Investigation of Lupeol as Anti-Melanoma Agent: An In Vitro-In Ovo Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cellular Viability and Morphology Assessment
2.4. Nuclear Morphology Evaluation
2.5. Immunofluorescence
2.6. Wound Healing Assay
2.7. Chorioallantoic Membrane (CAM) Assay
2.8. Statistical Analysis
3. Results
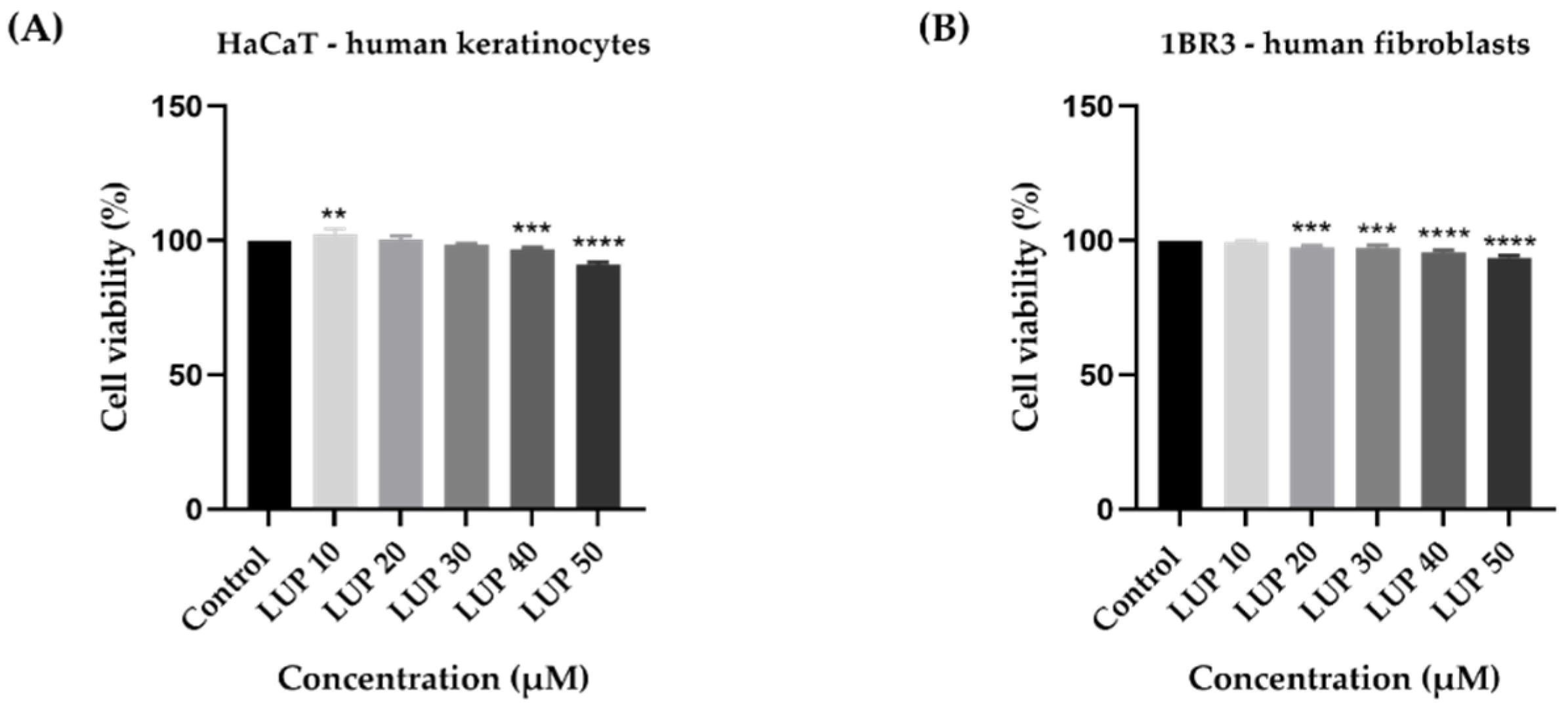
3.1. Cellular Viability and Morphology Assessment
3.2. Nuclear Morphology Evaluation
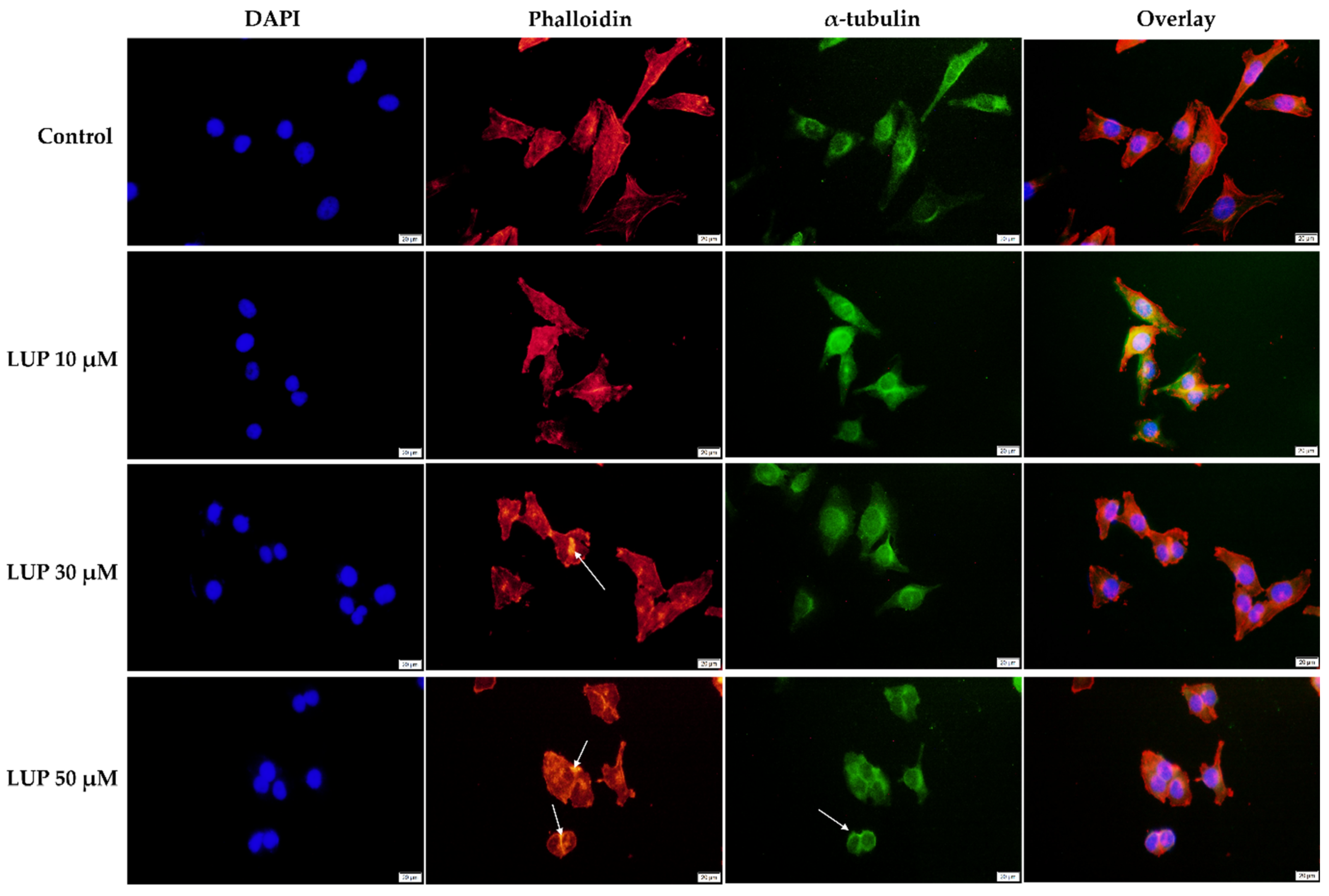
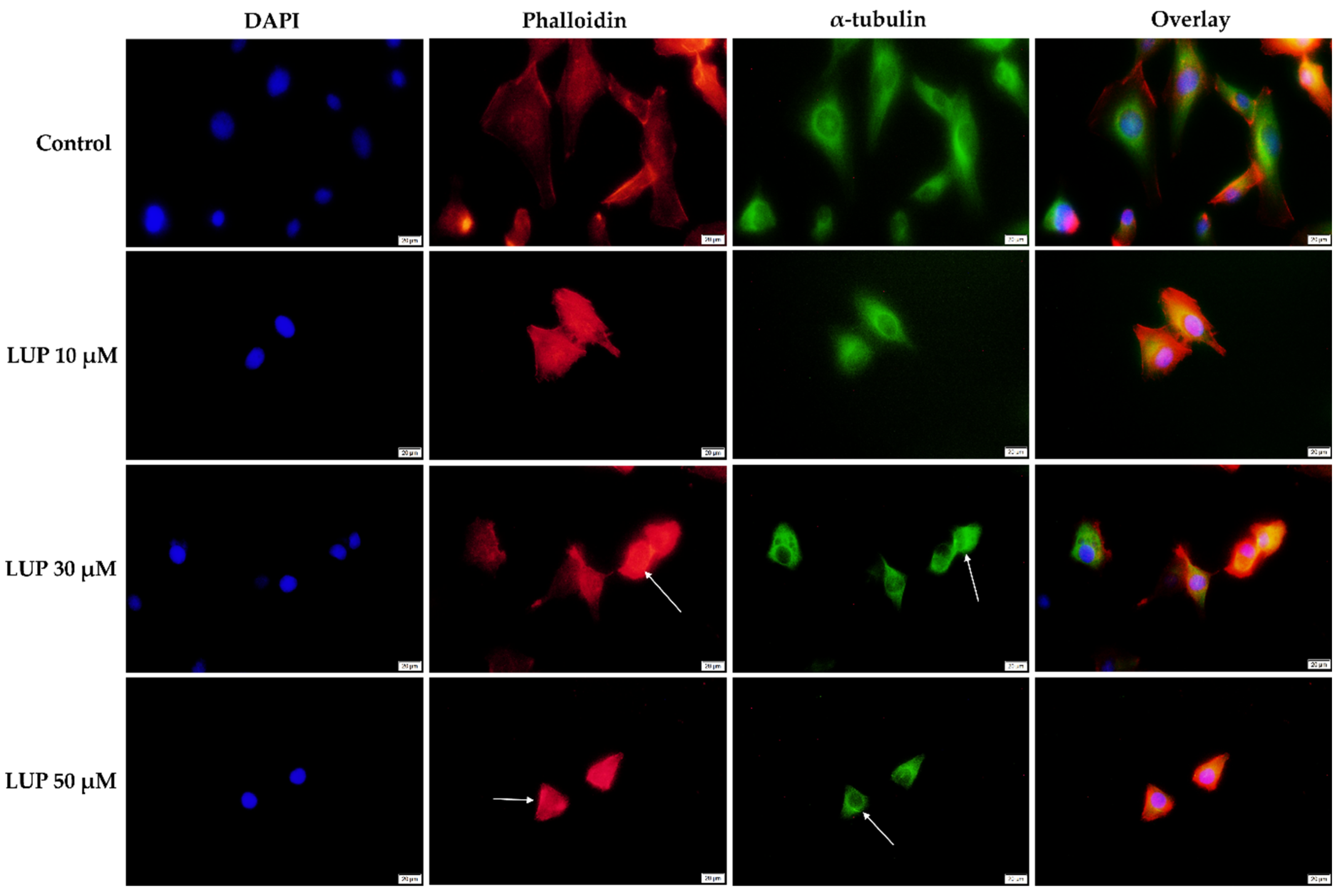
3.3. Immunofluorescence
3.4. Wound Healing Assay
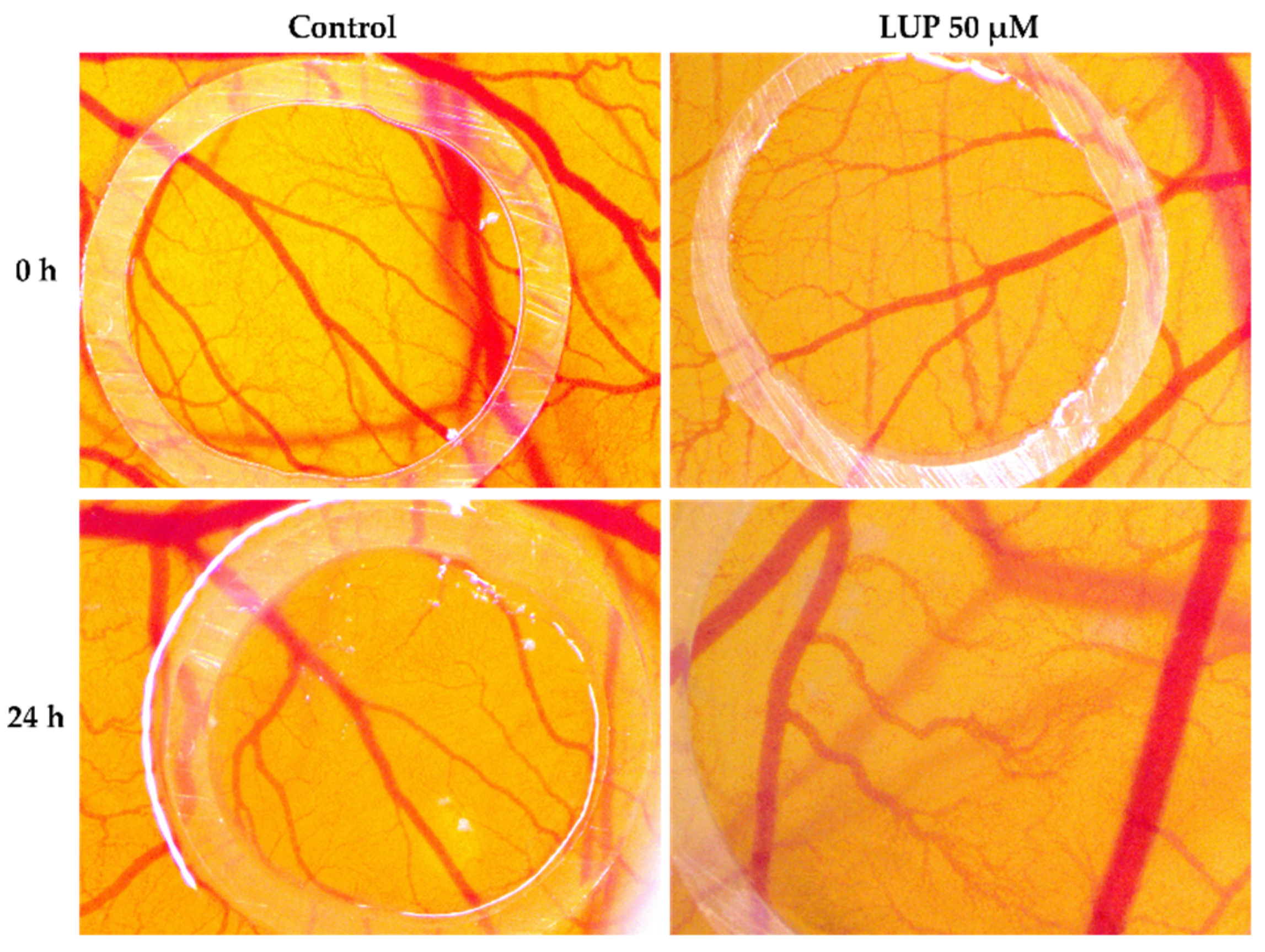
3.5. Chorioallantoic Membrane (CAM) Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2021, 141, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.; George, J.; Aucoin, D.; Bower, J.; Burrell, S.; Gilbert, R.; Bower, N. The Pathogenesis and Clinical Management of Cutaneous Melanoma: An Evidence-Based Review. J. Med. Imaging Radiat. Sci. 2019, 50, 460–469.e1. [Google Scholar] [CrossRef]
- Naik, P.P. Cutaneous Malignant Melanoma: A Review of Early Diagnosis and Management. World J. Oncol. 2021, 12, 7–19. [Google Scholar] [CrossRef]
- Gordon, R. Skin cancer: An overview of epidemiology and risk factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef]
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and Risk Factors of Melanoma. Surg. Clin. N. Am. 2020, 100, 1–12. [Google Scholar] [CrossRef]
