Management of Localized Muscle-Invasive Bladder Cancer from a Multidisciplinary Perspective: Current Position of the Spanish Oncology Genitourinary (SOGUG) Working Group
Abstract
:1. Introduction
2. Subclassification of Urothelial Carcinoma in Different Molecular Groups
Challenges and Recommendations
- To establish a correct classification of histopathological subtypes of MIBC, particularly the identification of subtypes with prognostic, molecular and therapeutic implications.
- Immunohistochemical techniques should be used in order to distinguish between basal and luminal subtypes.
- In relation to the molecular classification of MIBC, luminal subtypes as well as stroma-rich and neuroendocrine-like ones are difficult to characterize.
- PD-L1 is still the most relevant biomarker in urothelial cancer, and the association of its expression with some variants merits further investigation.
3. Integrated Assessment of Patient Candidates for Radical Cystectomy
Challenges and Recommendations
- In patients older than 80 years, it is necessary to include a geriatric assessment, and evaluation of comorbidities and frailty. The presence of comorbid diseases may be more relevant than the age per se.
- In the framework of ERAS protocols, although postoperative measures are the most relevant, preoperative assessment of candidates for radical cystectomy by the corresponding specialists is recommended, as well as the adequate preparation of patients regarding his/her condition and imitations after surgery.
- Outcomes could be improved by centralization of care in high-volume centers with experienced multidisciplinary teams.
4. Bladder Preservation and New Molecular Classifications
Challenges and Recommendations
- Bladder preservation may be an adequate strategy in the management of MIBC based on selection of the appropriate candidates.
- Bladder preservation does not compete with radical cystectomy, it is simply a complementary alternative.
- Bladder preservation strategies cannot be implemented in clinical practice without the presence of a urologist responsible for performing three main activities for success: maximal TUR, follow-up cystectomy and salvage cystectomy.
- Transdisciplinary collaboration needs to be potentiated for generating, sharing, and integrating knowledge, coordination of patient’s care activities, and to converge in the research effort.
5. Neoadjuvant Chemotherapy and Integration of Immunotherapy
Challenges and Recommendations
- Improvement of clinical staging, better definition of prognostic groups based on molecular subtypes, and identification of biomarkers potentially associated with maximum benefit from neoadjuvant chemotherapy are areas for further research.
- A current challenge in the management of MIBC is to improve the selection of patients likely to be candidates for immunotherapy with checkpoint inhibitors in the neoadjuvant setting.
- Patients with complete response (pT0) after neoadjuvant treatment may be suitable for bladder preservation procedures.
- Neoadjuvant therapy is not a widespread practice, although patients treated with neoadjuvant chemotherapy/immunotherapy present a better condition for undergoing cystectomy.
- The implementation of multidisciplinary teams will extend the use of neoadjuvant therapy and improve outcomes.
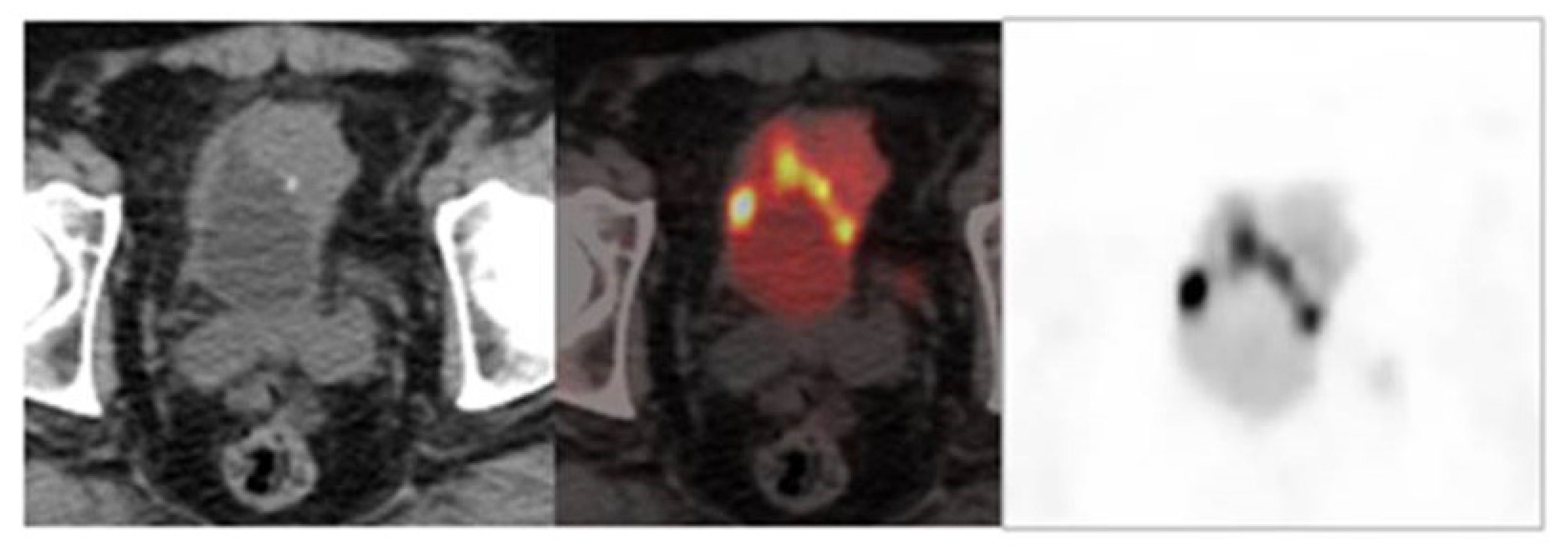
6. Role of Imaging Techniques in Staging, Assessment of Response, and Follow-Up
6.1. Nuclear Medicine
Challenges and Recommendations
- Optimization of FDG-PET/CT reliability in staging of MIBC.
