G.; Ciprani, D.; Mazzariol, A.; Secchettin, E.; Bonamini, D.; Gasparini, C.; et al. Preoperative surveillance rectal swab is associated with an increased risk of infectious complications in pancreaticoduodenectomy and directs antimicrobial prophylaxis: An antibiotic stewardship strategy? HPB 2018, 20, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Hirata, M.; Takishima, T.; Ohwada, T.; Shimazu, S.; Kakita, A. Mechanically assisted intraoperative peritoneal lavage for generalized peritonitis as a result of perforation of the upper part of the gastrointestinal tract. J. Am. Coll. Surg. 1994, 179, 443–448. [Google Scholar]
- Sugiura, T.; Mizuno, T.; Okamura, Y.; Ito, T.; Yamamoto, Y.; Kawamura, I.; Kurai, H.; Uesaka, K. Impact of bacterial contamination of the abdominal cavity during pancreaticoduodenectomy on surgical-site infection. Br. J. Surg. 2015, 102, 1561–1566. [Google Scholar] [CrossRef]
- Sourrouille, I.; Gaujoux, S.; Lacave, G.; Bert, F.; Dokmak, S.; Belghiti, J.; Paugam-Burtz, C.; Sauvanet, A. Five days of postoperative antimicrobial therapy decreases infectious complications following pancreaticoduodenectomy in patients at risk for bile contamination. HPB 2013, 15, 473–480. [Google Scholar] [CrossRef]
- Parikh, J.A.; Beane, J.D.; Kilbane, E.M.; Milgrom, D.P.; Pitt, H.A. Is American College of Surgeons NSQIP organ space infection a surrogate for pancreatic fistula? J. Am. Coll. Surg. 2014, 219, 1111–1116. [Google Scholar] [CrossRef]
- Donald, G.W.; Sunjaya, D.; Lu, X.; Chen, F.; Clerkin, B.; Eibl, G.; Li, G.; Tomlinson, J.S.; Donahue, T.R.; Reber, H.A.; et al. Perioperative antibiotics for surgical site infection in pancreaticoduodenectomy: Does the SCIP-approved regimen provide adequate coverage? Surgery 2013, 154, 190–196. [Google Scholar] [CrossRef]
