Combined Exoscopic and Endoscopic Technique for Craniofacial Resection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
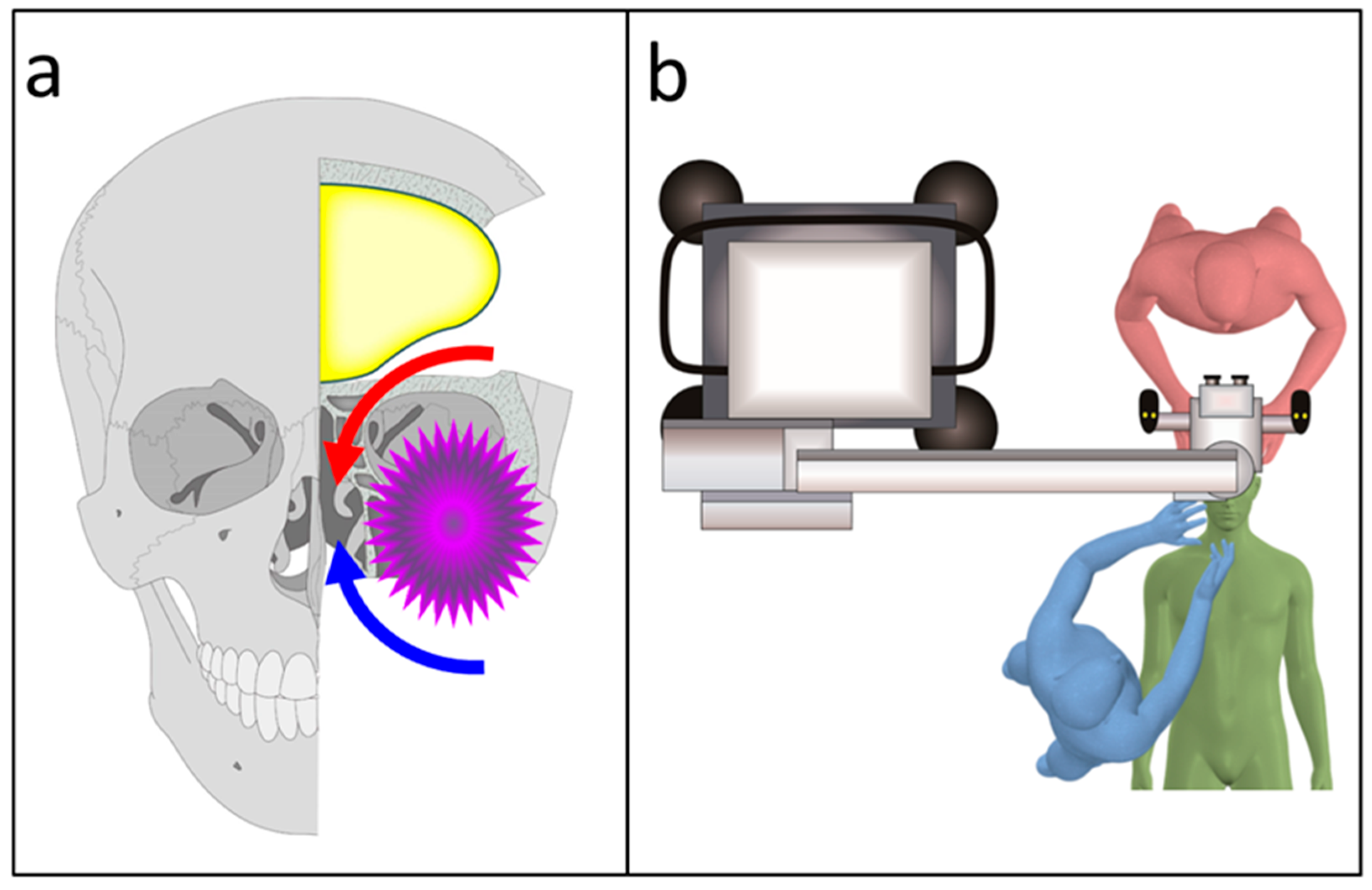
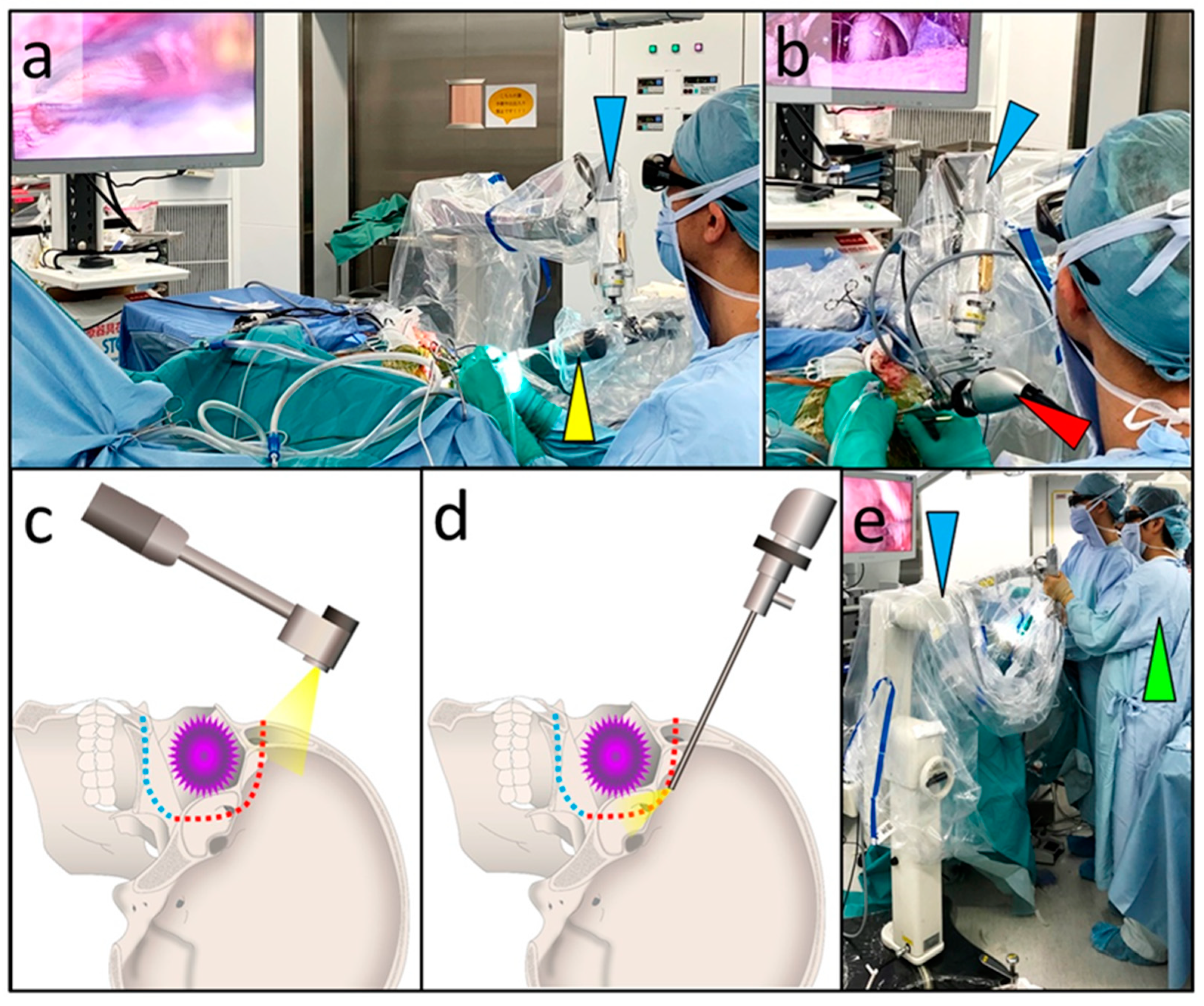
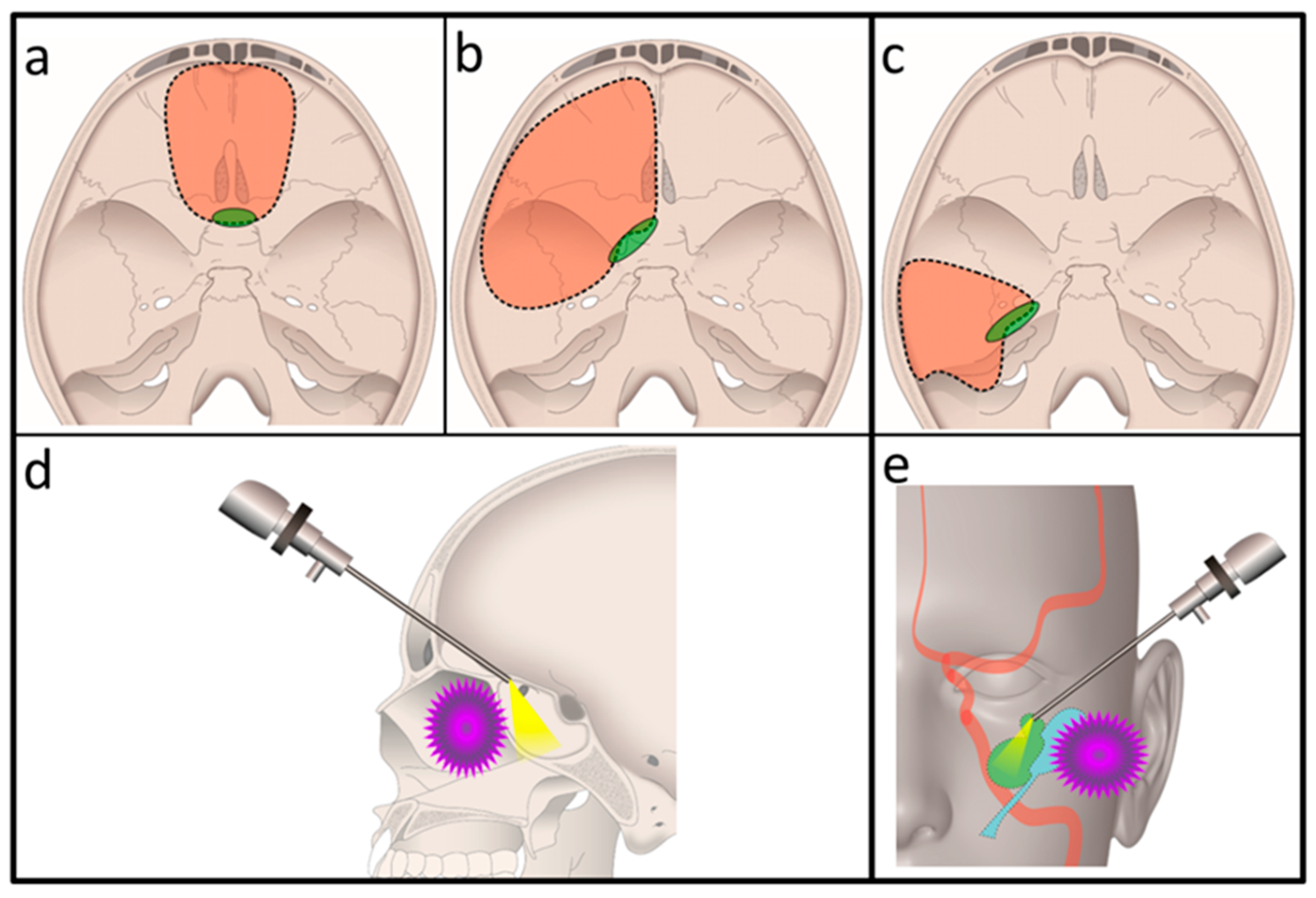
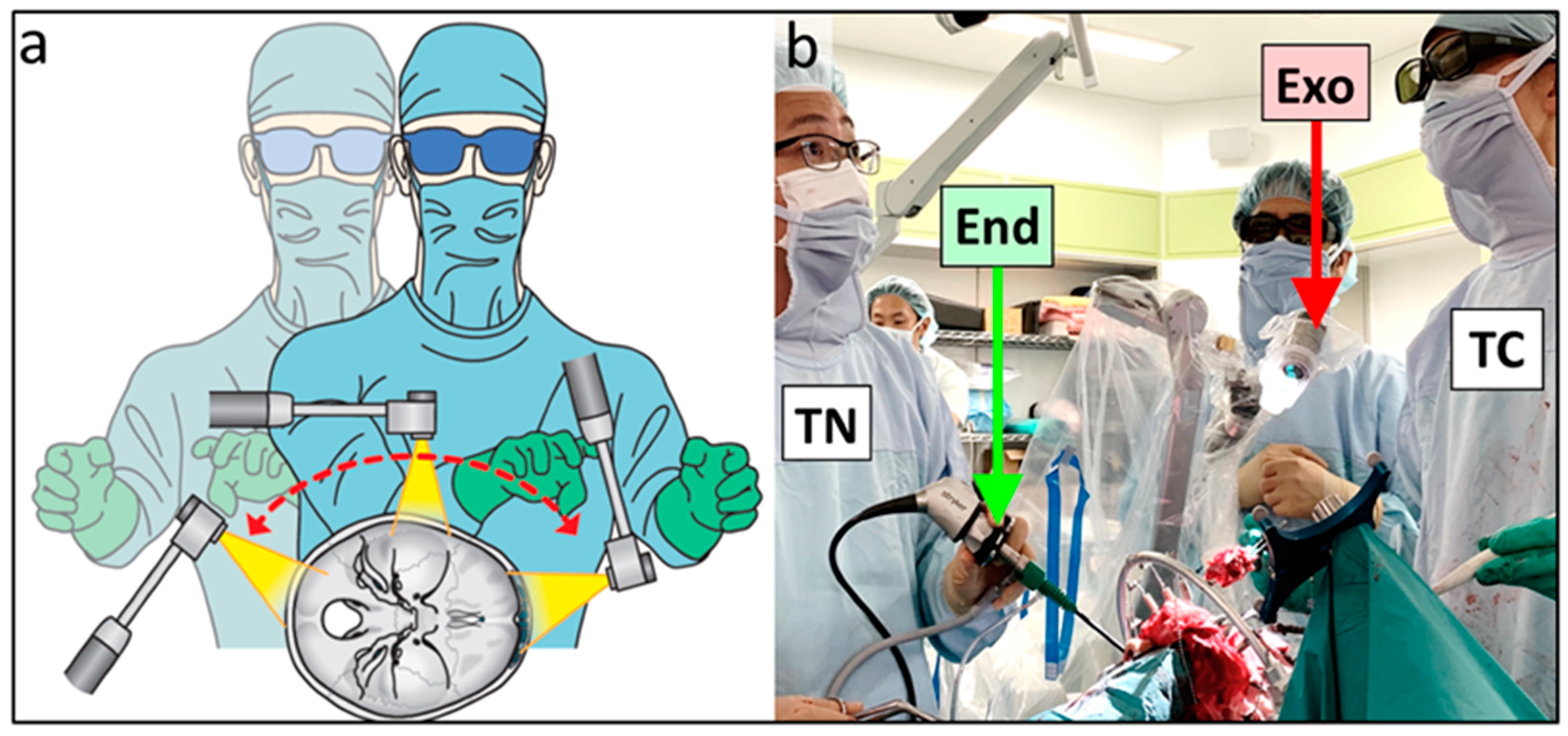
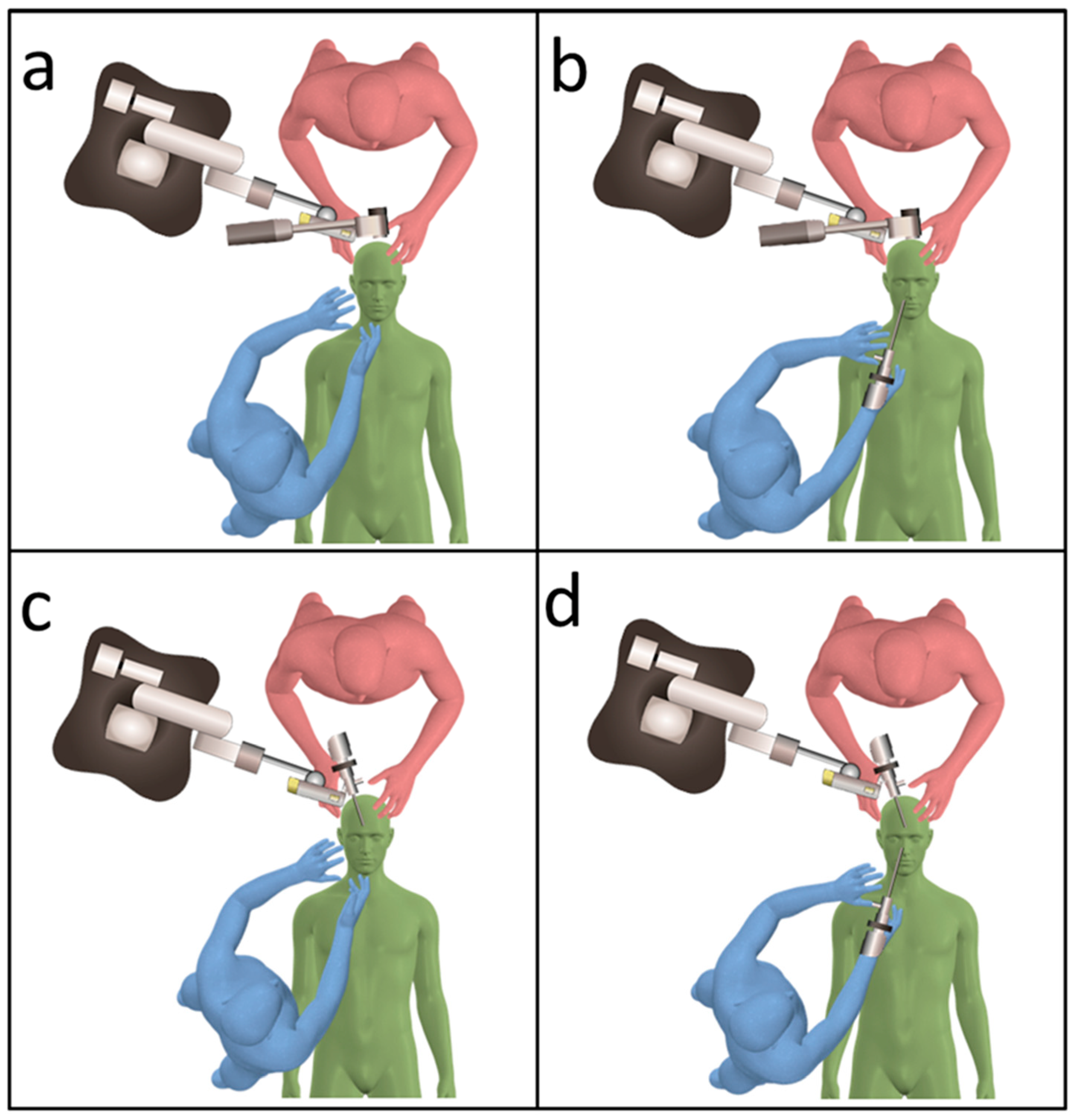
2.2. Surgical Techniques Used in the Transcranial Approach
2.3. Treatment Strategy for the Head and Neck Malignant Tumor
3. Results
3.1. Patient Characteristics
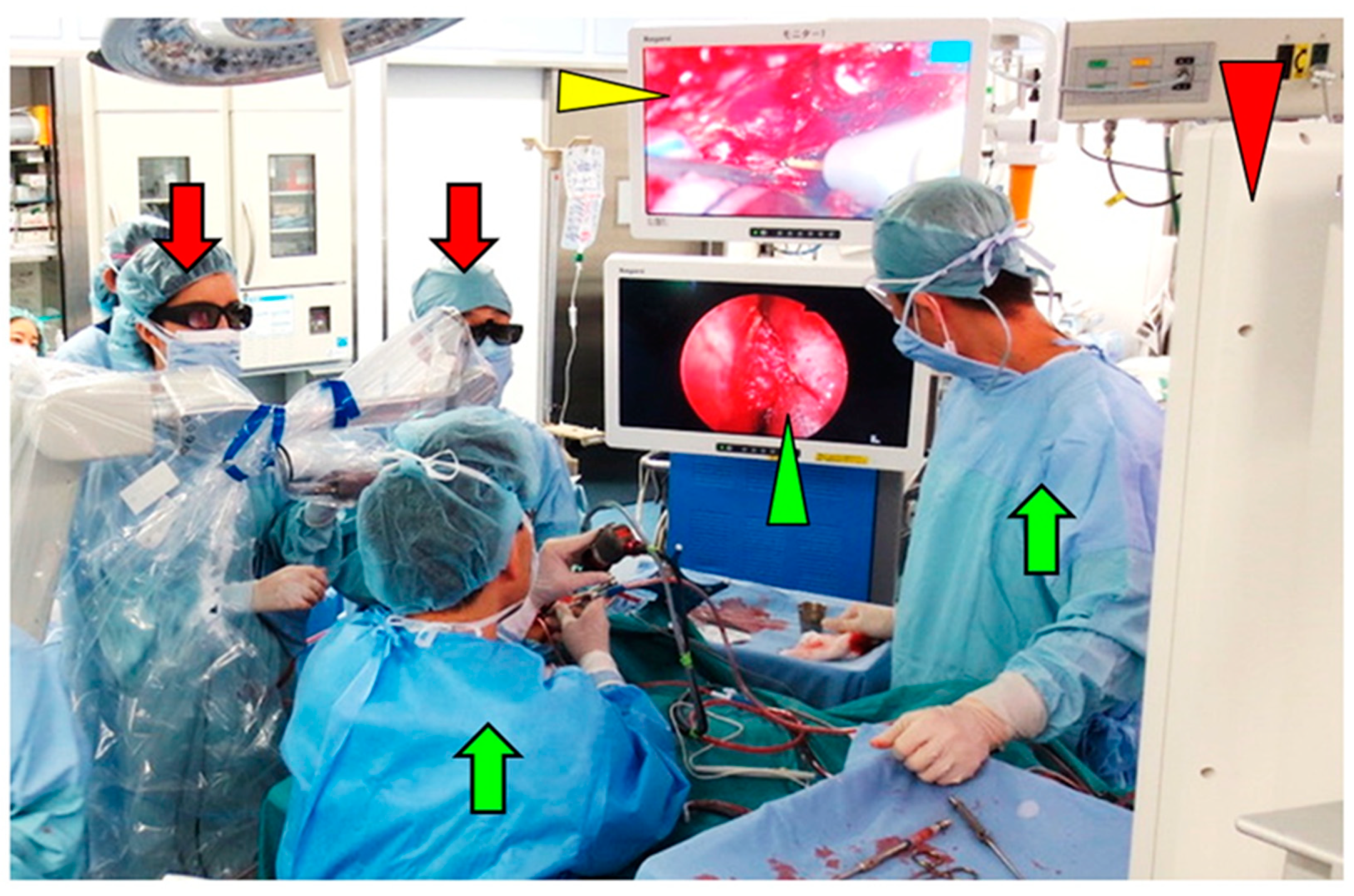
3.2. Intraoperative Findings
3.3. Operation Time and Intraoperative Blood Loss
3.4. Combined Exoscopic and Endoscopic Technique in the Transcranial Approach
3.5. Postoperative Findings
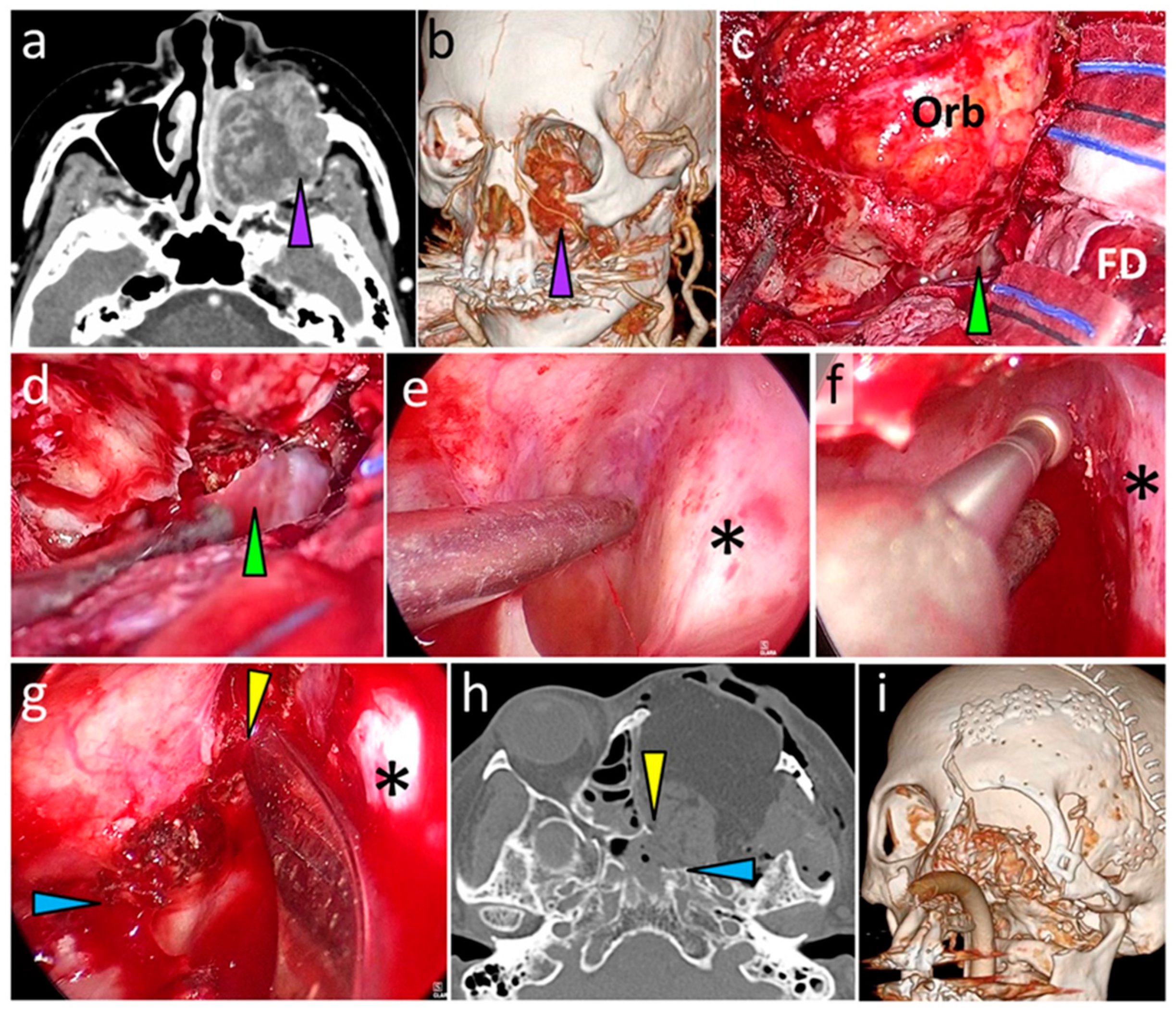
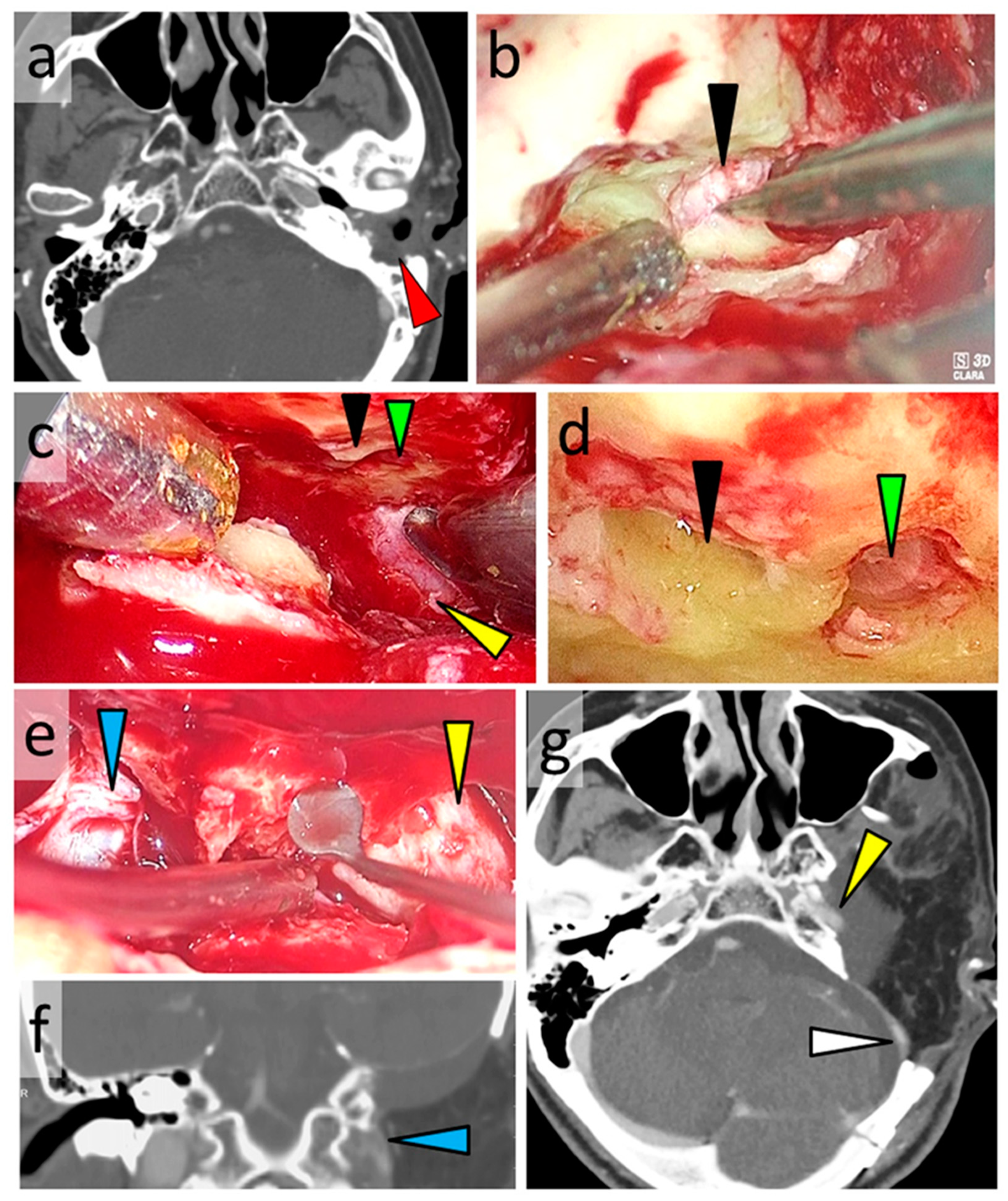
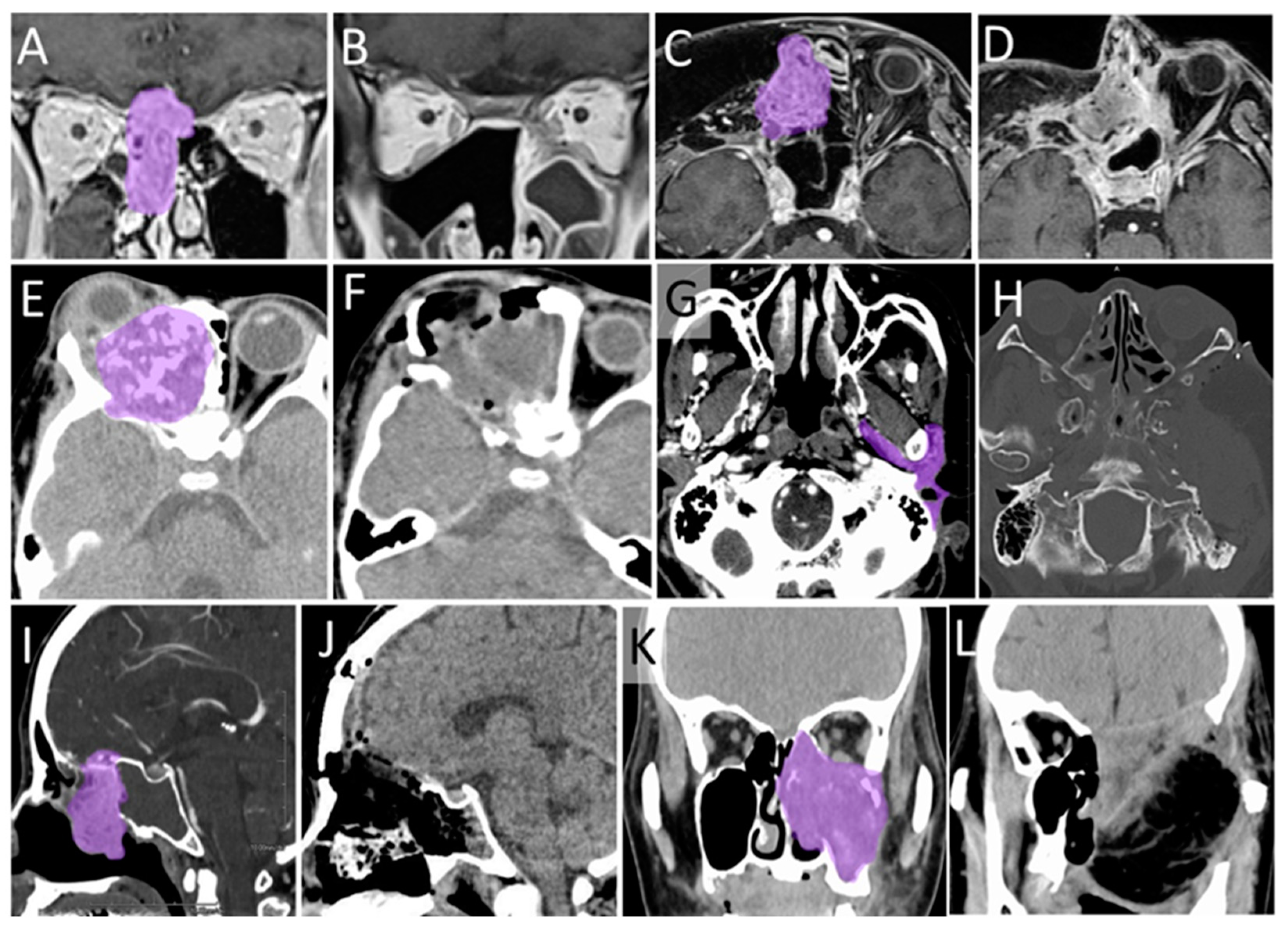
3.6. Typical Cases
3.6.1. Case 1
3.6.2. Case 2
3.7. The Other Cases
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grant, R.N. The incidence of and mortality from cancer in the United States. Prog. Clin. Cancer 1970, 4, 34–47. [Google Scholar]