- Design of prospective studies aimed to compare the value of different radioimaging techniques in parallel, and to define the impact of FDG-PET/CT in the selection of patients.
- To determine which imaging technique is most effective for predicting response after neoadjuvant chemotherapy or a guide for endoscopic biopsy.
- To unify the methodology for acquisition and interpretation of FDG-PET/CT.
- To define predictive risk models in which FDG-PET/CT would have the highest reliability in the detection of recurrence.
- To perform prospective studies for the comparison in parallel of FDG-PET/CT with other techniques for detecting recurrence in intermediate/high-risk patients.
6.2. Radiology
Challenges and Recommendations
- DWI has a promising role in the assessment of response to treatment, but evidence based on prospective studies is needed.
- MRI should be optimized for the differentiation of recurrence versus inflammation and fibrosis, as well as for the assessment of lymph node status.
- The interaction of different radiological techniques in the diagnosis of MIBC should be improved.
- MRI is the technique of choice for local staging, but it needs to be widely available and reproducible among hospitals.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cumberbatch, M.G.K.; Noon, A.P. Epidemiology, aetiology and screening of bladder cancer. Transl. Androl. Urol. 2019, 8, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Dall’Era, M.A.; Cheng, L.; Pan, C.X. Contemporary management of muscle-invasive bladder cancer. Expert Rev. Anticancer Ther. 2012, 12, 941–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apolo, A.B.; Vogelzang, N.J.; Theodorescu, D. New and promising strategies in the management of bladder cancer. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 105–112. [Google Scholar] [CrossRef]
- Gust, K.M.; Rebhan, K.; Resch, I.; Shariat, S.F.; Necchi, A. Immune checkpoint inhibition in muscle-invasive and locally advanced bladder cancer. Curr. Opin. Urol. 2020, 30, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltran, A.; Cimadamore, A.; Blanca, A.; Massari, F.; Vau, N.; Scarpelli, M.; Cheng, L.; Montironi, R. Immune checkpoint inhibitors for the treatment of bladder Cancer. Cancers 2021, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Walk, E.E. The role of pathologists in the era of personalized medicine. Arch. Pathol. Lab. Med. 2009, 133, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltran, A.; Henriques, V.; Montironi, R.; Cimadamore, A.; Raspollini, M.R.; Cheng, L. Variants and new entities of bladder cancer. Histopathology 2019, 74, 77–96. [Google Scholar] [CrossRef] [Green Version]
- College of American Pathologists. Protocol for the Examination of Specimens from Patients with Carcinoma of the Urinary Bladder. Available online: https://documents.cap.org/protocols/cp-urinary-bladder-17protocol-4010.pdf (accessed on 18 June 2021).