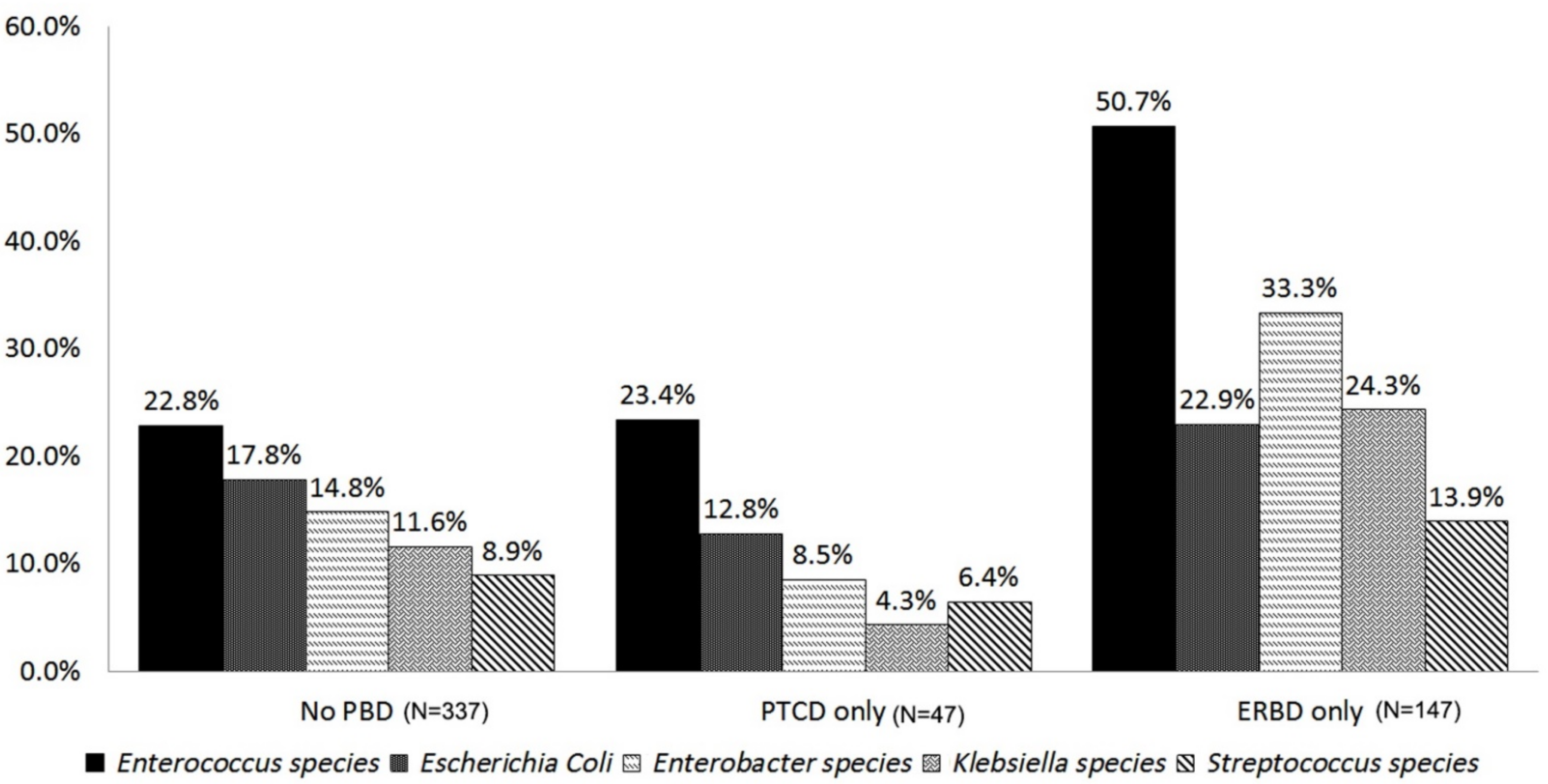

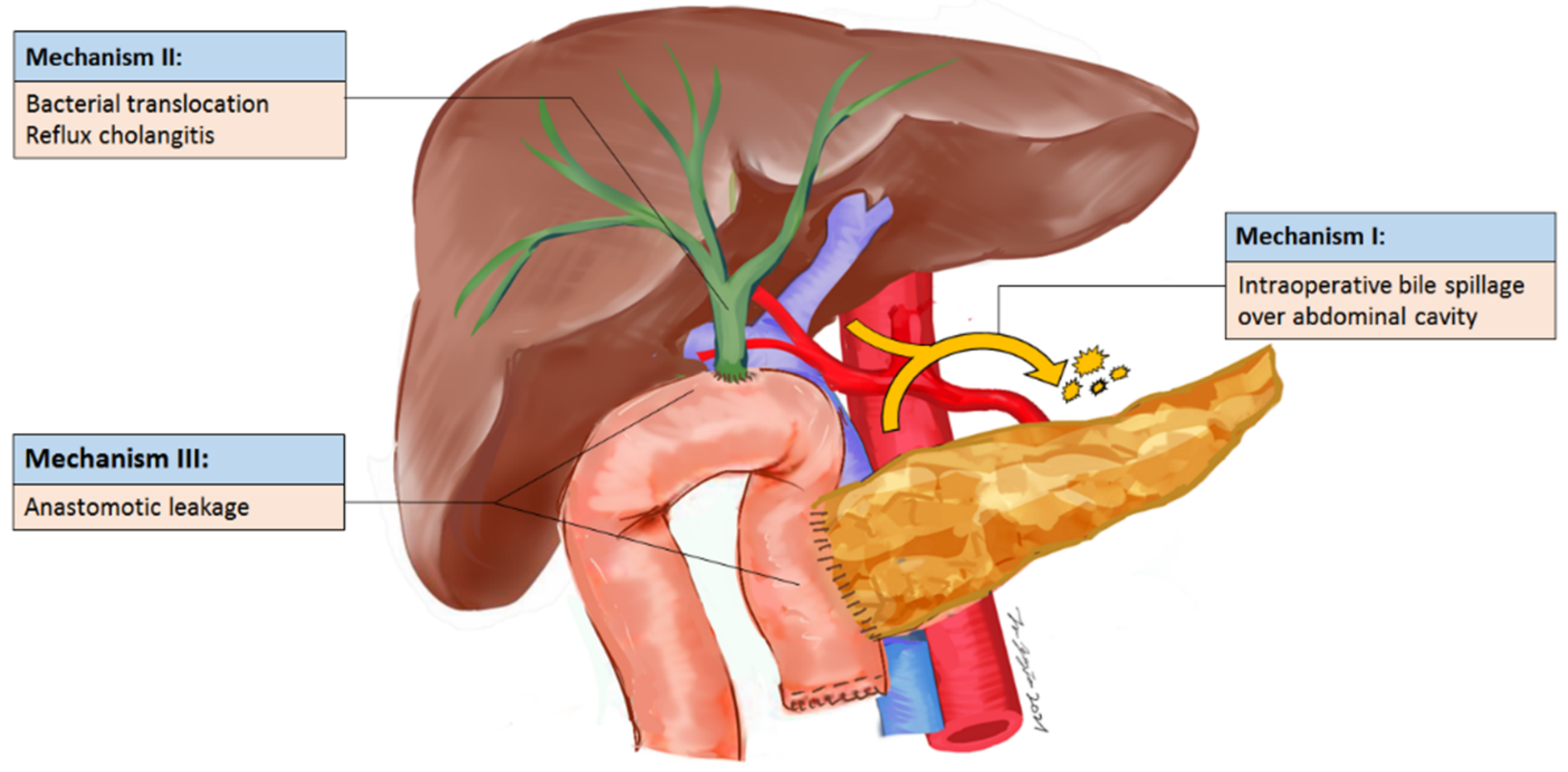
| Variable | Bile Contamination (n = 539) | ||
|---|---|---|---|
| Clinical Variable | No (n = 106) | Yes (n = 433) | p Value |
| Age, y, median (IQR) | 60.8 (52.5–70.2) | 66.4 (57.1–74.0) | 0.006 |
| Gender | 0.630 | ||
| Female | 46 (43.4%) | 199 (46.0%) | |
| Male | 60 (56.6%) | 234 (54.0%) | |
| BMI, median (IQR) | 22.2 (20.4–24.6) | 22.5 (20.7–24.6) | 0.630 |
| Charlson comorbidity index score | 0.063 | ||
| ≤2 | 27 (25.5%) | 76 (17.6%) | |
| >2 | 79 (74.5%) | 357 (82.4%) | |
| ASA physical status | 0.720 | ||
| <3 | 35 (33.0%) | 151 (34.9%) | |
| ≥3 | 71 (67.0%) | 282 (65.1%) | |
| Pancreatitis history | 15 (14.2%) | 51 (11.8%) | 0.500 |
| Pathology | 0.960 | ||
| Periampullary cancer | 68 (64.2%) | 279 (64.4%) | |
| Non periampullary cancer | |||
| Benign or low malignant neoplasm * | 27 (25.5%) | 100 (23.1%) | |
| Chronic pancreatitis | 10 (9.4%) | 50 (11.6%) | |
| Neuroendocrine tumor | 1 (0.9%) | 4 (0.9%) | |
| Preoperative T-BIL level (mg/dL, median, range) | |||
| T-BIL Maximum at any time | 0.9 (0.6–5.8) | 2.6 (1.0–9.8) | <0.001 |
| T-BIL, Maximum within 2 day before surgery | 0.9 (0.6–3.2) | 1.4 (0.9–2.9) | 0.001 |
| Preoperative usage of antibiotics within 30 days | 53 (50.0%) | 303 (70.0%) | <0.001 |
| Preoperative biliary drainage | 21 (19.8%) | 181 (41.8%) | <0.001 |
| Percutaneous transhepatic cholangiography & drainage | 18 (17.0%) | 39 (9.0%) | 0.061 |
| Endoscopic retrograde biliary drainage | 3 (2.8%) | 142 (32.8%) | <0.001 |
| Operation time, min, median (IQR) | 223.5 (194.0–268.0) | 260.0 (225.0–310.0) | <0.001 |
