The Benefit of Intraoperative Magnetic Resonance Imaging in Endoscopic and Microscopic Transsphenoidal Resection of Recurrent Pituitary Adenomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Follow-Up
2.2. OR Setup and MRI
2.3. MRI Volumetric Assessment
2.4. Surgical Procedure
2.5. Surgical Complications
2.6. Data Analysis
3. Results
3.1. General Characteristics
3.2. Extent of Resection
3.3. Additional Resection of Tumor Remnant Identified on iMRI
3.4. Endocrine Outcome and Visual Improvement
3.5. Complications
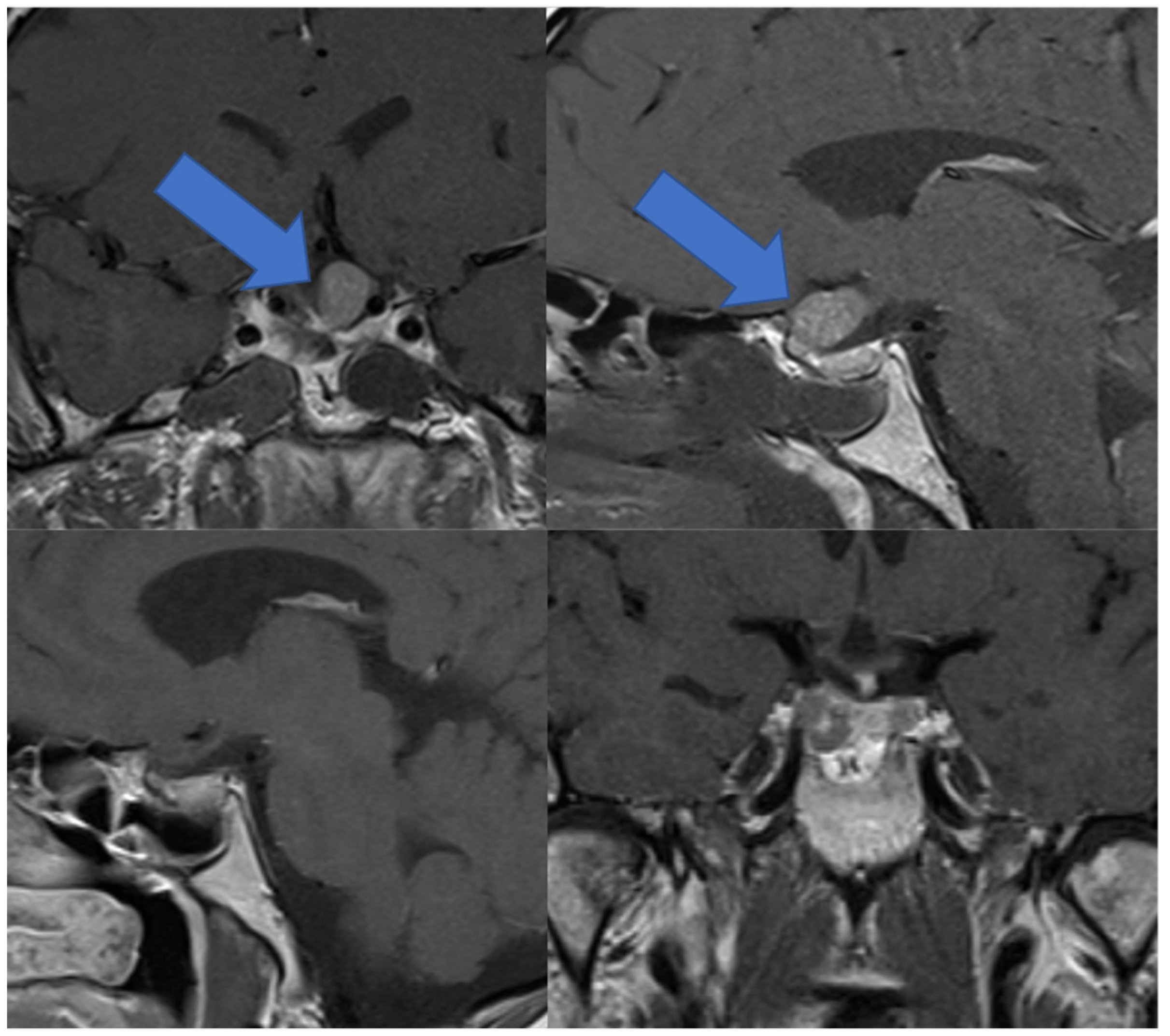
3.6. Illustrative Case
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hlaváč, M.; Knoll, A.; Mayer, B.; Braun, M.; Karpel-Massler, G.; Etzrodt-Walter, G.; Coburger, J.; Wirtz, C.R.; Paľa, A. Ten years’ experience with intraoperative mri-assisted transsphenoidal pituitary surgery. Neurosurg. Focus 2020, 48, E14. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, P.T.; Evans, J.A.; Zipfel, G.J.; Chole, R.A.; Uppaluri, R.; Haughey, B.H.; Getz, A.E.; Silverstein, J.; Rich, K.M.; Kim, A.H.; et al. Combined high-field intraoperative magnetic resonance imaging and endoscopy increase extent of resection and progression-free survival for pituitary adenomas. Pituitary 2015, 18, 72–85. [Google Scholar] [CrossRef] [Green Version]
- Pomeraniec, I.J.; Dallapiazza, R.F.; Xu, Z.; Jane, J.A.; Sheehan, J.P. 115 Early vs late gamma knife radiosurgery following transsphenoidal resection for nonfunctioning pituitary macroadenomas. Neurosurgery 2015, 62, 202. [Google Scholar] [CrossRef]