- Ahmad, I.; Sansom, O.J.; Leung, H.Y. Exploring molecular genetics of bladder cancer: Lessons learned from mouse models. Dis. Model. Mech. 2012, 5, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majewski, T.; Lee, S.; Jeong, J.; Yoon, D.-S.; Kram, A.; Kim, M.-S.; Tuziak, T.; Bondaruk, J.; Lee, S.; Park, W.-S.; et al. Understanding the development of human bladder cancer by using a whole-organ genomic mapping strategy. Lab. Investig. 2008, 88, 694–721. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.-L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadhania, V.; Zhang, M.; Zhang, L.; Bondaruk, J.; Majewski, T.; Siefker-Radtke, A.; Guo, C.C.; Dinney, C.; Cogdell, D.E.; Zhang, S.; et al. Meta-Analysis of the Luminal and Basal Subtypes of Bladder Cancer and the Identification of Signature Immunohistochemical Markers for Clinical Use. EBioMedicine 2016, 12, 105–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satyal, U.; Sikder, R.K.; McConkey, D.; Plimack, E.R.; Abbosh, P.H. Clinical implications of molecular subtyping in bladder cancer. Curr. Opin. Urol. 2019, 29, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Seiler, R.; Ashab, H.A.D.; Erho, N.; van Rhijn, B.W.; Winters, B.; Douglas, J.; Van Kessel, K.E.; van de Putte, E.E.F.; Sommerlad, M.; Wang, N.Q.; et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur. Urol. 2017, 72, 544–554. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef] [Green Version]
- Zajac, M.; Scott, M.; Ratcliffe, M.; Scorer, P.; Barker, C.; Al-Masri, H.; Rebelatto, M.C.; Walker, J. Concordance among four commercially available, validated programmed cell death ligand-1 assays in urothelial carcinoma. Diagn. Pathol. 2019, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Warrick, J.I.; Kaag, M.; Raman, J.D.; Chan, W.; Tran, T.; Kunchala, S.; Shuman, L.; DeGraff, D.; Chen, G. FOXA1 and CK14 as markers of luminal and basal subtypes in histologic variants of bladder cancer and their associated conventional urothelial carcinoma. Virchows Arch. 2017, 471, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Majewski, T.; Zhang, L.; Yao, H.; Bondaruk, J.; Wang, Y.; Zhang, S.; Wang, Z.; Lee, J.G.; Lee, S.; et al. Dysregulation of EMT Drives the Progression to Clinically Aggressive Sarcomatoid Bladder Cancer. Cell Rep. 2019, 27, 1781–1793.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin. Cancer Res. 2017, 23, 4242–4250. [Google Scholar] [CrossRef] [Green Version]
- Chahal, R.; Sundaram, S.; Iddenden, R.; Forman, D.; Weston, P.; Harrison, S. A Study of the Morbidity, Mortality and Long-Term Survival Following Radical Cystectomy and Radical Radiotherapy in the Treatment of Invasive Bladder Cancer in Yorkshire. Eur. Urol. 2003, 43, 246–257. [Google Scholar] [CrossRef]
- Llorente, C.; Guijarro, A.; Hernandez, V.; Fernández-Conejo, G.; Perez-Fernandez, E.; Pocock, S. Effect of hospital volume on 90-day mortality after radical cystectomy for bladder cancer in Spain. World J. Urol. 2020, 38, 1221–1228. [Google Scholar] [CrossRef]
- Shabsigh, A.; Korets, R.; Vora, K.C.; Brooks, C.M.; Cronin, A.M.; Savage, C.; Raj, G.; Bochner, B.; Dalbagni, G.; Herr, H.W.; et al. Defining Early Morbidity of Radical Cystectomy for Patients with Bladder Cancer Using a Standardized Reporting Methodology. Eur. Urol. 2009, 55, 164–176. [Google Scholar] [CrossRef]
- Izquierdo, L.; Peri, L.; Leon, P.; Ramírez-Backhaus, M.; Manning, T.; Alcaraz, A.; Rouprêt, M.; Solsona, E.; Rubio, J.; Sengupta, S.; et al. The role of cystectomy in elderly patients—A multicentre analysis. BJU Int. 2015, 116 (Suppl. 3), 73–79. [Google Scholar] [CrossRef] [Green Version]
- Scarberry, K.; Berger, N.G.; Scarberry, K.B.; Agrawal, S.; Francis, J.J.; Yih, J.M.; Gonzalez, C.M.; Abouassaly, R. Improved surgical outcomes following radical cystectomy at high-volume centers influence overall survival. Urol. Oncol. 2018, 36, 308.e11–308.e17. [Google Scholar] [CrossRef] [PubMed]
