Optimal Breast Density Characterization Using a Three-Dimensional Automated Breast Densitometry System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Content and Investigated Items
2.3. Statistical Analysis
3. Results
3.1. Volpara Density by Age and Relationship between VDG and Age
3.2. Relationship between VDG and Diagnostic Accuracy
3.3. Diagnostic Accuracy by US for Patients Whose Tumors Were Non-Detected by MMG According to VDG
3.4. Impact of VDG on the Relationship between Non-Detected Cases by MMG and Presence of Invasion
3.5. Difference in Non-Detected Rate between Cases with and without Calcifications Requiring MMG Differentiation by VDG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyd, N.F.; Rommens, J.M.; Vogt, K.; Lee, V.; Hopper, J.L.; Yaffe, M.J.; Paterson, A.D. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005, 6, 798–808. [Google Scholar] [CrossRef]
- Harvey, J.A.; Bovbjerg, V.E. Quantitative assessment of mammographic breast density: Relationship with breast cancer risk. Radiology 2004, 230, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Jeffers, A.M.; Sieh, W.; Lipson, J.A.; Rothstein, J.H.; McGuire, V.; Whittemore, A.S.; Rubin, D.L. Breast cancer risk and mammographic density assessed with semiautomated and fully automated methods and BI-RADS. Radiology 2017, 282, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Kerlikowske, K.; Zhu, W.; Tosteson, A.N.; Sprague, B.L.; Tice, J.A.; Lehman, C.D.; Miglioretti, D.L. Identifying women with dense breasts at high risk for interval cancer: A cohort study. Ann. Intern. Med. 2015, 162, 673–681. [Google Scholar] [CrossRef] [Green Version]
- McCormack, V.A.; dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, C.; Schairer, C.; Wolfe, J.; Parekh, N.; Salane, M.; Brinton, L.A.; Hoover, R.; Haile, R. Mammographic features and breast cancer risk: Effects with time, age, and menopause status. J. Natl. Cancer Inst. 1995, 87, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, A.R.; Cuzick, J.; Buist, D.S.M.; Bowles, E.J.A. Long-term accuracy of breast cancer risk assessment combining classic risk factors and breast density. JAMA Oncol. 2018, 4, e180174. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, T.; Nakashima, K.; Kikuchi, M.; Kubota, K.; Suzuki, A.; Nakano, S.; Iwata, H. The Japanese Breast Cancer Society clinical practice guidelines for breast cancer screening and diagnosis. Breast Cancer 2020, 27, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zonderland, H.M.; Coerkamp, E.G.; Hermans, J.; van de Vijver, M.J.; van Voorthuisen, A.E. Diagnosis of breast cancer: Contribution of US as an adjunct to mammography. Radiology 1999, 213, 413–422. [Google Scholar] [CrossRef]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Mariapun, S.; Li, J.; Yip, C.H.; Taib, N.A.; Teo, S.H. Ethnic differences in mammographic densities: An Asian cross-sectional study. PLoS ONE 2015, 10, e0117568. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, N.; Mariapun, S.; Eriksson, M.; Tapia, J.; Kwan, P.Y.; Ho, W.K.; Harun, F.; Rahmat, K.; Czene, K.; Taib, N.A.M.; et al. Differences in mammographic density between Asian and Caucasian populations: A comparative analysis. Breast Cancer Res. Treat 2017, 161, 353–362. [Google Scholar] [CrossRef] [PubMed]
