Safety Related to the Timing of Radiotherapy and Immune Checkpoint Inhibitors in Patients with Advanced Non-Small Cell Lung Cancer: A Single Institutional Experience
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
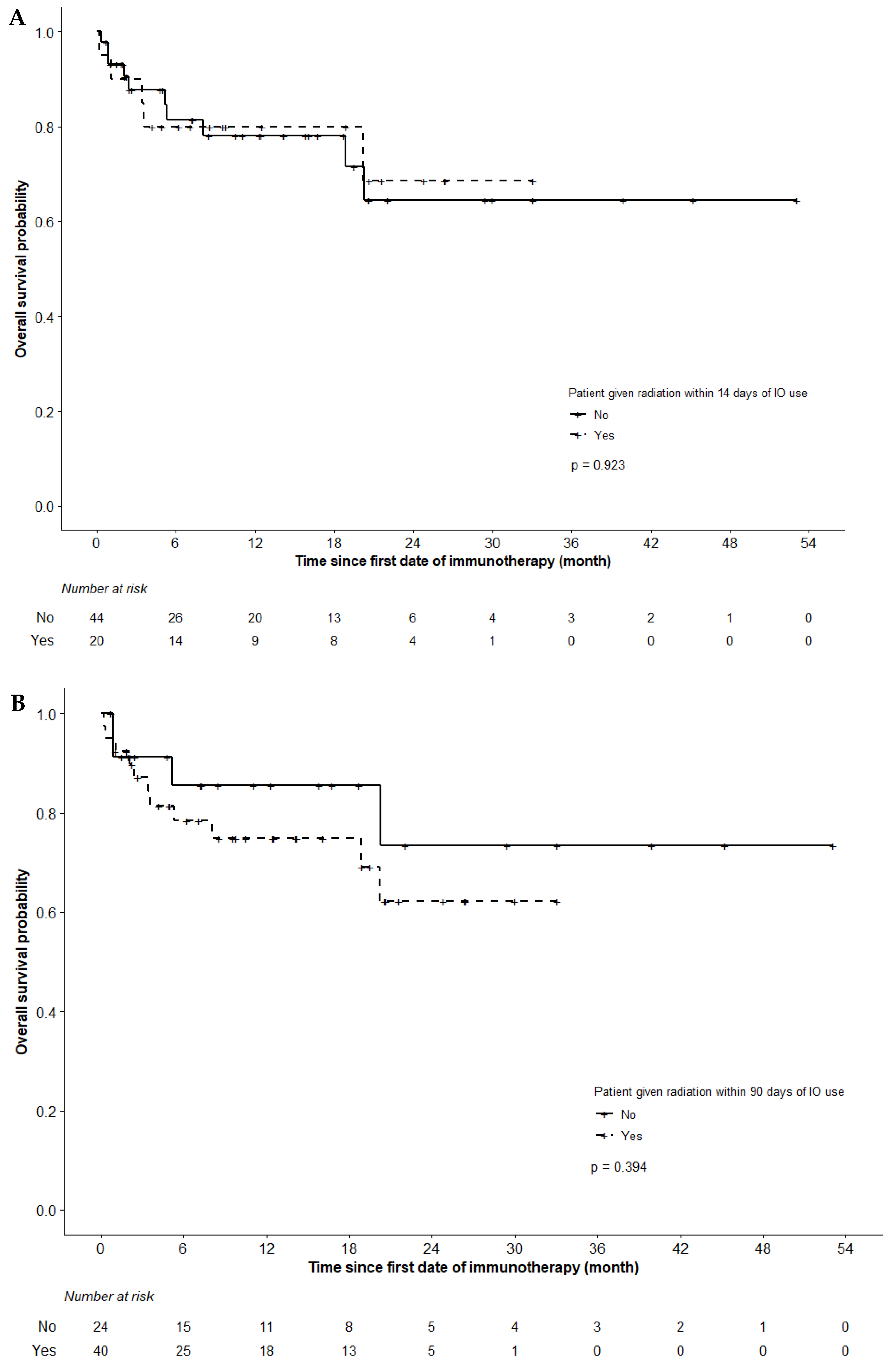
3. Results
3.1. Immunotherapy and Radiotherapy Details
3.2. Toxicities
3.3. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review (CSR), 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2017. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 18 August 2020).