- Bacciu, A.; Clemente, I.A.; Piccirillo, E.; Ferrari, S.; Sanna, M. Guidelines for Treating Temporal Bone Carcinoma Based on Long-Term Outcomes. Otol. Neurotol. 2013, 34, 898–907. [Google Scholar] [CrossRef]
- Dulguerov, P.; Jacobsen, M.S.; Allal, A.S.; Lehmann, W.; Calcaterra, T. Nasal and paranasal sinus carcinoma: Are we making progress? A series of 220 patients and a systematic review. Cancer 2001, 92, 3012–3029. [Google Scholar] [CrossRef]
- Hoppe, B.S.; Stegman, L.D.; Zelefsky, M.J.; Rosenzweig, K.E.; Wolden, S.L.; Patel, S.G. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting--the MSKCC experience. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.L.; Gu, F.M.; Dai, C.F.; Chen, B.; Li, H.W. Survival outcomes in surgical treatment of 72 cases of squamous cell carcinoma of the temporal bone. Otol. Neurotol. 2011, 32, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Iwami, K.; Fujii, M.; Kishida, Y.; Jinguji, S.; Yamada, M.; Bakhit, M.; Nishio, N.; Fujimoto, Y.; Ogawa, T.; Takanari, K.; et al. Role of transcranial sphenoidotomy in skull base surgery: Classification of surgical techniques based on the surgical anatomy of the sphenoid sinus. J. Neurosurg. 2019, 131, 1658–1667. [Google Scholar] [CrossRef]