- Domingues, B.; Lopes, J.; Soares, P.; Populo, H. Melanoma treatment in review. Immuno Targets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Júnior, R.G.; Ferraz, C.A.A.; e Silva, M.G.; de Lavor, É.M.; Rolim, L.A.; de Lima, J.T.; Fleury, A.; Picot, L.; de Souza Siqueira Quintans, J.; Quintans Júnior, L.J.; et al. Flavonoids: Promising Natural Products for Treatment of Skin Cancer (Melanoma). In Natural Products and Cancer Drug Discovery; Badria, F.A., Ed.; IntechOpen: Rijeka, Croatia, 2017; Available online: https://www.intechopen.com/chapters/54517 (accessed on 24 September 2021). [CrossRef]
- Luke, J.J.; Schwartz, G.K. Chemotherapy in the Management of Advanced Cutaneous Malignant Melanoma. Clin. Dermatol. 2013, 31, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Chinembiri, T.N.; Du Plessis, L.H.; Gerber, M.; Hamman, J.H.; Du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef]
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.A.; Ali, S.; Asrar, M. Plant derived anticancer agents: A green approach towards skin cancers. Biomed. Pharmacother. 2018, 103, 1643–1651. [Google Scholar] [CrossRef]
- Kim, T.; Amaria, R.N.; Spencer, C.; Reuben, A.; Cooper, Z.A.; Wargo, J.A. Combining targeted therapy and immune checkpoint inhibitors in the treatment of metastatic melanoma. Cancer Biol. Med. 2014, 11, 237–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akaike, T.; Nghiem, P.; Lacouture, M.E. CME Part I: Immune checkpoint inhibitors to treat cutaneous malignancies. J. Am. Acad. Dermatol. 2020, 83, 1239–1253. [Google Scholar] [CrossRef]