- Karl, A.; Buchner, A.; Becker, A.; Staehler, M.; Seitz, M.; Khoder, W.; Schneevoigt, B.; Weninger, E.; Rittler, P.; Grimm, T.; et al. A New Concept for Early Recovery after Surgery for Patients Undergoing Radical Cystectomy for Bladder Cancer: Results of a Prospective Randomized Study. J. Urol. 2014, 191, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Llorente, C.; Guijarro, A.; Hernández, V.; Fernández-Conejo, G.; Passas, J.; Aguilar, L.; Tejido, A.; Moralejo, M.; Subirá, D.; González-Enguita, C.; et al. Outcomes of an enhanced recovery after radical cystectomy program in a prospective multicenter study: Compliance and key components for success. World J. Urol. 2020, 38, 3121–3129. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Jani, A.B.; Efstathiou, J.A.; Shipley, W.U. Bladder Preservation Strategies. Hematol. Oncol. Clin. N. Am. 2015, 29, 289–300. [Google Scholar] [CrossRef]
- Giacalone, N.J.; Shipley, W.U.; Clayman, R.H.; Niemierko, A.; Drumm, M.; Heney, N.M.; Michaelson, M.D.; Lee, R.J.; Saylor, P.J.; Wszolek, M.F.; et al. Long-term Outcomes After Bladder-preserving Tri-modality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur. Urol. 2017, 71, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Grabenbauer, G.G.; Kühn, R.; Papadopoulos, T.; Dunst, J.; Meyer, M.; Schrott, K.M.; Sauer, R. Combined-Modality Treatment and Selective Organ Preservation in Invasive Bladder Cancer: Long-Term Results. J. Clin. Oncol. 2002, 20, 3061–3071. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Hussain, S.; Hall, E.; Jenkins, P.; Tremlett, J.; Rawlings, C.; Crundwell, M.; Sizer, B.; Sreenivasan, T.; Hendron, C.; et al. Radiotherapy with or without Chemotherapy in Muscle-Invasive Bladder Cancer. N. Engl. J. Med. 2012, 366, 1477–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppin, C.M.; Gospodarowicz, M.K.; James, K.; Tannock, I.F.; Zee, B.C.-Y.; Carson, J.; Pater, J.; Sullivan, L.D. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. J. Clin. Oncol. 1996, 14, 2901–2907. [Google Scholar] [CrossRef]
- Song, Y.P.; Mistry, H.; Choudhury, A.; Hoskin, P.; More, S. Long-term outcomes of hypoxia modification in bladder preservation: Update from BCON trial. J. Clin. Oncol. 2019, 37 (Suppl. 7), 356. [Google Scholar] [CrossRef]
- Coen, J.J.; Zhang, P.; Saylor, P.J.; Lee, C.T.; Wu, C.-L.; Parker, W.; Lautenschlaeger, T.; Zietman, A.L.; Efstathiou, J.A.; Jani, A.B.; et al. Bladder Preservation With Twice-a-Day Radiation Plus Fluorouracil/Cisplatin or Once Daily Radiation Plus Gemcitabine for Muscle-Invasive Bladder Cancer: NRG/RTOG 0712—A Randomized Phase II Trial. J. Clin. Oncol. 2019, 37, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.L.; Deal, A.M.; Ladoire, S.; Créhange, G.; Galsky, M.D.; Rosenberg, J.E.; Bellmunt, J.; Wimalasingham, A.; Wong, Y.-N.; Harshman, L.C.; et al. Patterns of Bladder Preservation Therapy Utilization for Muscle-Invasive Bladder Cancer. Bladder Cancer 2016, 2, 405–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuschke, M.; Thames, H.D. Hyperfractionated radiotherapy of human tumors: Overview of the randomized clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 259–267. [Google Scholar] [CrossRef]
- Horwich, A.; Dearnaley, D.; Huddart, R.; Graham, J.; Bessell, E.; Mason, M.; Bliss, J. A randomised trial of accelerated radiotherapy for localised invasive bladder cancer. Radiother. Oncol. 2005, 75, 34–43. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, J.; González, D.; van Rhoon, G.C.; van Dijk, J.D.; van Putten, W.L.; Hart, A.A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef]
- D’Rummo, K.A.; TenNapel, M.J.; Shen, X. The Impact of Radiotherapy Facility Volume on the Survival and Guideline Concordance of Patients with Muscle-invasive Bladder Cancer Receiving Bladder-preservation Therapy. Am. J. Clin. Oncol. 2019, 42, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Premo, C.; Apolo, A.B.; Agarwal, P.K.; Citrin, D.E. Trimodality Therapy in Bladder Cancer: Who, What, and When? Urol. Clin. North Am. 2015, 42, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, D.T.; Mouw, K.W.; Feng, F.Y.; Shipley, W.U.; A Efstathiou, J. Molecular biomarkers in bladder preservation therapy for muscle-invasive bladder cancer. Lancet Oncol. 2018, 19, e683–e695. [Google Scholar] [CrossRef]