- Nishio, N.; Fujimoto, Y.; Fujii, M.; Saito, K.; Hiramatsu, M.; Maruo, T. Craniofacial Resection for T4 Maxillary Sinus Carcinoma: Managing Cases with Involvement of the Skull Base. Otolaryngol. Head Neck. Surg. 2015, 153, 231–238. [Google Scholar] [CrossRef]
- Kawahara, N.; Sasaki, T.; Asakage, T.; Nakao, K.; Sugasawa, M.; Asato, H. Long-term outcome following radical temporal bone resection for lateral skull base malignancies: A neurosurgical perspective. J. Neurosurg. 2008, 108, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Iwami, K.; Fujii, M.; Nishio, N.; Maruo, T.; Fujimoto, Y.; Takanari, K.; Kamei, Y.; Yamada, M.; Ogawa, T.; Osuka, K.; et al. Skull base invasion patterns of malignant head and neck tumors: A neurosurgical perspective. J. Neurol. Surg. Part. B Skull Base 2020, 82, e120–e130. [Google Scholar] [CrossRef] [PubMed]
- Belloch, J.P.; Rovira, V.; Llacer, J.L.; Riesgo, P.A.; Cremades, A. Fluorescence-guided surgery in high grade gliomas using an exoscope system. Acta Neurochir. 2014, 156, 653–660. [Google Scholar] [CrossRef]
- Belykh, E.; George, L.; Zhao, X.; Carotenuto, A.; Moreira, L.B.; Yagmurlu, K.; Bozkurt, B.; Byvaltsev, V.; Nakaji, P.; Preul, M.C. Microvascular anastomosis under 3D exoscope or endoscope magnification: A proof-of-concept study. Surg. Neurol. Int. 2018, 9, 115. [Google Scholar]