- van Gils, C.H.; Otten, J.D.; Verbeek, A.L.; Hendriks, J.H. Short communication: Breast parenchymal patterns and their changes with age. Br. J. Radiol. 1995, 68, 1133–1135. [Google Scholar] [CrossRef]
- Maskarinec, G.; Pagano, I.; Lurie, G.; Wilkens, L.R.; Kolonel, L.N. Mammographic density and breast cancer risk: The multiethnic cohort study. Am. J. Epidemiol. 2005, 162, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Sawada, T.; Akashi, S.; Nakamura, S.; Kuwayama, T.; Enokido, K.; Yoshida, M.; Hashimoto, R.; Ide, T.; Masuda, H.; Taruno, K.; et al. Digital volumetric measurement of mammographic density and the risk of overlooking cancer in Japanese women. Breast Cancer 2017, 24, 708–713. [Google Scholar] [CrossRef]
- Nishiyama, K.; Taira, N.; Mizoo, T.; Kochi, M.; Ikeda, H.; Iwamoto, T.; Shien, T.; Doihara, H.; Ishihara, S.; Kawai, H.; et al. Influence of breast density on breast cancer risk: A case control study in Japanese women. Breast Cancer 2020, 27, 277–283. [Google Scholar] [CrossRef]
- Sung, H.; Rosenberg, P.S.; Chen, W.Q.; Hartman, M.; Lim, W.Y.; Chia, K.S.; Yang, X.R. Female breast cancer incidence among Asian and Western populations: More similar than expected. J. Natl. Cancer Inst. 2015, 107, 107. [Google Scholar] [CrossRef] [Green Version]
- American College of Radiology. Handlungsempfehlungen und Monitoring. In ACR bi-RADS®-Atlas der Mammadiagnostik Richtlinien zu Befundung, 1st ed.; Springer: Heidelberg/Berlin, Germany, 2016. [Google Scholar]
- Sprague, B.L.; Conant, E.F.; Onega, T.; Garcia, M.P.; Beaber, E.F.; Herschorn, S.D.; Lehman, C.D.; Tosteson, A.N.; Lacson, R.; Schnall, M.D.; et al. Variation in mammographic breast density assessments among radiologists in clinical practice: A multicenter observational study. Ann. Intern. Med. 2016, 165, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Melnikow, J.; Fenton, J.J.; Whitlock, E.P.; Miglioretti, D.L.; Weyrich, M.S.; Thompson, J.H.; Shah, K. Supplemental screening for breast cancer in women with dense breasts: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 268–278. [Google Scholar] [CrossRef]
- Gweon, H.M.; Youk, J.H.; Kim, J.A.; Son, E.J. Radiologist assessment of breast density by BI-RADS categories versus fully automated volumetric assessment. AJR Am. J. Roentgenol. 2013, 201, 692–697. [Google Scholar] [CrossRef]
- Youk, J.H.; Gweon, H.M.; Son, E.J.; Kim, J.A. Automated volumetric breast density measurements in the era of the BI-RADS Fifth Edition: A Comparison with Visual Assessment. AJR Am. J. Roentgenol. 2016, 206, 1056–1062. [Google Scholar] [CrossRef]
- Ekpo, E.U.; McEntee, M.F. Measurement of breast density with digital breast tomosynthesis–a systematic review. Br. J. Radiol. 2014, 87, 20140460. [Google Scholar] [CrossRef] [Green Version]