- Buchfelder, M.; Schlaffer, S.-M. Intraoperative magnetic resonance imaging for pituitary adenomas. Front. Horm. Res. 2016, 45, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.H. Intraoperative magnetic resonance imaging and pituitary surgery. J. Neurosurg. 2014, 120, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Pala, A.; Knoll, A.; Brand, C.; Etzrodt-Walter, G.; Coburger, J.; Wirtz, C.R.; Hlavac, M. The value of intraoperative magnetic resonance imaging in endoscopic and microsurgical transsphenoidal pituitary adenoma resection. World Neurosurg. 2017, 102, 144–150. [Google Scholar] [CrossRef]
- Hlavac, M.; Knoll, A.; Etzrodt-Walter, G.; Sommer, F.; Scheithauer, M.; Coburger, J.; Wirtz, C.R.; Pala, A. Intraoperative MRI in transsphenoidal resection of invasive pituitary macroadenomas. Neurosurg. Rev. 2019, 42, 737–743. [Google Scholar] [CrossRef]
- Knosp, E.; Steiner, E.; Kitz, K.; Matula, C. Pituitary adenomas with invasion of the cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery 1993, 33, 610–618. [Google Scholar] [CrossRef]
- Sheehan, J.; Lee, C.-C.; Bodach, M.E.; Tumialan, L.M.; Oyesiku, N.M.; Patil, C.G.; Litvack, Z.; Zada, G.; Aghi, M.K. Congress of neurological surgeons systematic review and evidence-based guideline for the management of patients with residual or recurrent nonfunctioning pituitary adenomas. Neurosurgery 2016, 79, E539–E540. [Google Scholar] [CrossRef] [Green Version]
- Almutairi, R.D.; Muskens, I.S.; Cote, D.J.; Dijkman, M.D.; Kavouridis, V.K.; Crocker, E.; Ghazawi, K.; Broekman, M.L.D.; Smith, T.R.; Mekary, R.A.; et al. Gross total resection of pituitary adenomas after endoscopic vs. microscopic transsphenoidal surgery: A meta-analysis. Acta Neurochir. 2018, 160, 1005–1021. [Google Scholar] [CrossRef]
