Small Particle DEBIRI TACE as Salvage Therapy in Patients with Liver Dominant Colorectal Cancer Metastasis: Retrospective Analysis of Safety and Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Statistical Analysis
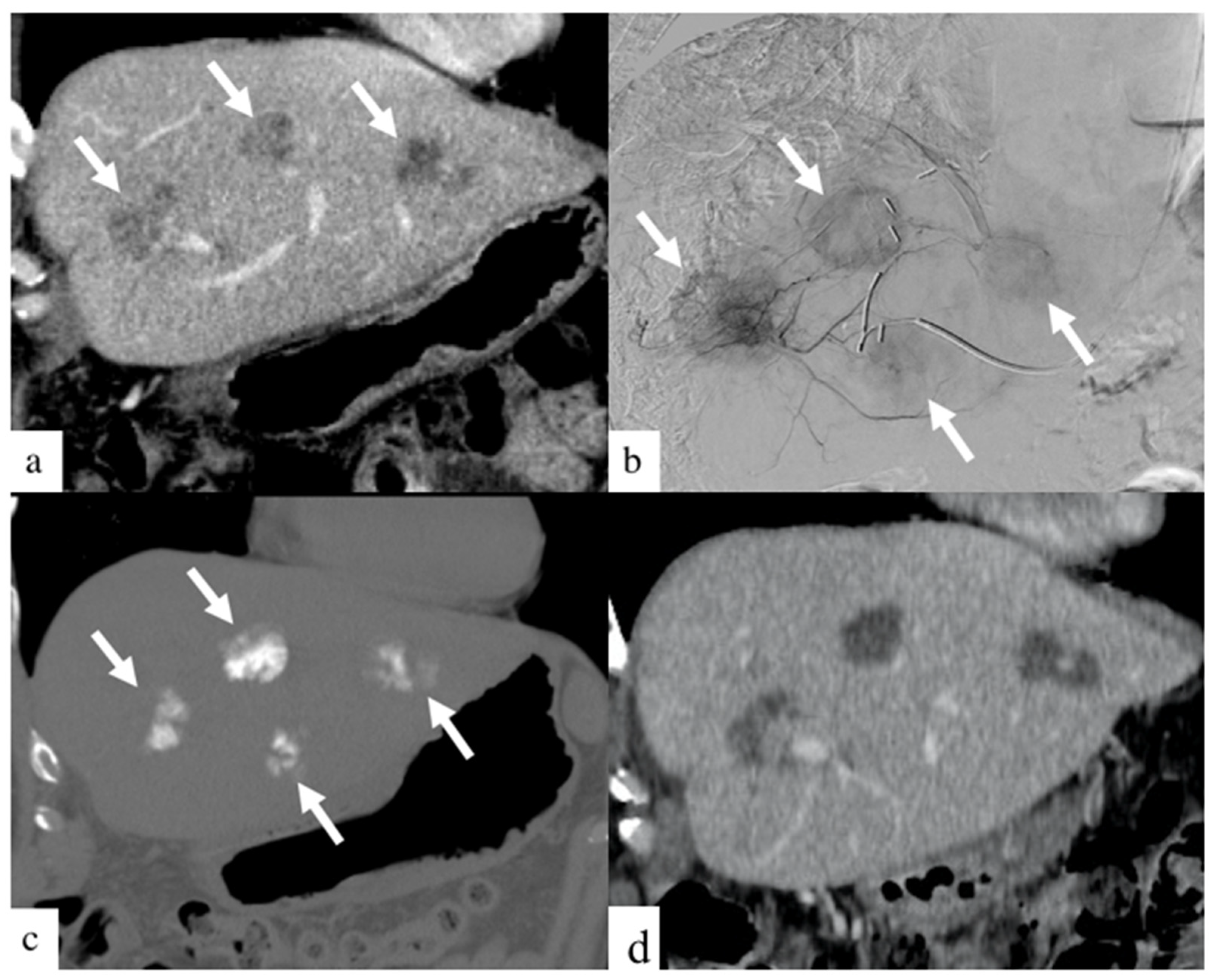
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Available online: Canadian-cancer-statistics-2019-en.pdf (accessed on 28 November 2021).
