Durable Response to Brentuximab Vedotin Plus Cyclophosphamide, Doxorubicin, and Prednisone (BV-CHP) in a Patient with CD30-Positive PTCL Arising as a Post-Transplant Lymphoproliferative Disorder (PTLD)
Abstract
:1. Introduction
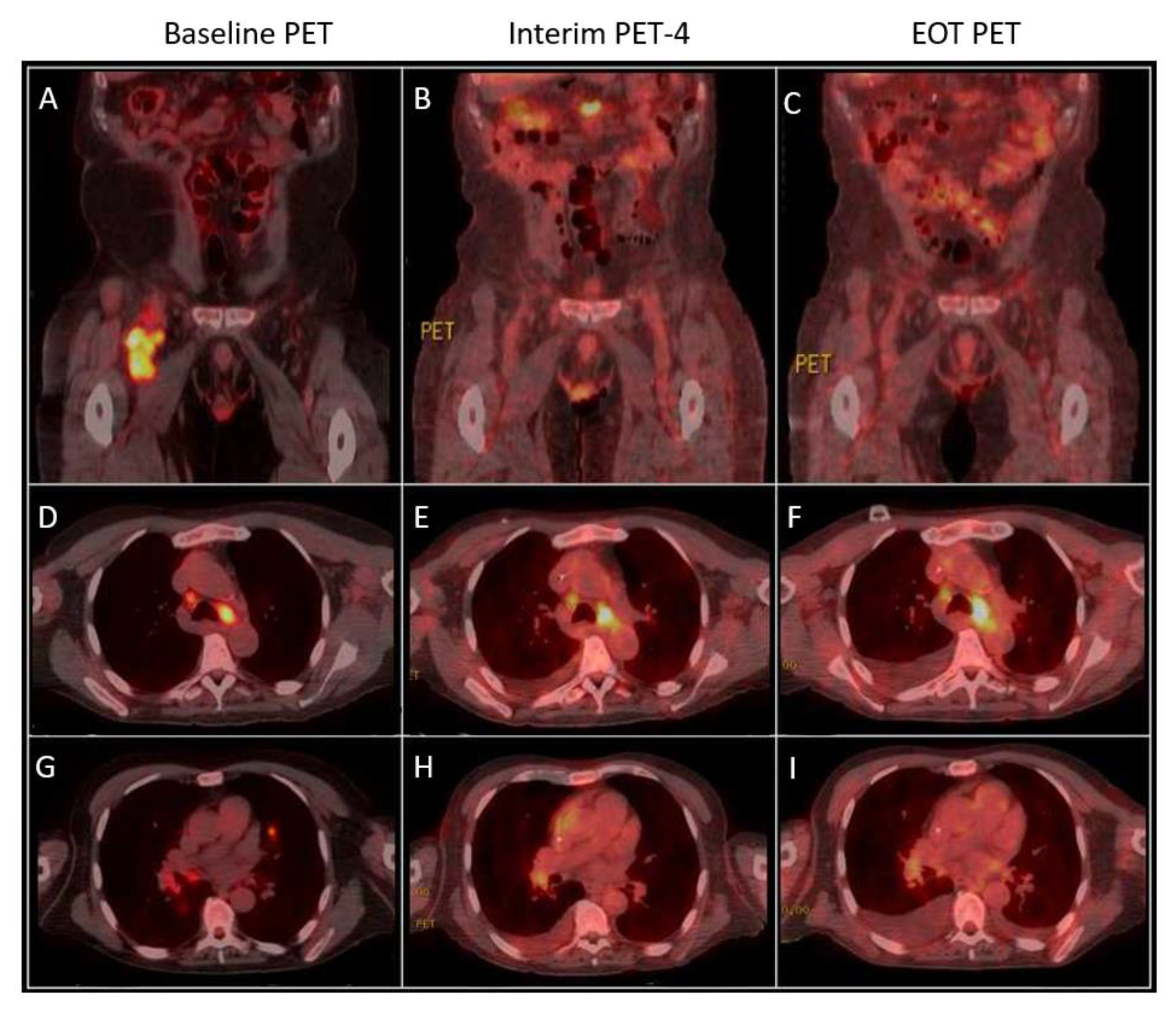
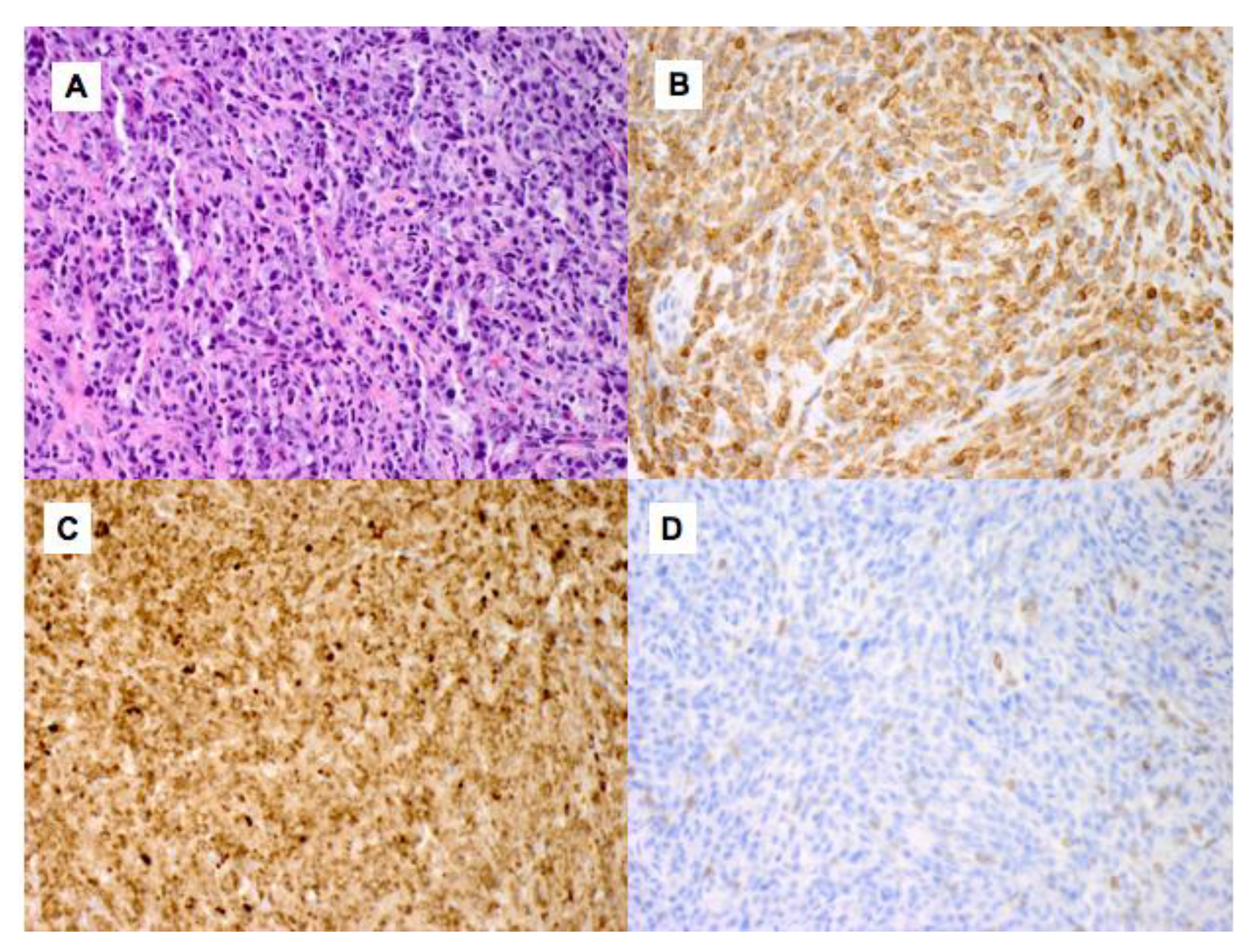
2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caillard, S.; Porcher, R.; Provot, F.; Dantal, J.; Choquet, S.; Durrbach, A.; Morelon, E.; Moal, V.; Janbon, B.; Alamartine, E.; et al. Post-transplantation lymphoproliferative disorder after kidney transplantation: Report of a nationwide French registry and the development of a new prognostic score. Am. J. Clin. Oncol. 2013, 31, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H. T-cell and NK-cell posttransplantation lymphoproliferative disorders. Am. J. Clin. Pathol. 2007, 127, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Draoua, H.Y.; Tsao, L.; Mancini, D.M.; Addonizio, L.J.; Bhagat, G.; Alobeid, B. T-cell post-transplantation lymphoproliferative disorders after cardiac transplantation: A single institutional experience. Br. J. Haematol. 2004, 127, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Montanari, F.; Bhagat, G.; Clark-Garvey, S.; Seshan, V.; Zain, J.; Diefenbach, C.; Mccormick, E.; Crook, M.; Conroy, M.; O’connor, O.A. Monomorphic T-cell post-transplant lymphoproliferative disorders exhibit markedly inferior outcomes compared to monomorphic B-cell post-transplant lymphoproliferative disorders. Leuk. Lymphoma 2010, 51, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Margolskee, E.; Jobanputra, V.; Jain, P.; Chen, J.; Ganapathi, K.; Nahum, O.; Levy, B.; Morscio, J.; Murty, V.; Tousseyn, T.; et al. Genetic landscape of T- and NK-cell post-transplant lymphoproliferative disorders. Oncotarget 2016, 7, 37636–37648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herreman, A.; Dierickx, D.; Morscio, J.; Camps, J.; Bittoun, E.; Verhoef, G.; De Wolf-Peeters, C.; Sagaert, X.; Tousseyn, T. Clinicopathological characteristics of posttransplant lymphoproliferative disorders of T-cell origin: Single-center series of nine cases and meta-analysis of 147 reported cases. Leuk. Lymphoma 2013, 54, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Advani, R.H.; Bartlett, N.L.; Jacobsen, E.D.; Sharman, J.P.; O’Connor, O.A.; Siddiqi, T.; Kennedy, D.A.; Oki, Y. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood 2014, 123, 3095–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barta, S.K.; Gong, J.Z.; Porcu, P. Brentuximab vedotin in the treatment of CD30+ PTCL. Blood 2019, 134, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Fanale, M.A.; Horwitz, S.M.; Forero-Torres, A.; Bartlett, N.L.; Advani, R.H.; Pro, B.; Chen, R.W.; Davies, A.; Illidge, T.; Huebner, D.; et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: Results of a phase I study. Am. J. Clin. Oncol. 2014, 32, 3137–3143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanale, M.A.; Horwitz, S.M.; Forero-Torres, A.; Bartlett, N.L.; Advani, R.H.; Pro, B.; Chen, R.W.; Davies, A.; Illidge, T.; Uttarwar, M.; et al. Five-year outcomes for frontline brentuximab vedotin with CHP for CD30-expressing peripheral T-cell lymphomas. Blood 2018, 131, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weyden, C.A.; Pileri, S.A.; Feldman, A.L.; Whisstock, J.; Prince, H.M. Understanding CD30 biology and therapeutic targeting: A historical perspective providing insight into future directions. Blood Cancer J. 2017, 79, e603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dierickx, D.; Tousseyn, T.; Gheysens, O. How I treat posttransplant lymphoproliferative disorders. Blood 2015, 126, 2274–2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mansour, Z.; Nelson, B.P.; Evens, A.M. Post-transplant lymphoproliferative disease (PTLD): Risk factors, diagnosis, and current treatment strategies. Curr. Hematol. Malig. Rep. 2013, 8, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dierickx, D.; Habermann, T.M. Post-transplantation lymphoproliferative disorders in adults. N. Engl. J. Med. 2018, 378, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Sabattini, E.; Bacci, F.; Sagramoso, C.; Pileri, S.A. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: An overview. Pathologica 2010, 102, 83–87. [Google Scholar] [PubMed]