- Chang, E.F.; Sughrue, M.E.; Zada, G.; Wilson, C.B.; Blevins, L.S.; Kunwar, S. Long term outcome following repeat transsphenoidal surgery for recurrent endocrine-inactive pituitary adenomas. Pituitary 2010, 13, 223–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, H.; Kshettry, V.R.; Siu, A.; Belinsky, I.; Farrell, C.J.; Nyquist, G.; Rosen, M.; Evans, J.J. Extent of resection, visual, and endocrinologic outcomes for endoscopic endonasal surgery for recurrent pituitary adenomas. World Neurosurg. 2017, 102, 35–41. [Google Scholar] [CrossRef]
- Pala, A.; König, R.; Hlavac, M.; Wirtz, C.R.; Coburger, J. Does the routine use of intraoperative mri prolong progression free survival in low-grade glioma surgery? A retrospective study. Innov. Neurosurg. 2015, 3, 67–74. [Google Scholar] [CrossRef]
- Coburger, J.; Merkel, A.; Scherer, M.; Schwartz, F.; Gessler, F.; Roder, C.; Pala, A.; König, R.; Bullinger, L.; Nagel, G.; et al. Low-grade glioma surgery in intraoperative magnetic resonance imaging: Results of a multicenter retrospective assessment of the german study group for intraoperative magnetic resonance imaging. Neurosurgery 2016, 78, 775–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–766. [Google Scholar] [CrossRef] [Green Version]
- Soneru, C.P.; Riley, C.A.; Hoffman, K.; Tabaee, A.; Schwartz, T.H. Intra-operative MRI vs endoscopy in achieving gross total resection of pituitary adenomas: A systematic review. Acta Neurochir. 2019, 161, 1683–1698. [Google Scholar] [CrossRef]