- Brenner, D.R.; Heer, E.; Sutherland, R.L.; Ruan, Y.; Tinmouth, J.; Heitman, S.J.; Hilsden, R.J. National Trends in Colorectal Cancer Incidence Among Older and Younger Adults in Canada. JAMA Netw. Open 2019, 2, e198090. [Google Scholar] [CrossRef]
- Kekelidze, M.; D’Errico, L.; Pansini, M.; Tyndall, A.; Hohmann, J. Colorectal cancer: Current imaging methods and future perspectives for the diagnosis, staging and therapeutic response evaluation. World J. Gastroenterol. 2013, 19, 8502–8514. [Google Scholar] [CrossRef]
- Primrose, J.N. Treatment of colorectal metastases: Surgery, cryotherapy, or radiofrequency ablation. Gut 2002, 50, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Helling, T.S.; Martin, M. Cause of death from liver metastases in colorectal cancer. Ann. Surg. Oncol. 2014, 21, 501–506. [Google Scholar] [CrossRef]
- Abbas, S.; Lam, V.; Hollands, M. Ten-Year Survival after Liver Resection for Colorectal Metastases: Systematic Review and Meta-Analysis. ISRN Oncol. 2011, 2011, 763245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gorgen, A.; Muaddi, H.; Zhang, W.; McGilvray, I.; Gallinger, S.; Sapisochin, G. The New Era of Transplant Oncology: Liver Transplantation for Nonresectable Colorectal Cancer Liver Metastases. Can. J. Gastroenterol. Hepatol. 2018, 2018, 9531925. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Okamoto, K.; Kasi, P.M.; Kawakami, H. Molecular Biomarkers in the Personalized Treatment of Colorectal Cancer. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2016, 14, 651–658. [Google Scholar] [CrossRef]
- Gustavsson, B.; Carlsson, G.; Machover, D.; Petrelli, N.; Roth, A.; Schmoll, H.-J.; Tveit, K.-M.; Gibson, F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin. Colorectal. Cancer 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, G.; Sarti, D.; Nani, R.; Aliberti, C.; Fiorentini, C.; Guadagni, S. Updates of colorectal cancer liver metastases therapy: Review on DEBIRI. Hepatic Oncol. 2020, 7, HEP16. [Google Scholar] [CrossRef]
- Eichler, K.; Zangos, S.; Mack, M.G.; Hammerstingl, R.; Gruber-Rouh, T.; Gallus, C.; Vogl, T.J. First human study in treatment of unresectable liver metastases from colorectal cancer with irinotecan-loaded beads (DEBIRI). Int. J. Oncol. 2012, 41, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.G.; Scoggins, C.R.; Schreeder, M.; Rilling, W.S.; Laing, C.J.; Tatum, C.M.; Kelly, L.R.; Garcia-Monaco, R.D.; Sharma, V.R.; Crocenzi, T.S.; et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer 2015, 121, 3649–3658. [Google Scholar] [CrossRef]