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; qMurakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; De Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.-J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients with Advanced Non–Small-Cell Lung Cancer Treated with Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Leighl, N.B.; Hellmann, M.D.; Hui, R.; Carcereny, E.; Felip, E.; Ahn, M.-J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir. Med. 2019, 7, 347–357. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Perez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.; Balagamwala, E.H.; Chen, A.; Creach, K.M.; Giaccone, G.; Koshy, M.; Zaky, S.; Rodrigues, G. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract. Radiat. Oncol. 2018, 8, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.; Cushman, T.R.; Tang, C.; Welsh, J.W. Toxicity of radiation and immunotherapy combinations. Adv. Radiat. Oncol. 2018, 3, 506–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Theelen, W.S.M.E.; Peulen, H.M.U.; Lalezari, F.; Van Der Noort, V.; De Vries, J.F.; Aerts, J.G.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.L.N.; De Langen, A.J.; et al. Effect of Pembrolizumab after Stereotactic Body Radiotherapy vs. Pembrolizumab Alone on Tumor Response in Patients with Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Centanni, M.; Moes, D.J.A.R.; Trocóniz, I.F.; Ciccolini, J.; van Hasselt, J.G.C. Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin. Pharmacokinet. 2019, 58, 835–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voong, K.R.; Hazell, S.Z.; Fu, W.; Hu, C.; Lin, C.T.; Ding, K.; Suresh, K.; Hayman, J.; Hales, R.K.; Alfaifi, S.; et al. Relationship Between Prior Radiotherapy and Checkpoint-Inhibitor Pneumonitis in Patients with Advanced Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2019, 20, e470–e479. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Niemierko, A.; Hwang, K.L.; Hubbeling, H.; Schapira, E.; Gainor, J.F.; Keane, F.K. Clinical Outcomes in Patients with Metastatic Lung Cancer Treated with PD-1/PD-L1 Inhibitors and Thoracic Radiotherapy. JAMA Oncol. 2018, 4, 253–255. [Google Scholar] [CrossRef] [PubMed]

| Interval 1 | Interval 2 | ||||||
|---|---|---|---|---|---|---|---|
| Total (N = 64) | Within (N = 20) | Outside (N = 44) | p * | Within (N = 40) | Outside (N = 24) | p * | |
| Age (years) | 0.0091 | 0.3177 | |||||
| Median | 70 | 65 | 73 | 69 | 71 | ||
| Interquartiles | 64–77 | 61–69 | 67–79 | 63–76 | 67–79 | ||
| Females (%) | 29 (45%) | 11 (55%) | 18 (41%) | 0.4171 | 21 (53%) | 8 (33%) | 0.1953 |
| Histology (%) | 0.6835 | 0.4598 | |||||
| Adenocarcinoma | 56 (88%) | 18 (90%) | 38 (86%) | 36 (90%) | 20 (83%) | ||
| Squamous cell carcinoma | 8 (13%) | 2 (10%) | 6 (14%) | 4 (10%) | 4 (17%) | ||
| Stage (AJCC 8th) | 0.4968 | 0.4350 | |||||
| IVA-B | 63 (98%) | 20 (100%) | 43 (98%) | 39 (98%) | 24 (100%) | ||
| IIIB | 1 (2%) | 0 (0%) | 1 (2%) | 1 (2%) | 0 (0%) | ||
| ECOG performance score (%) | 0.9684 | 0.6832 | |||||
| 0 | 14 (22%) | 5 (25%) | 9 (20%) | 10 (25%) | 4 (17%) | ||