- Coburger, J.; König, R.; Seitz, K.; Bäzner, U.; Wirtz, C.R.; Hlavac, M. Determining the utility of intraoperative magnetic resonance imaging for transsphenoidal surgery: A retrospective study. J. Neurosurg. 2014, 120, 346–356. [Google Scholar] [CrossRef] [Green Version]
- Esquenazi, Y.; Essayed, W.I.; Singh, H.; Mauer, E.; Ahmed, M.; Christos, P.J.; Schwartz, T.H. Endoscopic endonasal versus microscopic transsphenoidal surgery for recurrent and/or residual pituitary adenomas. World Neurosurg. 2017, 101, 186–195. [Google Scholar] [CrossRef]
- Davies, B.M.; Carr, E.; Soh, C.; Gnanalingham, K.K. Assessing size of pituitary adenomas: A comparison of qualitative and quantitative methods on MR. Acta Neurochir. 2016, 158, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Little, A.S.; Kelly, D.F.; White, W.L.; Gardner, P.A.; Fernandez-Miranda, J.C.; Chicoine, M.R.; Barkhoudarian, G.; Chandler, J.P.; Prevedello, D.M.; Liebelt, B.D.; et al. Results of a prospective multicenter controlled study comparing surgical outcomes of microscopic versus fully endoscopic transsphenoidal surgery for nonfunctioning pituitary adenomas: The transsphenoidal extent of resection (TRANSSPHER) study. J. Neurosurg. 2019, 62, 1–11. [Google Scholar] [CrossRef]
- Schaberg, M.R.; Anand, V.K.; Schwartz, T.H.; Cobb, W. Microscopic versus endoscopic transnasal pituitary surgery. Curr. Opin. Otolaryngol. Head Neck Surg. 2010, 18, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Berkmann, S.; Schlaffer, S.; Nimsky, C.; Fahlbusch, R.; Buchfelder, M. Follow-up and long-term outcome of nonfunctioning pituitary adenoma operated by transsphenoidal surgery with intraoperative high-field magnetic resonance imaging. Acta Neurochir. 