- Majolo, F.; de Oliveira Becker Delwing, L.K.; Marmitt, D.J.; Bustamante-Filho, I.C.; Goettert, M.I. Medicinal plants and bioactive natural compounds for cancer treatment: Important advances for drug discovery. Phytochem. Lett. 2019, 31, 196–207. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Sarek, J.; Kvasnica, M.; Vlk, M.; Urban, M.; Dzubak, P.; Hajduch, M. The Potential of Triterpenoids in the Treatment of Melanoma. In Research on Melanoma—A Glimpse into Current Directions and Future Trends; Murph, M., Ed.; IntechOpen: Rijeka, Croatia, 2011; Available online: https://www.intechopen.com/chapters/19095 (accessed on 6 September 2021). [CrossRef][Green Version]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Cui, X.; Cui, H.; Jin, Y.; Jin, W.; Sun, H. Geraniol and lupeol inhibit growth and promote apoptosis in human hepatocarcinoma cells through the MAPK signaling pathway. J. Cell. Biochem. 2019, 120, 5033–5041. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hong, D.; Qian, Y.; Tu, X.; Wang, K.; Yang, X.; Shao, S.; Kong, X.; Lou, Z.; Jin, L. Lupeol inhibits growth and migration in two human colorectal cancer cell lines by suppression of wnt–β-catenin pathway. Onco Targets Ther. 2018, 11, 7987–7999. [Google Scholar] [CrossRef]
- Min, T.R.; Park, H.J.; Ha, K.T.; Chi, G.Y.; Choi, Y.H.; Park, S.H. Suppression of EGFR/STAT3 activity by lupeol contributes to the induction of the apoptosis of human non-small cell lung cancer cells. Int. J. Oncol. 2019, 55, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; Belardinelli, E.; Fabiano, A.; Santoni, T.; Di Stefano, R. Effect of different chitosan derivatives on in vitro scratch wound assay: A comparative study. Int. J. Biol. Macromol. 