| 1 | 36 (56%) | 11 (55%) | 25 (57%) | 20 (50%) | 16 (67%) | ||
| 2 | 11 (17%) | 3 (15%) | 8 (18%) | 8 (20%) | 3 (12%) | ||
| 3 | 3 (5%) | 1 (5%) | 2 (5%) | 2 (5%) | 1 (4%) | ||
| Ethnicity (%) | 0.2150 | 0.0140 | |||||
| Caucasian | 50 (78%) | 13 (65%) | 37 (84%) | 27 (68%) | 23 (96%) | ||
| East Asian | 8 (13%) | 4 (20%) | 4 (20%) | 8 (20%) | 0 (0%) | ||
| Others | 6 (9%) | 3 (15%) | 3 (15%) | 5 (12%) | 1 (4%) | ||
| Smoking history (%) | 0.8327 | 0.7158 | |||||
| Yes | 48 (75%) | 16 (80%) | 32 (73%) | 31 (78%) | 17 (71%) | ||
| No | 15 (23%) | 4 (20%) | 11 (25%) | 8 (20%) | 7 (29%) | ||
| Unknown | 1 (2%) | 0 (0%) | 1 (2%) | 1 (2%) | 0 (0%) | ||
| PD-L1 status (%) | 0.2126 | 0.0301 | |||||
| <1% | 9 (14%) | 1 (5%) | 8 (18%) | 3 (7%) | 6 (25%) | ||
| 1–49% | 11 (17%) | 6 (30%) | 5 (12%) | 8 (20%) | 3 (13%) | ||
| ≥50% | 32 (50%) | 9 (45%) | 23 (52%) | 18 (45%) | 14 (58%) | ||
| Unknown | 12 (19%) | 4 (20%) | 8 (18%) | 11 (28%) | 1 (4%) | ||
| EGFR status (%) | 0.9245 | 0.6472 | |||||
| Positive | 9 (14%) | 3 (15%) | 6 (14%) | 7 (17%) | 2 (8%) | ||
| Negative | 41 (64%) | 12 (60%) | 29 (66%) | 25 (63%) | 16 (67%) | ||
| Unknown | 14 (22%) | 5 (25%) | 9 (25%) | 8 (20%) | 6 (25%) | ||
| ALK status (%) | 0.1005 | 0.3433 | |||||
| Positive | 1 (2%) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (4%) | ||
| Negative | 48 (75%) | 12 (60%) | 36 (82%) | 29 (73%) | 19 (79%) | ||
| Unknown | 15 (23%) | 8 (40%) | 7 (16%) | 11 (27%) | 4 (17%) | ||
| Prior chemotherapy (%) | 0.8713 | 0.2409 | |||||
| Yes | 57 (89%) | 18 (90%) | 39 (89%) | 34 (85%) | 23 (96%) | ||
| No | 7 (11%) | 2 (10%) | 5 (11%) | 6 (15%) | 1 (4%) | ||
| Prior targeted therapy (%) | 0.1649 | 0.8247 | |||||
| Yes | 6 (9%) | 0 (0%) | 6 (14%) | 4 (10%) | 2 (8%) | ||
| No | 58 (91%) | 20 (100%) | 38 (86%) | 36 (90%) | 22 (92%) | ||
| ICI agent (%) | 0.3632 | 0.0391 | |||||
| Nivolumab | 29 (45%) | 10 (50%) | 19 (43%) | 19 (48%) | 10 (42%) | ||
| Pembrolizumab | 24 (38%) | 9 (45%) | 15 (34%) | 18 (45%) | 6 (25%) | ||
| Atezolizumab | 6 (9%) | 0 (0%) | 6 (14%) | 1 (2%) | 5 (21%) | ||
| Durvalumab | 5 (8%) | 1 (5%) | 4 (9%) | 2 (5%) | 3 (12%) | ||
| Duration of ICI use (months) | 0.4647 | 0.6703 | |||||
| Median | 4.2 | 5.6 | 3.7 | 3.9 | 4.4 | ||
| Interquartiles | 2.1–12.8 | 2.8–12.7 | 1.5–12.9 | 2.3–12.7 | 1.5–12.9 | ||
| Steroid use after ICI (%) | 26 (41%) | 3 (15%) | 35 (80%) | <0.0001 | 16 (40%) | 22 (92%) | <0.0001 |
| Radiotherapy dose regimen (%) | 0.7389 | 0.3819 | |||||
| Palliative | 16 (25%) | 5 (25%) | 11 (25%) | 11 (28%) | 5 (21%) | ||
| Radical conventional | 10 (16%) | 2 (10%) | 8 (18%) | 4 (10%) | 6 (25%) | ||
| Ablative | 9 (14%) | 4 (20%) | 5 (11%) | 7 (17%) | 2 (8%) | ||
| More than 1 regimen above | 29 (45%) | 9 (45%) | 20 (45%) | 18 (45%) | 11 (46%) | ||
| Radiotherapy technique | 0.7490 | 0.2000 | |||||
| 2/3D-CRT | 8 (13%) | 2 (10%) | 6 (14%) | 5 (13%) | 3 (13%) | ||
| VMAT/IMRT/GammaKnife | 29 (45%) | 8 (40%) | 21 (48%) | 15 (38%) | 14 (58%) | ||
| Both | 27 (42%) | 10 (50%) | 17 (39%) | 20 (50%) | 7 (29%) | ||
| Radiotherapy target site (%) | 0.3904 | 0.2285 | |||||