- Fiorentini, G.; Aliberti, C.; Tilli, M.; Mulazzani, L.; Graziano, F.; Giordani, P.; Mambrini, A.; Montagnani, F.; Alessandroni, P.; Catalano, V.; et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: Final results of a phase III study. Anticancer Res. 2012, 32, 1387–1395. [Google Scholar]
- Mauri, G.; Varano, G.M.; Della Vigna, P.; Bonomo, G.; Monfardini, L.; Zampino, M.G.; Ravenda, P.S.; Orsi, F. Transarterial Embolization with Small-Size Particles Loaded with Irinotecan for the Treatment of Colorectal Liver Metastases: Results of the MIRACLE III Study. Cardiovasc. Intervent. Radiol. 2018, 41, 1708–1715. [Google Scholar] [CrossRef]
- Akinwande, O.K.; Philips, P.; Duras, P.; Pluntke, S.; Scoggins, C.; Martin, R.C.G. Small versus large-sized drug-eluting beads (DEBIRI) for the treatment of hepatic colorectal metastases: A propensity score matching analysis. Cardiovasc. Intervent. Radiol. 2015, 38, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Sato, T.; Nishiofuku, H.; Masada, T.; Tatsumoto, S.; Marugami, N.; Otsuji, T.; Kanno, M.; Koyama, F.; Sho, M.; et al. Selective TACE with irinotecan-loaded 40 μm microspheres and FOLFIRI for colorectal liver metastases: Phase I dose escalation pharmacokinetic study. BMC Cancer 2019, 19, 758. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Rouh, T.; Marko, C.; Thalhammer, A.; Nour-Eldin, N.-E.; Langenbach, M.; Beeres, M.; Naguib, N.N.; Zangos, S.; Vogl, T.J. Current strategies in interventional oncology of colorectal liver metastases. Br. J. Radiol. 2016, 89, 20151060. [Google Scholar] [CrossRef] [PubMed]
- Bhutiani, N.; Akinwande, O.; Martin, R.C.G. Efficacy and Toxicity of Hepatic Intra-Arterial Drug-Eluting (Irinotecan) Bead (DEBIRI) Therapy in Irinotecan-Refractory Unresectable Colorectal Liver Metastases. World J. Surg. 2016, 40, 1178–1190. [Google Scholar] [CrossRef]
- Di Noia, V.; Basso, M.; Marsico, V.; Cerchiaro, E.; Rossi, S.; D’Argento, E.; Strippoli, A.; Schinzari, G.; Iezzi, R.; Cassano, A.; et al. DEBIRI plus capecitabine: A treatment option for refractory liver-dominant metastases from colorectal cancer. Future Oncol. Lond. Engl. 2019, 15, 2349–2360. [Google Scholar] [CrossRef]
- Aliberti, C.; Fiorentini, G.; Muzzio, P.C.; Pomerri, F.; Tilli, M.; Dallara, S.; Benea, G. Trans-arterial chemoembolization of metastatic colorectal carcinoma to the liver adopting DC Bead®, drug-eluting bead loaded with irinotecan: Results of a phase II clinical study. Anticancer Res. 2011, 31, 4581–4587. [Google Scholar]
- Zener, R.; Yoon, H.; Ziv, E.; Covey, A.; Brown, K.T.; Sofocleous, C.T.; Thornton, R.H.; Boas, F.E. Outcomes After Transarterial Embolization of Neuroendocrine Tumor Liver Metastases Using Spherical Particles of Different Sizes. Cardiovasc. Intervent. Radiol. 2019, 42, 569–576. [Google Scholar] [CrossRef]