- Choudhury, A.; Nelson, L.D.; Teo, M.T.; Chilka, S.; Bhattarai, S.; Johnston, C.F.; Elliott, F.; Lowery, J.; Taylor, C.F.; Churchman, M.; et al. MRE11 Expression Is Predictive of Cause-Specific Survival following Radical Radiotherapy for Muscle-Invasive Bladder Cancer. Cancer Res. 2010, 70, 7017–7026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurberg, J.R.; Brems-Eskildsen, A.S.; Nordentoft, I.K.; Fristrup, N.; Schepeler, T.; Ulhøi, B.P.; Agerbaek, M.; Hartmann, A.; Bertz, S.; Wittlinger, M.; et al. Expression of TIP60 (tat-interactive protein) and MRE11 (meiotic recombination 11 homolog) predict treatment-specific outcome of localised invasive bladder cancer. BJU Int. 2012, 110 Pt C, E1228–E1236. [Google Scholar] [CrossRef]
- Teo, M.T.W.; Dyrskjøt, L.; Nsengimana, J.; Buchwald, C.; Snowden, H.; Morgan, J.; Jensen, J.B.; Knowles, M.A.; Taylor, G.; Barrett, J.; et al. Next-generation sequencing identifies germline MRE11A variants as markers of radiotherapy outcomes in muscle-invasive bladder cancer. Ann. Oncol. 2014, 25, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Huddart, R.A.; Birtle, A.; Maynard, L.; Beresford, M.; Blazeby, J.; Donovan, J.; Kelly, J.D.; Kirkbank, T.; McLaren, D.; Mead, G.M.; et al. Clinical and patient-reported outcomes of SPARE—A randomised feasibility study of selective bladder preservation versus radical cystectomy. BJU Int. 2017, 120, 639–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galsky, M.D.; Daneshmand, S.; Chan, K.G.; Dorff, T.B.; Cetnar, J.P.; O Neil, B.; D’Souza, A.; Mamtani, R.; Kyriakopoulos, C.; Garcia, P.; et al. Phase 2 trial of gemcitabine, cisplatin, plus nivolumab with selective bladder sparing in patients with muscle- invasive bladder cancer (MIBC): HCRN GU 16-257. J. Clin. Oncol. 2021, 39, 4503. [Google Scholar] [CrossRef]
- Balar, A.V.; Milowsky, M.I.; O’Donnell, P.H.; Alva, A.S.; Kollmeier, M.; Rose, T.L.; Pitroda, S.; Kaffenberger, S.D.; Rosenberg, J.E.; Francese, K.; et al. Pembrolizumab (pembro) in combination with gemcitabine (Gem) and concurrent hypofractionated radiation therapy (RT) as bladder sparing treatment for muscle-invasive urothelial cancer of the bladder (MIBC): A multicenter phase 2 trial. J. Clin. Oncol. 2021, 39, 4504. [Google Scholar] [CrossRef]
- del Muro, X.G.; Valderrama, B.P.; Medina, A.; Cuellar, M.A.; Etxaniz, O.; Sarrió, R.G.; Juan-Fita, M.J.; Ferrer, F.; Rodríguez, I.M.; Lendínez-Cano, G.; et al. Phase II trial of durvalumab plus tremelimumab with concurrent radiotherapy (RT) in patients (pts) with localized muscle invasive bladder cancer (MIBC) treated with a selective bladder preservation approach: IMMUNOPRESERVE-SOGUG trial. J. Clin. Oncol. 2021, 39, 4505. [Google Scholar] [CrossRef]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 2005, 48, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.; Vogelzang, N.J.; Trump, D.L.; White, R.W.D.; Sarosdy, M.F.; Wood, D.P.; Raghavan, D.; et al. Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Tully, C.M.; Zabor, E.C.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; Donat, S.M.; Russo, P.; Ostrovnaya, I.; Regazzi, A.M.; et al. Neoadjuvant Gemcitabine-Cisplatin Plus Radical Cystectomy-Pelvic Lymph Node Dissection for Muscle-invasive Bladder Cancer: A 12-year Experience. Clin. Genitourin. Cancer 2020, 18, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Pettus, J.A., 4th; Herr, H.W.; Bochner, B.; Dalbagni, G.; Donat, S.; Russo, P.; Boyle, M.G.; Milowsky, M.I.; Bajorin, D.F. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: A retrospective experience. Cancer 2008, 113, 2471–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeshchina, O.; Badalato, G.M.; Wosnitzer, M.S.; Hruby, G.; RoyChoudhury, A.; Benson, M.C.; Petrylak, D.P.; McKiernan, J.M. Relative Efficacy of Perioperative Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Adriamycin, and Cisplatin in the Management of Locally Advanced Urothelial Carcinoma of the Bladder. Urology 2012, 79, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Jacobus, S.; Bellmunt, J.; Qu, A.; Appleman, L.; Tretter, C.; Bubley, G.J.; Stack, E.C.; Signoretti, S.; Walsh, M.; et al. Neoadjuvant Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin With Pegfilgrastim Support in Muscle-Invasive Urothelial Cancer: Pathologic, Radiologic, and Biomarker Correlates. J. Clin. Oncol. 