- Bossard, C.; Dobay, M.P.; Parrens, M.; Lamant, L.; Missiaglia, E.; Haioun, C.; Martin, A.; Fabiani, B.; Delarue, R.; Tournilhac, O.; et al. Immunohistochemistry as a valuable tool to assess CD30 expression in peripheral T-cell lymphomas: High correlation with mRNA levels. Blood 2014, 124, 2983–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Johnson, W.T.; Kartan, S.; Gonsalves, A.S.; Fenkel, J.M.; Gong, J.Z.; Porcu, P. Durable Response to Brentuximab Vedotin Plus Cyclophosphamide, Doxorubicin, and Prednisone (BV-CHP) in a Patient with CD30-Positive PTCL Arising as a Post-Transplant Lymphoproliferative Disorder (PTLD). Curr. Oncol. 2021, 28, 5067-5072. https://doi.org/10.3390/curroncol28060426
Hong J, Johnson WT, Kartan S, Gonsalves AS, Fenkel JM, Gong JZ, Porcu P. Durable Response to Brentuximab Vedotin Plus Cyclophosphamide, Doxorubicin, and Prednisone (BV-CHP) in a Patient with CD30-Positive PTCL Arising as a Post-Transplant Lymphoproliferative Disorder (PTLD). Current Oncology. 2021; 28(6):5067-5072. https://doi.org/10.3390/curroncol28060426
Chicago/Turabian StyleHong, Jennifer, William T. Johnson, Saritha Kartan, Anitha S. Gonsalves, Jonathan M. Fenkel, Jerald Z. Gong, and Pierluigi Porcu. 2021. "Durable Response to Brentuximab Vedotin Plus Cyclophosphamide, Doxorubicin, and Prednisone (BV-CHP) in a Patient with CD30-Positive PTCL Arising as a Post-Transplant Lymphoproliferative Disorder (PTLD)" Current Oncology 28, no. 6: 5067-5072. https://doi.org/10.3390/curroncol28060426
APA StyleHong, J., Johnson, W. T., Kartan, S., Gonsalves, A. S., Fenkel, J. M., Gong, J. Z., & Porcu, P. (2021). Durable Response to Brentuximab Vedotin Plus Cyclophosphamide, Doxorubicin, and Prednisone (BV-CHP) in a Patient with CD30-Positive PTCL Arising as a Post-Transplant Lymphoproliferative Disorder (PTLD). Current Oncology, 28(6), 5067-5072. https://doi.org/10.3390/curroncol28060426