- Krishnan, K.G.; Scholler, K.; Uhl, E. Application of a Compact High-Definition Exoscope for Illumination and Magnification in High-Precision Surgical Procedures. World Neurosurg. 2017, 97, 652–660. [Google Scholar] [CrossRef]
- Sack, J.; Steinberg, J.A.; Rennert, R.C.; Hatefi, D.; Pannell, J.S.; Levy, M.; Khalessi, A.A. Initial Experience Using a High-Definition 3-Dimensional Exoscope System for Microneurosurgery. Oper. Neurosurg. 2018, 14, 395–401. [Google Scholar] [CrossRef]
- Beez, T.; Munoz-Bendix, C.; Beseoglu, K.; Steiger, H.J.; Ahmadi, S.A. First Clinical Applications of a High-Definition Three-Dimensional Exoscope in Pediatric Neurosurgery. Cureus 2018, 10, e2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossini, Z.; Cardia, A.; Milani, D.; Lasio, G.B.; Fornari, M.; D’Angelo, V. VITOM 3D: Preliminary Experience in Cranial Surgery. World Neurosurg. 2017, 107, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Oertel, J.M.; Burkhardt, B.W. Vitom-3D for Exoscopic Neurosurgery: Initial Experience in Cranial and Spinal Procedures. World Neurosurg. 2017, 105, 153–162. [Google Scholar] [CrossRef] [PubMed]