| Intracranial only | 4 (6%) | 1 (5%) | 3 (7%) | 4 (10%) | 0 (0%) | ||
| Extracranial only | 36 (56%) | 9 (45%) | 27 (61%) | 20 (50%) | 16 (67%) | ||
| Both | 24 (38%) | 10 (50%) | 14 (32%) | 16 (40%) | 8 (33%) | ||
| Variable | All Patients (N = 64) | Received RT in Interval 1 (N = 20) | No RT in Interval 1 (N = 44) | Received RT in Interval 2 (N = 40) | No RT in Interval 2 (N = 24) |
|---|---|---|---|---|---|
| Median RT courses prescribed (range) | 3 (1–31) | 5 (1–17) | 2 (1–31) | 5 (1–17) | 2 (1–31) |
| Median RT prescription dose in Gy (range) | 20 (8–78) | 20 (8–78) | 20 (8–66) | 20 (8–78) | 23 (8–66) |
| Median RT fractions per regimen (range) | 2 (1–39) | 2 (1–39) | 2 (1–33) | 2 (1–39) | 4 (1–33) |
| Median BED10 in Gy10 (range) | 50 (14–106) | 50 (14–94) | 50 (14–106) | 53 (14–106) | 50 (14–106) |
| Median BED10 within Intervals in Gy10 (range) | N/A | Interval 1: 35 (14–94) | N/A | Interval 2: 50 (14–106) | N/A |
| Patients with intracranial RT (%) | 28 (44%) | 11 (55%) | 17 (39%) | 20 (50%) | 8 (33%) |
| Patients with Grade ≥2 toxicities (%) | 18 (28%) | 7 (35%) | 11 (25%) | 12 (30%) | 6 (25%) |
| Grade | ||||
|---|---|---|---|---|
| Toxicity | 2 | 3 | 4 | 5 |
| Pneumonitis | 1 (2%) | 5 (8%) | 0 (0%) | 0 (0%) |
| Nausea/emesis | 2 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hepatitis | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Dermatitis | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pericardial effusion | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Thrombocytopenia | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Hypothyroidism | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Colitis | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Encephalitis | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Arthritis | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Adrenitis | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Esophagitis | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Fever | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Variable | OR | 95% CI | p-Value | |
|---|---|---|---|---|
| Age | 1.01 | 0.96 | 1.08 | 0.54 |
| Histology (SCC vs. Adenocarcinoma) | 3.00 | 0.64 | 14.29 | 0.15 |
| ECOG score | 0.27 | |||
| 0 vs. ≥2 | 1.64 | 0.23 | 14.26 | 0.88 |
| 1 vs. ≥2 | 3.39 | 0.77 | 24.00 | 0.11 |
| PD-L1 status | 0.33 | |||
| <1% vs. ≥50% | 1.79 | 0.32 | 8.83 | 0.97 |
| 1–49% vs. ≥50% | 2.98 | 0.68 | 13.12 | 0.27 |
| RT within 14 days of ICI use (Interval 1) | 1.62 | 0.50 | 5.07 | 0.41 |
| RT within 90 days of ICI use (Interval 2) | 1.29 | 0.42 | 4.26 | 0.67 |
| RT regimen within Interval 1 | 0.21 | |||
| Ablative vs. Palliative | 1.28 | 0.18 | 8.98 | 0.80 |
| Radical conventional vs. Palliative | 6.42 | 0.33 | 124.20 | 0.22 |
| More than 1 regimen vs. Palliative | 0.07 | 0.01 | 0.84 | 0.04 * |
| RT regimen within Interval 2 | 0.05 | |||
| Ablative vs. Palliative | 0.90 | 0.22 | 3.64 | 0.88 |
| Radical conventional vs. Palliative | 1.98 | 0.40 | 9.92 | 0.41 |
| More than 1 regimen vs. Palliative | 0.17 | 0.04 | 0.73 | 0.02 * |
| Intracranial RT in Interval 1 | 0.20 | 0.02 | 1.38 | 0.98 |