2015, 76, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Simu, S.; Marcovici, I.; Dobrescu, A.; Malita, D.; Dehelean, C.A.; Coricovac, D.; Olaru, F.; Draghici, G.A.; Navolan, D. Insights into the behavior of triple-negative mda-mb-231 breast carcinoma cells following the treatment with 17β-ethinylestradiol and levonorgestrel. Molecules 2021, 26, 2776. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016, 778–781. [Google Scholar] [CrossRef]
- Tsai, F.S.; Lin, L.W.; Wu, C.R. Lupeol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 145–175. [Google Scholar] [CrossRef]
- Siddique, H.R.; Saleem, M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Beserra, F.P.; Xue, M.; De Azevedo Maia, G.L.; Rozza, A.L.; Pellizzon, C.H.; Jackson, C.J. Lupeol, a pentacyclic triterpene, promotes migration, wound closure, and contractile effect in vitro: Possible involvement of PI3K/Akt and p38/ERK/MAPK pathways. Molecules 2018, 23, 2819. [Google Scholar] [CrossRef]
- Beserra, F.P.; Gushiken, L.F.S.; Vieira, A.J.; Bérgamo, D.A.; Bérgamo, P.L.; de Souza, M.O.; Hussni, C.A.; Takahira, R.K.; Nóbrega, R.H.; Martinez, E.R.M.; et al. From inflammation to cutaneous repair: Topical application of lupeol improves skin wound healing in rats by modulating the cytokine levels, NF-κB, Ki-67, growth factor expression, and distribution of collagen fibers. Int. J. Mol. Sci. 2020, 21, 4952. [Google Scholar] [CrossRef]