- De Divitiis, O.; D’Avella, E.; Denaro, L.; Somma, T.; Sacco, M.; D’Avella, D. Vitom 3D: Preliminary experience with intradural extramedullary spinal tumors. J. Neurosurg Sci. 2019. [Google Scholar] [CrossRef]
- Burkhardt, B.W.; Csokonay, A.; Oertel, J.M. 3D-exoscopic visualization using the VITOM-3D in cranial and spinal neurosurgery. What are the limitations? Clin. Neurol. Neurosurg. 2020, 198, 106101. [Google Scholar] [CrossRef]
- Iwami, K.; Watanabe, T.; Yokota, M.; Hara, M.; Osuka, K.; Miyachi, S. Feasibility of underwater microvascular decompression for hemifacial spasm: A technical note. Acta Neurochir 2021, 163, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Minoda, R.; Miwa, T. Non-microscopic Middle Ear Cholesteatoma Surgery: A Case Report of a Novel Head-Up Approach. Otol. Neurotol. 2019, 40, 777–781. [Google Scholar] [CrossRef]
- Nishio, N.; Fujii, M.; Hayashi, Y.; Hiramatsu, M.; Maruo, T.; Iwami, K.; Kamei, Y.; Yagi, S.; Takanari, K.; Fujimoto, Y. Preoperative surgical simulation and validation of the line of resection in anterolateral craniofacial resection of advanced sinonasal sinus carcinoma. Head Neck 2017, 39, 512–519. [Google Scholar] [CrossRef]