- Muaddi, H.; D’Angelica, M.; Wiseman, J.T.; Dillhoff, M.; Latchana, N.; Roke, R.; Ko, Y.; Carpizo, D.; Do, K.S.; Fields, R.C.; et al. Safety and feasibility of initiating a hepatic artery infusion pump chemotherapy program for unresectable colorectal liver metastases: A multicenter, retrospective cohort study. J. Surg. Oncol. 2021, 123, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Spina, J.C.; Hume, I.; Pelaez, A.; Peralta, O.; Quadrelli, M.; Monaco, R.G. Expected and Unexpected Imaging Findings after 90Y Transarterial Radioembolization for Liver Tumors. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc 2019, 39, 578–595. [Google Scholar] [CrossRef]
- Wasan, H.S.; Gibbs, P.; Sharma, N.K.; Taieb, J.; Heinemann, V.; Ricke, J.; Peeters, M.; Findlay, M.; Weaver, A.; Mills, J.; et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): A combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017, 18, 1159–1171. [Google Scholar] [CrossRef]
- Neugut, A.I.; Lin, A.; Raab, G.T.; Hillyer, G.C.; Keller, D.; O’Neil, D.S.; Accordino, M.K.; Kiran, R.P.; Wright, J.; Hershman, D.L. FOLFOX and FOLFIRI use in Stage IV Colon Cancer: Analysis of SEER-Medicare Data. Clin. Colorectal. Cancer 2019, 18, 133–140. [Google Scholar] [CrossRef]
- de Mestier, L.; Manceau, G.; Neuzillet, C.; Bachet, J.B.; Spano, J.P.; Kianmanesh, R.; Vaillant, J.C.; Bouché, O.; Hannoun, L.; Karoui, M. Primary tumor resection in colorectal cancer with unresectable synchronous metastases: A review. World J. Gastrointest. Oncol. 2014, 6, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef]

| Variable | Characteristics | No. Patient (%) or Value |
|---|---|---|
| Population | ||
| n | 36 | |
| Age, year | 64 ± 13 | |
| Gender, n (%) | M/F | 27 (75%)/9 (25%) |
| ECOG, n (%) | Group 0/group 1/group 2/unknown | 18 (50%)/13 (36%)/2 (6%)/3 (8%) |
| Colorectal Cancer | ||
| Primary Cancer | ||
| Location, n (%) | Right-sided/left-sided/multifocal | 8 (22%)/27 (75%)/1 (3%) |
| Primary CRC resection, n (%) | Resected/in situ | 29 (81%)/7 (19%) |
| Mutation status, n (%) | KRAS mutated/wild-type/unknown | 14 (39%)/14 (39%)/8 (22%) |
| Metastatic Liver Disease | ||
| Presentation, n (%) | Synchronous/metachronous | 21 (58%)/15 (42%) |
| Liver dominance, n (%) | Liver only/dominant | 23 (64%)/13 (36%) |
| Liver lobe, n (%) | Bilobar/unilobar right/unilobar left | 34 (94%)/2 (6%)/0 (0%) |
| Tumoral burden, n (%) | <25%/25–50%/50–75%/>75% | 10 (28%)/19 (53%)/3 (11%)/3 (8%) |
| Lines of Treatment | ||
| Conventional systemic therapy | ||
| Neo adjuvant lines, n (%) | 0/1/2/≥3 lines | 27 (75%)/9 (25%)/0 (0%)/0 (0%) |