- Babu, T.S.; Michael, B.P.; Jerard, C.; Vijayakumar, N.; Ramachandran, R. Study on the anti metastatic and anti cancer activity of triterpene compound lupeol in human lung cancer. Int. J. Pharm. Sci. Res. 2019, 10, 721–727. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Sekar, V.; Majumder, B.; Mehrotra, D.G.; Banerjee, S.; Bhowmick, A.K.; Alam, N.; Mandal, G.K.; Biswas, J.; Majumder, P.K.; et al. CDKN2A-p53 mediated antitumor effect of Lupeol in head and neck cancer. Cell. Oncol. 2017, 40, 145–155. [Google Scholar] [CrossRef]
- Eldohaji, L.M.; Fayed, B.; Hamoda, A.M.; Ershaid, M.; Abdin, S.; Alhamidi, T.B.; Mohammad, M.G.; Omar, H.A.; Soliman, S.S.M. Potential targeting of Hep3B liver cancer cells by lupeol isolated from Avicennia marina. Arch. Pharm. 2021, 354, e2100120. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Murtaza, I.; Tarapore, R.S.; Suh, Y.; Adhami, V.M.; Johnson, J.J.; Siddiqui, I.A.; Khan, N.; Asim, M.; Hafeez, B.; et al. Lupeol inhibits proliferation of human prostate cancer cells by targeting β-catenin signaling. Carcinogenesis 2009, 30, 808–817. [Google Scholar] [CrossRef]
- Saleem, M.; Maddodi, N.; Zaid, M.A.; Khan, N.; Bin Hafeez, B.; Asim, M.; Suh, Y.; Yun, J.M.; Setaluri, V.; Mukhtar, H. Lupeol inhibits growth of highly aggressive human metastatic melanoma cells in vitro and in vivo by inducing apoptosis. Clin. Cancer Res. 2008, 14, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, R.S.; Siddiqui, I.A.; Saleem, M.; Adhami, V.M.; Spiegelman, V.S.; Mukhtar, H. Specific targeting of wnt/β-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis 2010, 31, 1844–1853. [Google Scholar] [CrossRef]