- Kutz, J.W., Jr.; Mitchell, D.; Isaacson, B.; Roland, P.S.; Allen, K.P.; Sumer, B.D.; Barnett, S.; Truelson, J.M.; Myers, L.L. En bloc resection of the temporal bone and temporomandibular joint for advanced temporal bone carcinoma. Otolaryngol. Neck Surg. 2015, 152, 571–573. [Google Scholar] [CrossRef]
- Okada, T.; Saito, K.; Takahashi, M.; Hasegawa, Y.; Fujimoto, Y.; Terada, A.; Kamei, Y.; Yoshida, J. En bloc petrosectomy for malignant tumors involving the external auditory canal and middle ear: Surgical methods and long-term outcome. J. Neurosurg. 2008, 108, 97–104. [Google Scholar] [CrossRef]
- Mukoyama, N.; Nishio, N.; Kimura, H.; Kishi, S.; Tokura, T.; Kimura, H. Prospective Evaluation of Health-Related Quality of Life in Patients Undergoing Anterolateral Craniofacial Resection with Orbital Exenteration. J. Neurol. Surg B Skull Base. 2020, 81, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, S.; Takahashi, S.; Toda, M.; Yoshida, K.; Ogawa, K. Pros and Cons of the Exoscope for Otologic Surgery. Surg. Innov. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Corcione, F.; Silvestri, V.; Merola, G.; Dambra, M.; Lionetti, R.; Pirozzi, N. Use of the ORBEYE(TM) Exoscope in General Surgery: The Advent of Video-Assisted Open Surgery. Surg Innov. 2020, 28, 79–84. [Google Scholar] [CrossRef]
- Ahmad, F.I.; Mericli, A.F.; DeFazio, M.V.; Chang, E.I.; Hanasono, M.M.; Pederson, W.C.; Kaufman, M.; Selber, J.C. Application of the ORBEYE three-dimensional exoscope for microsurgical procedures. Microsurgery 2019, 40, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Iwami, K.; Fujii, M.; Nishio, N.; Maruo, T.; Yoshida, T.; Mukoyama, N. Surgical Classification of Radical Temporal Bone Resection and Transcranial Tympanotomy: A Retrospective Study from the Neurosurgical Perspective. World Neurosurg. 2021, 151, e192–e207. [Google Scholar] [CrossRef] [PubMed]
| Case No. | Type of CFR | Approach/ Observation Device | Operation Time (min) | Bleeding (mL) | Complication | PORT | FU (mo) | ||
|---|---|---|---|---|---|---|---|---|---|
| NS | HNS | Total | NS | ||||||
| 1 | Anterolateral | FT/ Exo + End | TF/ None | 1095 | 229 | 1670 | SSI, Sepsis | No | 14 |
| 2 | TBR | TS/ Exo + End | TF/ None | 925 | 284 | 437 | None | No | 8 |
| 3 | Anterior | BF/ Exo + End | TN/ End | 430 | 160 | 230 | None | Yes | 24 |