| Adjuvant lines before DEBIRI, n (%) | 0/1/2/≥3 lines | 5 (14%)/18 (50%)/11 (31%)/2 (6%) |
| Adjuvant lines total, n (%) | 0/1/2/≥3 lines | 1 (3%)/14 (39%)/12 (33%)/9 (25%) |
| Targeted systemic therapy | ||
| EGFR inhibitors, n(%) | 22 (61%) | |
| VEGF inhibitors, n(%) | 8 (22%) | |
| EGFR- and VEGF-inhibitors, n(%) | 4 (11%) | |
| No targeted therapy, n (%) | 10 (28%) | |
| Surgery | ||
| Liver resection, n (%) | 1/2 resections | 10 (28%)/2 (6%) |
| Intra-operative ablation, n (%) | 5 (14%) | |
| Ablation | ||
| Radiation therapy, n (%) | 1/2 treatments | 7 (19%)/1 (3%) |
| Percutaneous ablation, n (%) | 1/2/≥3 treatments | 6 (17%)/5 (14%)/4 (11%) |
| DEBIRI | ||
| Rounds of treatments, n (%) | 1/2 rounds of treatment | 31 (86%)/5 (14%) |
| Total number of sessions, n (%) | 1/2/3/≥4 sessions | 7 (19%)/9 (25%)/7 (19%)/13 (36%) |
| Early DEBIRI (≤12 months), n (%) | Alive/deceased patients | 1 (2.8%)/9 (25%) |
| Mean irinotecan/session, mg | 82 | |
| Bead size, n (%) | variable | 17 (47%)/11 (31%)/8 (22%) |
| Access, n (%) | radial/femoral/variable | 29 (81%)/5 (14%)/2 (6%) |
| Total lines after T0 1, n (%) | 1 line | 0 (0%) |
| 2 lines | 8 (22%) | |
| 3 lines | 6 (17%) | |
| 4 lines | 5 (14%) | |
| 5 lines | 3 (8%) | |
| 6 lines | 7 (19%) | |
| 7 lines | 5 (14%) | |
| ≥8 lines | 2 (6%) | |
| Variable | No. Patient (%) |
|---|---|
| Mean Length of Stay | |
| <1 day, n (%) | 1 (3%) |
| =1 day, n (%) | 33 (92%) |
| >1 day, n (%) | 2 (6%) |
| Readmission <1 month after DEBIRI | |
| All causes, n (%) | 5 (14%) |
| DEBIRI related, n (%) | 3 (8%) |
| Adverse events (Grade 3–4) | |
| Longer length of stay for pain management, n (%) | 2 (6%) |
| PES requiring readmission, n (%) | 2 (6%) |
| Liver abscess, n (%) | 1 (3%) |
| Variable | Value | No. Patient (%) or Months |
|---|---|---|
| Metachronous Presentation | 15 (42%) | |
| Time to metastatic disease, months | Median (min, max) | 10 (3, 50) |
| Mean ± SD | 14.1 ± 13.0 | |
| Deceased patient, n (%) | 27 (75%) | |
| OS (from diagnosis), months | Median (min, max) | 28 (13, 145) |
| Mean ± SD | 39.8 ± 29.7 | |
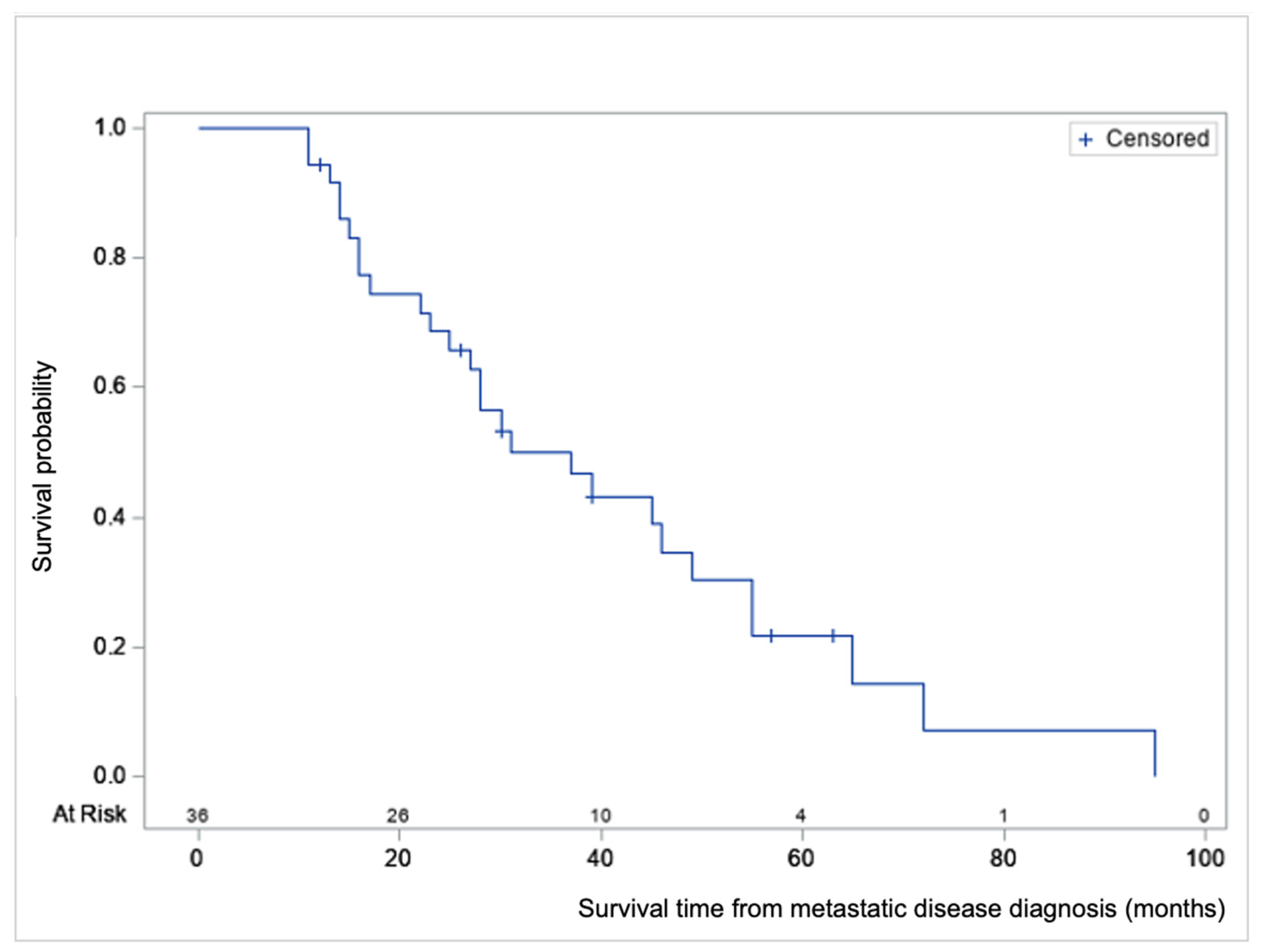
| Survival from T0 ‡ | ||
| All patients, months | Median (min, max) | 28 (11, 95) |
| Mean ± SD | 33.3 ± 21.1 | |
| Early DEBIRI § only, months | Mean ± SD | 15.6 ± 4.3 |
| Late DEBIRI only, months | Mean ± SD | 42.2 ± 20.6 |
| Time first DEBIRI—death, months | Median (min, max) | 10 (2, 36) |
| Mean ± SD | 11.6 ± 8.8 | |
| Alive patient, n (%) | 9 (25%) | |
| Follow-up time, months | Median (min, max) | 39 (12, 63) |
| Mean ± SD | 36.8 ± 15.8 |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (>60 vs. ≤60) | 1.17 | 0.51 to 2.68 | 0.7129 | |||