- Parasuraman, S. Toxicological screening. J. Pharmacol. Pharmacother. 2011, 2, 74–79. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, Z.; Ji, C.; Zhang, X.; Xu, J.; Wang, J. Novel Insights into the Role of the Cytoskeleton in Cancer. In Cytoskeleton—Structure, Dynamics, Function and Disease; Jimenez-Lopez, J.C., Ed.; IntechOpen: Rijeka, Croatia, 2017; Available online: https://www.intechopen.com/chapters/53708 (accessed on 28 August 2021). [CrossRef]
- Povea-Cabello, S.; Oropesa-Ávila, M.; de la Cruz-Ojeda, P.; Villanueva-Paz, M.; De La Mata, M.; Suárez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Cotán, D.; Ybot-González, P.; et al. Dynamic reorganization of the cytoskeleton during apoptosis: The two coffins hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef] [PubMed]
- Oropesa-Ávila, M.; de la Cruz-Ojeda, P.; Porcuna, J.; Villanueva-Paz, M.; Fernández-Vega, A.; de la Mata, M.; de Lavera, I.; Rivero, J.M.S.; Luzón–Hidalgo, R.; Álvarez-Córdoba, M.; et al. Two coffins and a funeral: Early or late caspase activation determines two types of apoptosis induced by DNA damaging agents. Apoptosis 2017, 22, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Mioc, M.; Avram, S.; Bercean, V.; Porcarasu, M.B.; Soica, C.; Susan, R.; Kurunczi, L. Synthesis, Characterization and Antiproliferative Activity Assessment of a Novel 1H-5-mercapto-1,2,4 Triazole Derivative. Rev. Chim. 2017, 68, 745–747. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-angiogenic therapy: Current challenges and future perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef]