| Intracranial RT in Interval 2 | 0.28 | 0.05 | 1.21 | 0.97 |
| A | OR | 95% CI | p-Value | B | OR | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| RT within 14 days of ICI use (Interval 1) | 2.35 | 0.47 | 15.31 | 0.34 | RT within 90 days of ICI use (Interval 2) | 1.79 | 0.40 | 8.93 | 0.48 |
| Age | 1.00 | 0.91 | 1.09 | 0.92 | Age | 0.98 | 0.90 | 1.07 | 0.71 |
| Histology (SCC vs. Adenocarcinoma) | 3.74 | 0.60 | 27.83 | 0.21 | Histology (SCC vs. Adenocarcinoma) | 3.79 | 0.63 | 26.85 | 0.20 |
| ECOG score | 0.36 | ECOG score | 0.36 | ||||||
| 0 vs. ≥2 | 2.62 | 0.22 | 79.96 | 0.91 | 0 vs. ≥2 | 2.01 | 0.18 | 45.05 | 0.93 |
| 1 vs. ≥2 | 5.53 | 0.58 | 181.76 | 0.15 | 1 vs. ≥2 | 4.79 | 0.55 | 108.46 | 0.15 |
| PD-L1 status | 0.20 | PD-L1 status | 0.18 | ||||||
| <1% vs. ≥50% | 2.12 | 0.36 | 12.67 | 0.41 | <1% vs. ≥50% | 2.05 | 0.36 | 11.71 | 0.42 |
| 1–49% vs. ≥50% | 2.01 | 0.58 | 7.01 | 0.27 | 1–49% vs. ≥50% | 2.11 | 0.62 | 7.20 | 0.23 |
| RT regimen | 0.12 | RT regimen | 0.12 | ||||||
| Ablative vs. Palliative | 0.74 | 0.14 | 3.78 | 0.71 | Ablative vs. Palliative | 0.65 | 0.12 | 3.52 | 0.62 |
| Radical conventional vs. Palliative | 0.70 | 0.14 | 3.48 | 0.67 | Radical conventional vs. Palliative | 0.75 | 0.15 | 3.70 | 0.72 |
| More than 1 regimen vs. Palliative | 0.33 | 0.08 | 1.31 | 0.11 | More than 1 regimen vs. Palliative | 0.35 | 0.09 | 1.37 | 0.13 |
| Intracranial RT in Interval 1 | 0.95 | 0.19 | 4.55 | 0.95 | Intracranial RT in Interval 2 | 0.92 | 0.19 | 4.56 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tjong, M.C.; Ragulojan, M.; Poon, I.; Louie, A.V.; Cheng, S.Y.; Doherty, M.; Zhang, L.; Ung, Y.; Cheung, P.; Cheema, P.K. Safety Related to the Timing of Radiotherapy and Immune Checkpoint Inhibitors in Patients with Advanced Non-Small Cell Lung Cancer: A Single Institutional Experience. Curr. Oncol. 2022, 29, 221-230. https://doi.org/10.3390/curroncol29010021
Tjong MC, Ragulojan M, Poon I, Louie AV, Cheng SY, Doherty M, Zhang L, Ung Y, Cheung P, Cheema PK. Safety Related to the Timing of Radiotherapy and Immune Checkpoint Inhibitors in Patients with Advanced Non-Small Cell Lung Cancer: A Single Institutional Experience. Current Oncology. 2022; 29(1):221-230. https://doi.org/10.3390/curroncol29010021
Chicago/Turabian StyleTjong, Michael C., Malavan Ragulojan, Ian Poon, Alexander V. Louie, Susanna Y. Cheng, Mark Doherty, Liying Zhang, Yee Ung, Patrick Cheung, and Parneet K. Cheema. 2022. "Safety Related to the Timing of Radiotherapy and Immune Checkpoint Inhibitors in Patients with Advanced Non-Small Cell Lung Cancer: A Single Institutional Experience" Current Oncology 29, no. 1: 221-230. https://doi.org/10.3390/curroncol29010021
APA StyleTjong, M. C., Ragulojan, M., Poon, I., Louie, A. V., Cheng, S. Y., Doherty, M., Zhang, L., Ung, Y., Cheung, P., & Cheema, P. K. (2022). Safety Related to the Timing of Radiotherapy and Immune Checkpoint Inhibitors in Patients with Advanced Non-Small Cell Lung Cancer: A Single Institutional Experience. Current Oncology, 29(1), 221-230. https://doi.org/10.3390/curroncol29010021