- Wanders, J.O.; Holland, K.; Veldhuis, W.B.; Mann, R.M.; Pijnappel, R.M.; Peeters, P.H.; van Gils, C.H.; Karssemeijer, N. Volumetric breast density affects performance of digital screening mammography. Breast Cancer Res. Treat. 2017, 162, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Brand, J.S.; Czene, K.; Shepherd, J.A.; Leifland, K.; Heddson, B.; Sundbom, A.; Eriksson, M.; Li, J.; Humphreys, K.; Hall, P. Automated measurement of volumetric mammographic density: A tool for widespread breast cancer risk assessment. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1764–1772. [Google Scholar] [CrossRef] [Green Version]
- Mandelson, M.T.; Oestreicher, N.; Porter, P.L.; White, D.; Finder, C.A.; Taplin, S.H.; White, E. Breast density as a predictor of mammographic detection: Comparison of interval- and screen-detected cancers. J. Natl. Cancer Inst. 2000, 92, 1081–1087. [Google Scholar] [CrossRef]
- Benson, S.R.; Blue, J.; Judd, K.; Harman, J.E. Ultrasound is now better than mammography for the detection of invasive breast cancer. Am. J. Surg. 2004, 188, 381–385. [Google Scholar] [CrossRef]
- Welch, H.G.; Prorok, P.C.; O’Malley, A.J.; Kramer, B.S. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N. Engl. J. Med. 2016, 375, 1438–1447. [Google Scholar] [CrossRef]
- Vourtsis, A.; Berg, W.A. Breast density implications and supplemental screening. Eur. Radiol. 2019, 29, 1762–1777. [Google Scholar] [CrossRef]
- Berg, W.A.; Blume, J.D.; Cormack, J.B.; Mendelson, E.B.; Lehrer, D.; Böhm-Vélez, M.; Pisano, E.D.; Jong, R.A.; Evans, W.P.; Morton, M.J.; et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008, 299, 2151–2163. [Google Scholar] [CrossRef]
- Ohuchi, N.; Suzuki, A.; Sobue, T.; Kawai, M.; Yamamoto, S.; Zheng, Y.F.; Shiono, Y.N.; Saito, H.; Kuriyama, S.; Tohno, E.; et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): A randomised controlled trial. Lancet 2016, 387, 341–348. [Google Scholar] [CrossRef]
- Harada-Shoji, N.; Suzuki, A.; Ishida, T.; Zheng, Y.F.; Narikawa-Shiono, Y.; Sato-Tadano, A.; Ohta, R.; Ohuchi, N. Evaluation of adjunctive ultrasonography for breast cancer detection among women aged 40–49 years with varying breast density undergoing screening mammography: A secondary analysis of a randomized clinical trial. JAMA Net. Open 2021, 4, e2121505. [Google Scholar] [CrossRef] [PubMed]
- Wilczek, B.; Wilczek, H.E.; Rasouliyan, L.; Leifland, K. Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. Eur. J. Radiol. 2016, 85, 1554–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, K.M.; Dean, J.; Comulada, W.S.; Lee, S.J. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur. Radiol. 2010, 20, 734–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Zelst, J.C.; Tan, T.; Clauser, P.; Domingo, A.; Dorrius, M.D.; Drieling, D.; Golatta, M.; Gras, F.; de Jong, M.; Pijnappel, R.; et al. Dedicated computer-aided detection software for automated 3D breast ultrasound; an efficient tool for the radiologist in supplemental screening of women with dense breasts. Eur. Radiol. 2018, 28, 2996–3006. [Google Scholar] [CrossRef] [Green Version]