| Sex (F vs. M) | 0.59 | 0.22 to 1.60 | 0.3010 | |||
| ECOG (Group 1 and 2 vs. Group 1) | 2.63 | 1.11 to 6.20 | 0.0276 | |||
| Total number of lines after T0 | 0.70 | 0.57 to 0.86 | 0.0007 | 0.65 | 0.504 to 0.837 | 0.0009 |
| Number chemo lines before DEBIRI | 0.77 | 0.47 to 1.26 | 0.2996 | |||
| Time to first DEBIRI (months from T0) | 0.89 | 0.83 to 0.94 | 0.0001 | |||
| Late (>12 months) vs. early (≤12 months) DEBIRI | 31.4 | 6.4 to 154.0 | <0.0001 | |||
| No use vs. use of VEGF- and/or EGFR-inhibitors | 0.63 | 0.27 to 1.46 | 0.2793 | |||
| Presence KRAS mutation vs. wild-type | 1.46 | 0.57 to 3.72 | 0.4314 | 1.742 | 0.643 to 4.716 | 0.2747 |
| CEA (>5 ng/mL vs. ≤5 ng/mL) | 1.59 | 0.57 to 4.40 | 0.3735 | |||
| Side of primary CRC (right side or multifocal vs. left) | 1.37 | 0.59 to 3.17 | 0.4690 | |||
| Primary resection (resected vs. non-resected) | 0.32 | 0.12 to 0.86 | 0.0235 | 0.241 | 0.065 to 0.892 | 0.0331 |
| Presentation (metachronous vs. synchronous) | 0.62 | 0.26 to 1.45 | 0.2674 | |||
| Liver resection (resected vs. non-resected) | 0.52 | 0.22 to 1.24 | 0.1377 | |||
| Disease dominance (liver dominant vs. liver only) | 1.03 | 0.44 to 2.41 | 0.9501 | |||
| Number of DEBIRI rounds (2 vs. 1 round) | 0.44 | 0.13 to 1.55 | 0.2023 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voizard, N.; Ni, T.; Kiss, A.; Pugash, R.; Raphael, M.J.; Coburn, N.; David, E. Small Particle DEBIRI TACE as Salvage Therapy in Patients with Liver Dominant Colorectal Cancer Metastasis: Retrospective Analysis of Safety and Outcomes. Curr. Oncol. 2022, 29, 209-220. https://doi.org/10.3390/curroncol29010020
Voizard N, Ni T, Kiss A, Pugash R, Raphael MJ, Coburn N, David E. Small Particle DEBIRI TACE as Salvage Therapy in Patients with Liver Dominant Colorectal Cancer Metastasis: Retrospective Analysis of Safety and Outcomes. Current Oncology. 2022; 29(1):209-220. https://doi.org/10.3390/curroncol29010020
Chicago/Turabian StyleVoizard, Nicolas, Tiffany Ni, Alex Kiss, Robyn Pugash, Michael Jonathon Raphael, Natalie Coburn, and Elizabeth David. 2022. "Small Particle DEBIRI TACE as Salvage Therapy in Patients with Liver Dominant Colorectal Cancer Metastasis: Retrospective Analysis of Safety and Outcomes" Current Oncology 29, no. 1: 209-220. https://doi.org/10.3390/curroncol29010020
APA StyleVoizard, N., Ni, T., Kiss, A., Pugash, R., Raphael, M. J., Coburn, N., & David, E. (2022). Small Particle DEBIRI TACE as Salvage Therapy in Patients with Liver Dominant Colorectal Cancer Metastasis: Retrospective Analysis of Safety and Outcomes. Current Oncology, 29(1), 209-220. https://doi.org/10.3390/curroncol29010020