| Intraabdominal abscess | 1 (0.9%) | 74 (17.1%) | <0.001 |
| Superficial surgical site infection | 4 (3.8%) | 14 (3.2%) | 0.780 |
| Clinically relevant POPF | 28 (26.4%) | 110 (25.4%) | 0.610 |
| Microorganisms | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Enterococcus species | 4.156 | 2.661–6.491 | <0.001 |
| Enterobacter species | 2.515 | 1.546–4.093 | <0.001 |
| Escherichia Coli | 2.333 | 1.384–3.933 | 0.001 |
| Klebsiella species | 1.195 | 0.697–2.048 | 0.517 |
| Streptococcus species | 2.796 | 1.502–5.206 | 0.001 |
| Citrobacter species | 3.376 | 1.686–6.762 | 0.001 |
| Pseudomonas Aeruginosa | 1.783 | 0.889–3.581 | 0.104 |
| Aeromonas species | 2.094 | 1.002–4.376 | 0.057 |
| Clostridium species | 0.728 | 0.316–1.676 | 0.455 |
| Proteus species | 1.099 | 0.469–2.573 | 0.827 |
| Candida species | 4.994 | 1.837–13.572 | 0.002 |
| Clinical Variant | Univariate p Value | Multivariate | ||
|---|---|---|---|---|
| Odds Ratio | 95% CI | p Value | ||
| Age | <0.001 | 1.023 | 1.004–1.041 | 0.014 |
| Male | 0.580 | 1.151 | 0.769–1.724 | 0.493 |
| ASA ≥ 3 | 0.990 | 0.862 | 0.544–1.366 | 0.529 |
| BMI | 0.240 | 1.005 | 0.946–1.067 | 0.867 |
| T-BIL, Maximum within 2 days before surgery | 0.140 | 0.969 | 0.905–1.039 | 0.386 |
| Antibiotic use within 30 days before surgery | 0.170 | 0.930 | 0.598–1.447 | 0.750 |
| Preoperative biliary drainage | 0.010 | |||
| Percutaneous transhepatic cholangiography and drainage (ref: no PBD) | 0.869 | 1.001 | 0.461–2.171 | 0.997 |
| Endoscopic retrograde biliary drainage (ref: no PBD) | <0.001 | 1.176 | 0.676–2.047 | 0.565 |
| CCI score > 2 | 0.105 | 1.610 | 0.812–3.193 | 0.172 |
| Periampullary cancer | 0.020 | 1.041 | 0.626–1.731 | 0.876 |
| Pancreatitis | 0.510 | 1.067 | 0.553–2.056 | 0.846 |