2014, 32, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Plimack, E.R.; Hoffman-Censits, J.H.; Viterbo, R.; Trabulsi, E.J.; Ross, E.A.; Greenberg, R.E.; Chen, D.Y.; Lallas, C.D.; Wong, Y.-N.; Lin, J.; et al. Accelerated Methotrexate, Vinblastine, Doxorubicin, and Cisplatin Is Safe, Effective, and Efficient Neoadjuvant Treatment for Muscle-Invasive Bladder Cancer: Results of a Multicenter Phase II Study with Molecular Correlates of Response and Toxicity. J. Clin. Oncol. 2014, 32, 1895–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zargar, H.; Shoshtari, K.Z.; Lotan, Y.; Shah, J.B.; Van Rhijn, B.W.; Daneshmand, S.; Spiess, P.E.; Black, P.; Fairey, L.S.M.C.A.S.; Mertens, L.S.; et al. Final Pathological Stage after Neoadjuvant Chemotherapy and Radical Cystectomy for Bladder Cancer—Does pT0 Predict Better Survival than pTa/Tis/T1? J. Urol. 2016, 195 Pt 1, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as Neoadjuvant Therapy before Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2018, 36, 3353–3360. Available online: https://doi-org.sire.ub.edu/10.1200/JCO.18.01148 (accessed on 28 June 2021). [CrossRef] [PubMed] [Green Version]
- Necchi, A.; Raggi, D.; Gallina, A.; Madison, R.; Colecchia, M.; Lucianò, R.; Montironi, R.; Giannatempo, P.; Farè, E.; Pederzoli, F.; et al. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur. Urol. 2020, 77, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Grande, E.; Guerrero, F.; Puente, J.; Galante, I.; Duran, I.; Dominguez, M.; Gordoa, T.A.; Burgos, J.; Font, A.; Pinto, A.; et al. DUTRENEO Trial: A Randomized Phase II Trial of DUrvalumab and TREmelimumab versus Chemotherapy as a NEOadjuvant Approach to Muscle-Invasive Urothelial Bladder Cancer (MIBC) Patients (pts) Prospectively Selected by an Interferon (INF)-Gamma Immune Signature. J. Clin. Oncol. 2020, 38, 5012. Available online: https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.5012 (accessed on 22 June 2021). [CrossRef]
- Bladder cancer: Diagnosis and management of bladder cancer: © NICE (2015) Bladder cancer: Diagnosis and management of bladder cancer. BJU Int. 2017, 120, 755–765. [CrossRef] [PubMed] [Green Version]
- Expert Panel on Urologic Imaging; van der Pol, C.; Sahni, V.A.; Eberhardt, S.C.; Oto, A.; Akin, O.; Alexander, L.; Allen, B.C.; Coakley, F.V.; Froemming, A.T.; et al. ACR Appropriateness Criteria ® Pretreatment Staging of Muscle-Invasive Bladder Cancer. J. Am. Coll. Radiol. 2018, 15, S150–S159. [Google Scholar] [CrossRef] [Green Version]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. Available online: https://jnccn.org/view/journals/jnccn/18/3/article-p329.xml (accessed on 23 June 2021).
- Einerhand, S.M.; van Gennep, E.J.; Mertens, L.S.; Hendricksen, K.; Donswijk, M.L.; van der Poel, H.G.; van Rhijn, B.W. 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in muscle-invasive bladder cancer. Curr. Opin. Urol. 2020, 30, 654–664. [Google Scholar] [CrossRef]
- Kollberg, P.; Almquist, H.; Bläckberg, M.; Cronberg, C.; Garpered, S.; Gudjonsson, S.; Kleist, J.; Lyttkens, K.; Patschan, O.; Liedberg, F. [(18)F]Fluorodeoxyglucose—Positron emission tomography/computed tomography improves staging in patients with high-risk muscle-invasive bladder cancer scheduled for radical cystectomy. Scand. J. Urol. 2014, 49, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.K.; Koo, P.J.; Kim, S.-J. Diagnostic Accuracy of F-18 FDG PET/CT for Preoperative Lymph Node Staging in Newly Diagnosed Bladder Cancer Patients: A Systematic Review and Meta-Analysis. Oncology 2018, 95, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Koo, P.J.; Pak, K.; Kim, I.