2014, 156, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Albano, L.; Losa, M.; Barzaghi, L.R.; Niranjan, A.; Siddiqui, Z.; Flickinger, J.C.; Lunsford, L.D.; Mortini, P. Gamma knife radiosurgery for pituitary tumors: A systematic review and meta-analysis. Cancers 2021, 13, 4998. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Starke, R.M.; Mathieu, D.; Young, B.; Sneed, P.K.; Chiang, V.L.; Lee, J.Y.K.; Kano, H.; Park, K.-J.; Niranjan, A.; et al. Gamma knife radiosurgery for the management of nonfunctioning pituitary adenomas: A multicenter study: Clinical article. J. Neurosurg. 2013, 119, 446–456. [Google Scholar] [CrossRef] [PubMed]

| Patient and Tumor Characteristics | Total |
|---|---|
| n | 59 |
| Age (Median) | 57 |
| Male Ratio | 71.2% (42) |
| Median Tumor Volume (cm3) | 3.3 |
| Gross Total Resection | 49.2% (29) |
| Non-Functioning Adenoma | 71.2% (42) |
| Knosp Grades 3–4 | 55.9% (33) |
| Endoscopic Technique | 44.1% (26) |
| Microscopic Technique | 55.9% (33) |
| Adenoma Remnant Resection after iMRI | 54.2% (32) |
| Variables | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.0 | 0.9–1.1 | 0.067 | |||
| Sex | 1.4 | 0.4–4.2 | 0.592 | |||
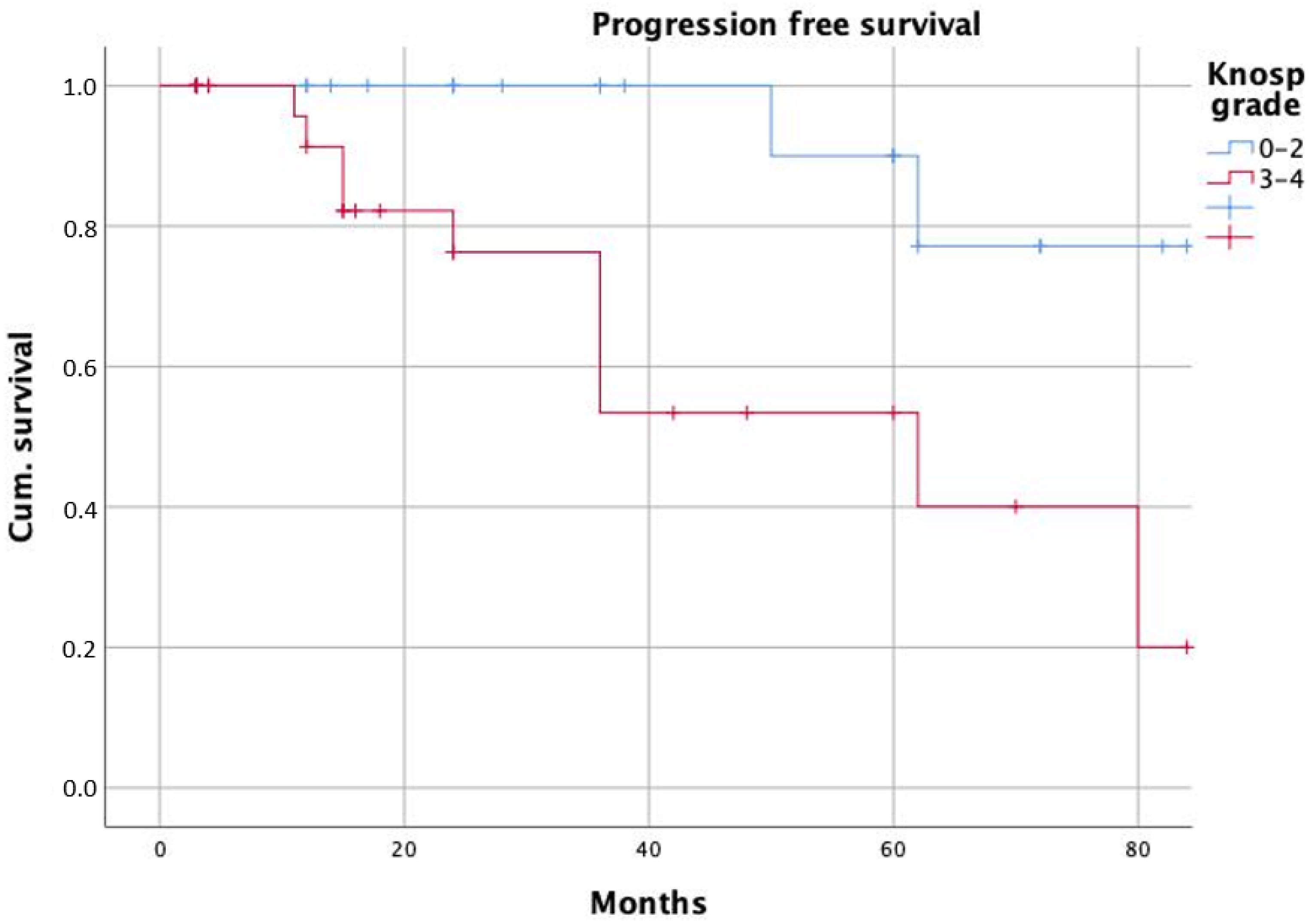
| Knosp grade | 3.8 | 1.3–11.5 | 0.017 | 6.6 | 1.2–36.1 | 0.031 |
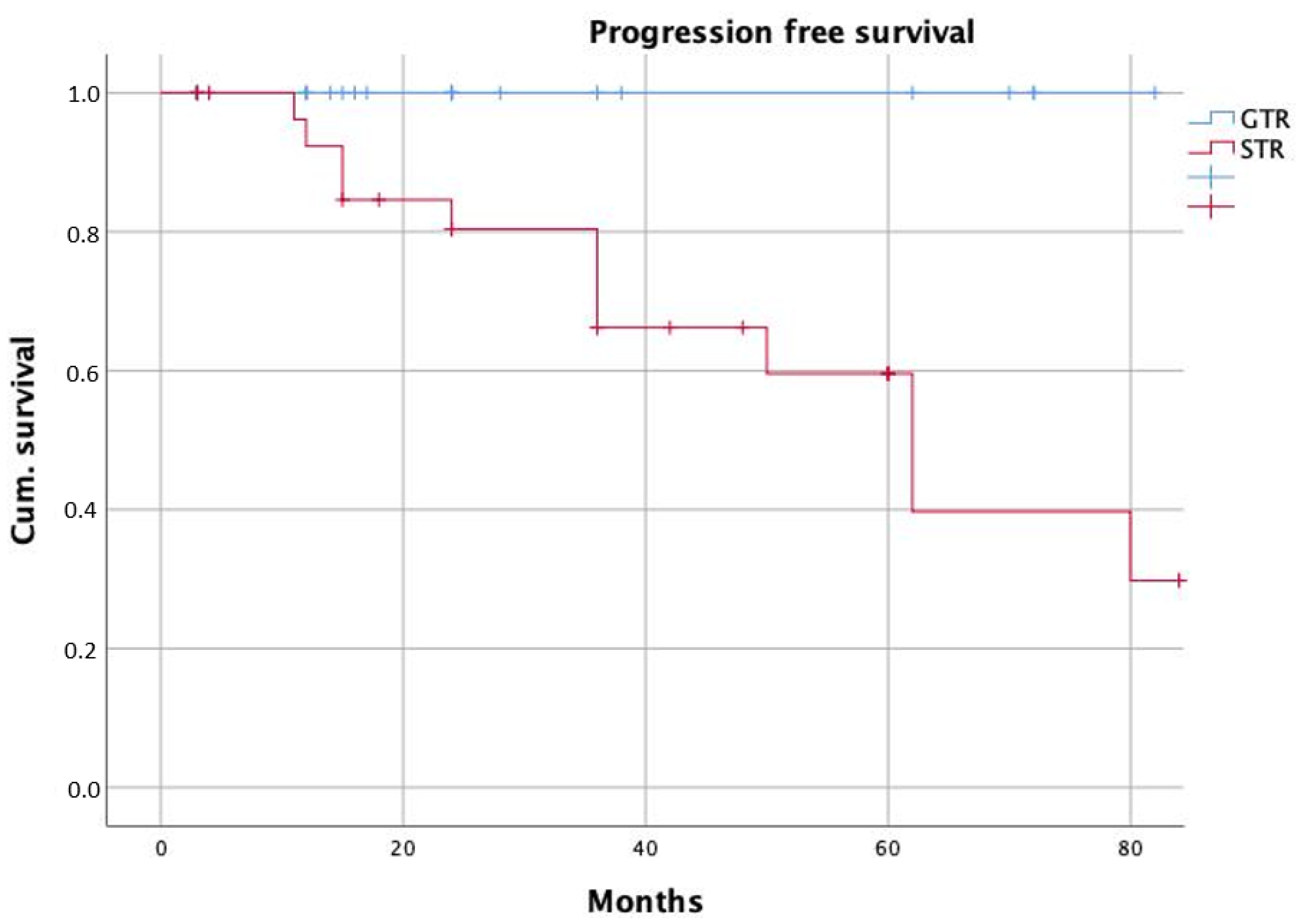
| Surgical technique | 5.2 | 1.7–15.8 | 0.004 | 6.2 | 1.1–34.9 | 0.038 |
| Tumor volume | 1.5 | 1.1–1.9 | 0.005 | 1.2 | 1.0–1.3 | 0.063 |
| Variables | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.0 | 0.98–1.1 | 0.255 | |||