| Clinically relevant postoperative pancreatic fistula | 0.610 | 0.882 | 0.591–1.316 | 0.539 |
| Microorganism | ||||
| Candida species | 0.002 | 4.666 | 1.677–12.981 | 0.003 |
| Enterococcus species | < 0.001 | 4.103 | 2.609–6.454 | <0.001 |
| Citrobacter species | 0.001 | 3.050 | 1.510–6.159 | 0.002 |
| Enterobacter species | <0.001 | 2.231 | 1.349–3.689 | 0.002 |
| Streptococcus species | 0.001 | 2.519 | 1.331–4.767 | 0.005 |
| Escherichia Coli | 0.001 | 2.215 | 1.336–3.671 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-J.; Ho, T.-W.; Wu, C.-H.; Kuo, T.-C.; Yang, C.-Y.; Wu, J.-M.; Tien, Y.-W. Specific Bile Microorganisms Caused by Intra-Abdominal Abscess on Pancreaticoduodenectomy Patients: A Retrospective Cohort Study. Curr. Oncol. 2022, 29, 111-121. https://doi.org/10.3390/curroncol29010009
Lin Y-J, Ho T-W, Wu C-H, Kuo T-C, Yang C-Y, Wu J-M, Tien Y-W. Specific Bile Microorganisms Caused by Intra-Abdominal Abscess on Pancreaticoduodenectomy Patients: A Retrospective Cohort Study. Current Oncology. 2022; 29(1):111-121. https://doi.org/10.3390/curroncol29010009
Chicago/Turabian StyleLin, Young-Jen, Te-Wei Ho, Chien-Hui Wu, Ting-Chun Kuo, Ching-Yao Yang, Jin-Ming Wu, and Yu-Wen Tien. 2022. "Specific Bile Microorganisms Caused by Intra-Abdominal Abscess on Pancreaticoduodenectomy Patients: A Retrospective Cohort Study" Current Oncology 29, no. 1: 111-121. https://doi.org/10.3390/curroncol29010009
APA StyleLin, Y.-J., Ho, T.-W., Wu, C.-H., Kuo, T.-C., Yang, C.-Y., Wu, J.-M., & Tien, Y.-W. (2022). Specific Bile Microorganisms Caused by Intra-Abdominal Abscess on Pancreaticoduodenectomy Patients: A Retrospective Cohort Study. Current Oncology, 29(1), 111-121. https://doi.org/10.3390/curroncol29010009