-J.; Kim, K. Diagnostic accuracy of C-11 choline and C-11 acetate for lymph node staging in patients with bladder cancer: A systematic review and meta-analysis. World J. Urol. 2018, 36, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Soubra, A.; Hayward, D.; Dahm, P.; Goldfarb, R.; Froehlich, J.; Jha, G.; Konety, B.R. The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: A single-institution study and a systematic review with meta-analysis. World J. Urol. 2016, 34, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. The Diagnostic Performance of MRI for Detection of Lymph Node Metastasis in Bladder and Prostate Cancer: An Updated Systematic Review and Diagnostic Meta-Analysis. Am. J. Roentgenol. 2018, 210, W95–W109. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Dogra, P.N.; Naswa, N.; Kumar, R. Diuretic 18F-FDG PET/CT imaging for detection and locoregional staging of urinary bladder cancer: Prospective evaluation of a novel technique. Eur. J. Nucl. Med. Mol. Imaging 2012, 40, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Brant, A.; Nichols, P.; Kates, M.; Reese, A.; Hahn, N.; Schoenberg, M.; Bivalacqua, T. Inaccuracy of clinical staging after neoadjuvant chemotherapy for muscle invasive bladder cancer. (Abstract MP78-10). J. Urol. 2018, 199, e1040–e1041. [Google Scholar] [CrossRef] [Green Version]
- Asghar, A.; Parker, D.; McGowan, T.; Greenberg, R.; Smaldone, M.; Chen, D.; Viterbo, R.; Uzzo, R.; Bloom, E.; Geynisman, D.; et al. Prospective evaluation of cystoscopy and bladder mapping reveals nearly a 30% miss rate for ≥PT2 pathology. (Abstract PD66-06). J. Urol. 2019, 201 (Suppl. 4), e1194. [Google Scholar]
- Kollberg, P.; Almquist, H.; Bläckberg, M.; Cwikiel, M.; Gudjonsson, S.; Lyttkens, K.; Patschan, O.; Liedberg, F. [18F]Fluorodeoxyglucose-positron emission tomography/computed tomography response evaluation can predict histological response at surgery after induction chemotherapy for oligometastatic bladder cancer. Scand. J. Urol. 2017, 51, 308–313. [Google Scholar] [CrossRef] [PubMed]
- van de Putte, E.E.F.; Vegt, E.; Mertens, L.S.; Bruining, A.; Hendricksen, K.; Van Der Heijden, M.S.; Horenblas, S.; Van Rhijn, B.W.G. FDG-PET/CT for response evaluation of invasive bladder cancer following neoadjuvant chemotherapy. Int. Urol. Nephrol. 2017, 49, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Soubra, A.; Gencturk, M.; Froelich, J.; Balaji, P.; Gupta, S.; Jha, G.; Konety, B.R. FDG-PET/CT for Assessing the Response to Neoadjuvant Chemotherapy in Bladder Cancer Patients. Clin. Genitourin. Cancer 2018, 16, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Eulitt, P.J.; Altun, E.; Sheikh, A.; Wong, T.Z.; Woods, M.E.; Rose, T.L.; Wallen, E.M.; Pruthi, R.S.; Smith, A.B.; Nielsen, M.E.; et al. Pilot Study of [18F] Fluorodeoxyglucose Positron Emission Tomography (FDG-PET)/Magnetic Resonance Imaging (MRI) for Staging of Muscle-invasive Bladder Cancer (MIBC). Clin. Genitourin. Cancer 2020, 18, 378–386.e1. [Google Scholar] [CrossRef]
- Panebianco, V.; Narumi, Y.; Altun, E.; Bochner, B.H.; Efstathiou, J.A.; Hafeez, S.; Huddart, R.; Kennish, S.; Lerner, S.; Montironi, R.; et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur. Urol. 2018, 74, 294–306. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Shang, Y.; Luan, T.; Duan, Y.; Wang, J.; Wang, H.; Hao, J. Evaluation of the value of the VI-RADS scoring system in assessing muscle infiltration by bladder cancer. Cancer Imaging 2020, 20, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Thoeny, H.C.; Bellin, M.-F.; Comperat, E.-M.; Thalmann, G.N. Vesical Imaging-Reporting and Data System (VI-RADS): Added Value for Management of Bladder Cancer Patients? Eur. Urol. 2018, 74, 307–308. [Google Scholar] [CrossRef] [PubMed]

| Candidates | Characteristics |
|---|---|
| Ideal candidate | T2 stage |
| No hydronephrosis | |
| No carcinoma in situ (CIS) | |
| Visibly complete TURBT | |
| Unifocal tumor | |
| Good bladder function and capacity | |
| Less than ideal candidate | T3a stage |
| Incomplete TURBT | |
| Poor bladder function or capacity | |
| Relative contraindications | T3b-T4a stage |
| Diffuse CIS | |
| Lymph node positive disease | |
| Absolute contraindications | T4b stage |
| Tumor-related hydronephrosis | |
| Prostatic stromal invasion | |