| Sex | 0.7 | 0.2–2.5 | 0.660 | |||
| Knosp grade | 2.4 | 0.8–7.0 | 0.119 | |||
| Surgical technique | 4.4 | 1.4–13.7 | 0.009 | 4.3 | 0.97–19.4 | 0.054 |
| Tumor volume | 1.6 | 1.2–2.1 | 0.004 | 1.5 | 1.1–2.1 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pala, A.; Knoll, A.; Schneider, M.; Etzrodt-Walter, G.; Karpel-Massler, G.; Wirtz, C.R.; Hlavac, M. The Benefit of Intraoperative Magnetic Resonance Imaging in Endoscopic and Microscopic Transsphenoidal Resection of Recurrent Pituitary Adenomas. Curr. Oncol. 2022, 29, 392-401. https://doi.org/10.3390/curroncol29010035
Pala A, Knoll A, Schneider M, Etzrodt-Walter G, Karpel-Massler G, Wirtz CR, Hlavac M. The Benefit of Intraoperative Magnetic Resonance Imaging in Endoscopic and Microscopic Transsphenoidal Resection of Recurrent Pituitary Adenomas. Current Oncology. 2022; 29(1):392-401. https://doi.org/10.3390/curroncol29010035
Chicago/Turabian StylePala, Andrej, Andreas Knoll, Max Schneider, Gwendolin Etzrodt-Walter, Georg Karpel-Massler, Christian Rainer Wirtz, and Michal Hlavac. 2022. "The Benefit of Intraoperative Magnetic Resonance Imaging in Endoscopic and Microscopic Transsphenoidal Resection of Recurrent Pituitary Adenomas" Current Oncology 29, no. 1: 392-401. https://doi.org/10.3390/curroncol29010035
APA StylePala, A., Knoll, A., Schneider, M., Etzrodt-Walter, G., Karpel-Massler, G., Wirtz, C. R., & Hlavac, M. (2022). The Benefit of Intraoperative Magnetic Resonance Imaging in Endoscopic and Microscopic Transsphenoidal Resection of Recurrent Pituitary Adenomas. Current Oncology, 29(1), 392-401. https://doi.org/10.3390/curroncol29010035