- Comstock, C.E.; Gatsonis, C.; Newstead, G.M.; Snyder, B.S.; Gareen, I.F.; Bergin, J.T.; Rahbar, H.; Sung, J.S.; Jacobs, C.; Harvey, J.A.; et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA 2020, 323, 746–756. [Google Scholar] [CrossRef]
- Gatta, G.; Cappabianca, S.; La Forgia, D.; Massafra, R.; Fanizzi, A.; Cuccurullo, V.; Brunese, S.; Tagliafico, A.; Grassi, R. Second-Generation 3D Automated Breast Ultrasonography (Prone ABUS) for Dense Breast Cancer Screening Integrated to Mammography: Effectiveness, Performance and Detection Rates. J. Pers. Med. 2021, 11, 875. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.H.; Moon, W.K. Automated breast ultrasound screening for dense breasts. Korean J. Radiol. 2020, 21, 15–24. [Google Scholar] [CrossRef]
- Massafra, R.; Bove, S.; Lorusso, V.; Biafora, A.; Comes, M.C.; Didonna, V.; La Forgia, D. Radiomic Feature Reduction Approach to Predict Breast Cancer by Contrast-Enhanced Spectral Mammography Images. Diagnostics 2021, 11, 684. [Google Scholar] [CrossRef]
- Bakker, M.F.; de Lange, S.V.; Pijnappel, R.M.; Mann, R.M.; Peeters, P.; Monninkhof, E.M.; Emaus, M.J.; Loo, C.E.; Bisschops, R.; Lobbes, M.; et al. DENSE Trial Study Group. Supplemental MRI Screening for Women with Extremely Dense Breast Tissue. N. Engl. J. Med. 2019, 381, 2091–2102. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schrading, S.; Strobel, K.; Schild, H.H.; Hilgers, R.D.; Bieling, H.B. Abbreviated breast magnetic resonance imaging (MRI): First postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J. Clin. Oncol. 2014, 32, 2304–2310. [Google Scholar] [CrossRef]
- Friedewald, S.M.; Rafferty, E.A.; Rose, S.L.; Durand, M.A.; Plecha, D.M.; Greenberg, J.S.; Hayes, M.K.; Copit, D.S.; Carlson, K.L.; Cink, T.M.; et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014, 311, 2499–2507. [Google Scholar] [CrossRef] [Green Version]
- Skaane, P.; Bandos, A.I.; Gullien, R.; Eben, E.B.; Ekseth, U.; Haakenaasen, U.; Izadi, M.; Jebsen, I.N.; Jahr, G.; Krager, M.; et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013, 267, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciatto, S.; Houssami, N.; Bernardi, D.; Caumo, F.; Pellegrini, M.; Brunelli, S.; Tuttobene, P.; Bricolo, P.; Fantò, C.; Valentini, M.; et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): A prospective comparison study. Lancet Oncol. 2013, 14, 583–589. [Google Scholar] [CrossRef]
- Lowry, K.P.; Coley, R.Y.; Miglioretti, D.L.; Kerlikowske, K.; Henderson, L.M.; Onega, T.; Sprague, B.L.; Lee, J.M.; Herschorn, S.; Tosteson, A.N.; et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Net. Open 2020, 3, e2011792. [Google Scholar] [CrossRef] [PubMed]
- Tollens, F.; Baltzer, P.A.T.; Dietzel, M.; Rübenthaler, J.; Froelich, M.F.; Kaiser, C.G. Cost-Effectiveness of Digital Breast Tomosynthesis vs. abbreviated Breast MRI for Screening Women with Intermediate Risk of Breast Cancer-How Low-Cost Must MRI Be? Cancers 2021, 13, 1241. [Google Scholar] [CrossRef]