| Prior pelvic radiation therapy | |
| Not being a candidate for chemotherapy |
| Mechanism | Biomarker | Value | Clinical Correlation |
|---|---|---|---|
| DNA repair genes | MRE11 | Predictive | Disease-specific survival |
| ERCC1 | Prognostic | Disease-specific survival | |
| Signal transduction genes | EGFR | Prognostic | Disease-specific survival |
| HER2 | Prognostic | Disease-specific survival | |
| VEGF | Prognostic | Overall survival | |
| Immune checkpoints | PD-L1 | Prognostic | Local recurrence-free survival |
| Molecular signatures | Hypoxia | Predictive | Local recurrence-free survival |
| Immune response | Predictive | Disease-specific survival |
| Study (Reference) | Patients | Scheme | IO | RTP | QMT | Primary Endpoint | Result |
|---|---|---|---|---|---|---|---|
| HCRN GU 16-257 [48] | 76 | QMT-IO | Nivolumab | No | Gem/Cis | cCR | cCR 48% BI-DFS 78% (1 year) |
| NCT02621151 [49] | 54 | IO-TUR- IO/CRTR-TUR | Pembrolizumab | 64 Gy (hypo) | Gem | BI-DFS (2 years) | cCR 80% BI-DFS 88% (1 year) |
| Immunopreserve- SOGUG [50] | 32 | TUR-IO/RTP-TUR | Durvalumab + tremelimumab | 64 Gy (conv) | No | cCR | cCR 78% BI-DFS 73% (1 year) |
| First Author (Reference) | Patients No. | Regimen | Pathological Stage | ||
|---|---|---|---|---|---|
| pT0N0, % | <pT2N0, % | ≥pT2, % | |||
| Grossman [52] | 126 | MVAC | 38 | 44 | 56 |
| Iyer [53] | 154 | Gemcitabine-cisplatin | 21 | 46 | 56 |
| Dash [54] | 42 | Gemcitabine-cisplatin | 26 | 36 | 64 |
| Yeshchina [55] | 37 | Gemcitabine-cisplatin | 25 | 50 | 50 |
| Choueiri [56] | 39 | ddMVAC | 26 | 49 | 51 |
| Plimack [57] | 44 | ddMVAC | 38 | 53 | 47 |
| First Author (Reference) | Technique | Number of Studies (Number of Patients) | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|
| Ha [68] | 18F-FDG-PET/CT | 14 (785) | 57 (49–64)) | 92 (87–95) |
| Kim [69] | C-11 choline and C-11 acetate PET/CT | 10 (282) | 66 (54–75) | 89 (76–95) |
| Soubra [70] | 18F-FDG-PET/CT | Single-center (78) | 56 (29–80) | 98 (91–100) |
| Woo [71] | MRI | 24 (2928) | 56 (42–69) (per-patient) | 94 (90–96) (per patient) |
| 57 (29–82) (per-lymph node | 97 (94–98) (per-lymph node) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Caamaño, A.; García Vicente, A.M.; Maroto, P.; Rodríguez Antolín, A.; Sanz, J.; Vera González, M.A.; Climent, M.Á.; on behalf of the Spanish Oncology Genitourinary (SOGUG) Multisiciplinary Working Group. Management of Localized Muscle-Invasive Bladder Cancer from a Multidisciplinary Perspective: Current Position of the Spanish Oncology Genitourinary (SOGUG) Working Group. Curr. Oncol. 2021, 28, 5084-5100. https://doi.org/10.3390/curroncol28060428
Gómez Caamaño A, García Vicente AM, Maroto P, Rodríguez Antolín A, Sanz J, Vera González MA, Climent MÁ, on behalf of the Spanish Oncology Genitourinary (SOGUG) Multisiciplinary Working Group. Management of Localized Muscle-Invasive Bladder Cancer from a Multidisciplinary Perspective: Current Position of the Spanish Oncology Genitourinary (SOGUG) Working Group. Current Oncology. 2021; 28(6):5084-5100. https://doi.org/10.3390/curroncol28060428
Chicago/Turabian StyleGómez Caamaño, Antonio, Ana M. García Vicente, Pablo Maroto, Alfredo Rodríguez Antolín, Julián Sanz, María Almudena Vera González, Miguel Ángel Climent, and on behalf of the Spanish Oncology Genitourinary (SOGUG) Multisiciplinary Working Group. 2021. "Management of Localized Muscle-Invasive Bladder Cancer from a Multidisciplinary Perspective: Current Position of the Spanish Oncology Genitourinary (SOGUG) Working Group" Current Oncology 28, no. 6: 5084-5100. https://doi.org/10.3390/curroncol28060428
APA StyleGómez Caamaño, A., García Vicente, A. M., Maroto, P., Rodríguez Antolín, A., Sanz, J., Vera González, M. A., Climent, M. Á., & on behalf of the Spanish Oncology Genitourinary (SOGUG) Multisiciplinary Working Group. (2021). Management of Localized Muscle-Invasive Bladder Cancer from a Multidisciplinary Perspective: Current Position of the Spanish Oncology Genitourinary (SOGUG) Working Group. Current Oncology, 28(6), 5084-5100. https://doi.org/10.3390/curroncol28060428