- Vijay Avin, B.R.; Prabhu, T.R.C. New role of lupeol in reticence of angiogenesis, the cellular parameter of neoplastic progression in tumorigenesis models through altered gene expression. Biochem. Biophys. Res. Commun. 2014, 448, 139–144. [Google Scholar] [CrossRef]
- Ambasta, R.K.; Jha, S.K.; Kumar, D.; Sharma, R.; Jha, N.K.; Kumar, P. Comparative study of anti-angiogenic activities of luteolin, lectin and lupeol biomolecules. J. Transl. Med. 2015, 13, 307. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bociort, F.; Macasoi, I.G.; Marcovici, I.; Motoc, A.; Grosu, C.; Pinzaru, I.; Petean, C.; Avram, S.; Dehelean, C.A. Investigation of Lupeol as Anti-Melanoma Agent: An In Vitro-In Ovo Perspective. Curr. Oncol. 2021, 28, 5054-5066. https://doi.org/10.3390/curroncol28060425
Bociort F, Macasoi IG, Marcovici I, Motoc A, Grosu C, Pinzaru I, Petean C, Avram S, Dehelean CA. Investigation of Lupeol as Anti-Melanoma Agent: An In Vitro-In Ovo Perspective. Current Oncology. 2021; 28(6):5054-5066. https://doi.org/10.3390/curroncol28060425
Chicago/Turabian StyleBociort, Flavia, Ioana Gabriela Macasoi, Iasmina Marcovici, Andrei Motoc, Cristina Grosu, Iulia Pinzaru, Crina Petean, Stefana Avram, and Cristina Adriana Dehelean. 2021. "Investigation of Lupeol as Anti-Melanoma Agent: An In Vitro-In Ovo Perspective" Current Oncology 28, no. 6: 5054-5066. https://doi.org/10.3390/curroncol28060425
APA StyleBociort, F., Macasoi, I. G., Marcovici, I., Motoc, A., Grosu, C., Pinzaru, I., Petean, C., Avram, S., & Dehelean, C. A. (2021). Investigation of Lupeol as Anti-Melanoma Agent: An In Vitro-In Ovo Perspective. Current Oncology, 28(6), 5054-5066. https://doi.org/10.3390/curroncol28060425