- Gardezi, S.J.S.; Elazab, A.; Lei, B.; Wang, T. Breast cancer detection and diagnosis using mammographic data: Systematic review. J. Med. Internet. Res. 2019, 21, e14464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, A.; Chang, J.M.; Shin, S.U.; Chu, A.J.; Cho, N.; Noh, D.Y.; Moon, W.K. Detection of noncalcified breast cancer in patients with extremely dense breasts using digital breast tomosynthesis compared with full-field digital mammography. Br. J. Radiol. 2019, 92, 20180101. [Google Scholar] [CrossRef]

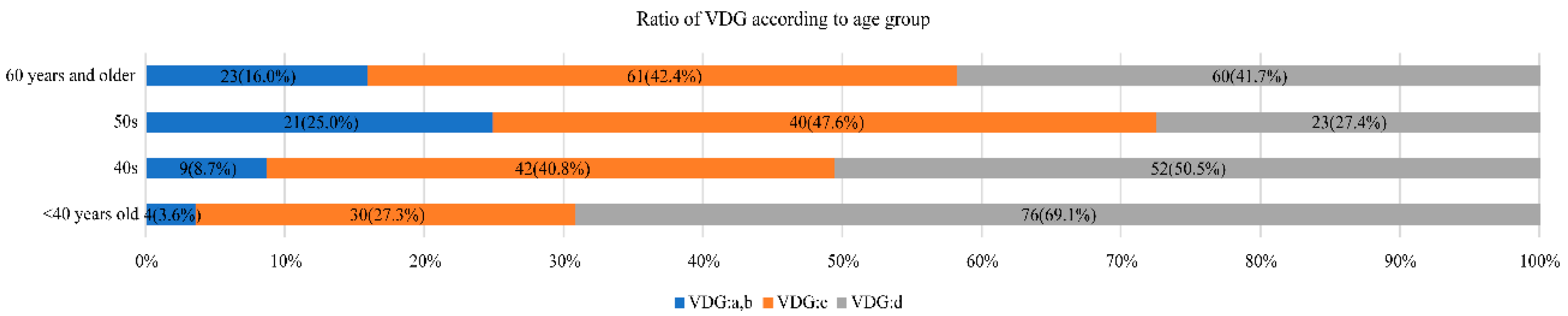


| Characteristic | Value | |
|---|---|---|
| Age | 56 [47, 70] | |
| Volpara density | 14.7% [9.2, 20.7] | |
| Suspicious calcifications | Yes | 193 (43.8%) |
| No | 248 (56.2%) | |
| MMG category | 1.2 (non-detected) | 82 (18.6%) |
| 3.4.5 (detected) | 359 (81.4%) | |
| US category | 1.2 (non-detected) | 48 (10.9%) |
| 3.4.5 (detected) | 393 (89.1%) | |
| Neoadjuvant chemotherapy/preoperative hormone therapy | Yes | 55 (12.5%) |
| No | 386 (87.5%) | |
| Invasion | Yes | 354 (80.3%) |
| No | 87 (19.7%) | |
| Size of invasion | 12.5 mm [5, 22] | |
| Method of diagnosis | Symptomatic | 81 (18.4%) |
| Screening | 147 (33.3%) | |
| Referred | 129 (29.3%) | |
| Others | 84 (19%) |
| Characteristics | Diagnosis by MMG Only | ||
|---|---|---|---|
| Suspicious calcifications | Detected | Non-detected | |
| Yes | 182 | 10 (5.2%) | |
| No | 177 | 72 (28.9%) | |
| Characteristics | Odds Ratio | 95% Confidence Interval | p | |
|---|---|---|---|---|
| Age | 0.99 | 0.97–1.01 | 0.3 | |
| VDG | 1.07 | 1.04–1.11 | <0.001 | |
| Suspicious calcifications | Yes | 1.00 (reference) | ||
| No | 9.2 | 4.68–19.85 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, R.; Yamauchi, T.; Akashi-Tanaka, S.; Matsuyanagi, M.; Taruno, K.; Sawada, T.; Kokaze, A.; Nakamura, S. Optimal Breast Density Characterization Using a Three-Dimensional Automated Breast Densitometry System. Curr. Oncol. 2021, 28, 5384-5394. https://doi.org/10.3390/curroncol28060448
Yoshida R, Yamauchi T, Akashi-Tanaka S, Matsuyanagi M, Taruno K, Sawada T, Kokaze A, Nakamura S. Optimal Breast Density Characterization Using a Three-Dimensional Automated Breast Densitometry System. Current Oncology. 2021; 28(6):5384-5394. https://doi.org/10.3390/curroncol28060448
Chicago/Turabian StyleYoshida, Reika, Takenori Yamauchi, Sadako Akashi-Tanaka, Misaki Matsuyanagi, Kanae Taruno, Terumasa Sawada, Akatsuki Kokaze, and Seigo Nakamura. 2021. "Optimal Breast Density Characterization Using a Three-Dimensional Automated Breast Densitometry System" Current Oncology 28, no. 6: 5384-5394. https://doi.org/10.3390/curroncol28060448
APA StyleYoshida, R., Yamauchi, T., Akashi-Tanaka, S., Matsuyanagi, M., Taruno, K., Sawada, T., Kokaze, A., & Nakamura, S. (2021). Optimal Breast Density Characterization Using a Three-Dimensional Automated Breast Densitometry System. Current Oncology, 28(6), 5384-5394. https://doi.org/10.3390/curroncol28060448