| 4 | Anterolateral | FT/ Exo + End | TF/ None | 1138 | 244 | 1030 | None | Yes | 14 |
| 5 | Anterior | BF/ Exo + End | TN/ End | 360 | 95 | 89 | None | No | 7 |
| 6 | TBR | Temporal/ Exo + End | TF/ None | 1010 | 192 | 996 | None | No | 4 |
| 7 | Anterior | BF/ Exo + End | TN/ End | 295 | 203 | 550 | None | Yes | 3 |
| 8 | Anterolateral | FT/ Exo + End | TF/ None | 1239 | 193 | 585 | None | No | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwami, K.; Watanabe, T.; Osuka, K.; Ogawa, T.; Miyachi, S.; Fujimoto, Y. Combined Exoscopic and Endoscopic Technique for Craniofacial Resection. Curr. Oncol. 2021, 28, 3945-3958. https://doi.org/10.3390/curroncol28050336
Iwami K, Watanabe T, Osuka K, Ogawa T, Miyachi S, Fujimoto Y. Combined Exoscopic and Endoscopic Technique for Craniofacial Resection. Current Oncology. 2021; 28(5):3945-3958. https://doi.org/10.3390/curroncol28050336
Chicago/Turabian StyleIwami, Kenichiro, Tadashi Watanabe, Koji Osuka, Tetsuya Ogawa, Shigeru Miyachi, and Yasushi Fujimoto. 2021. "Combined Exoscopic and Endoscopic Technique for Craniofacial Resection" Current Oncology 28, no. 5: 3945-3958. https://doi.org/10.3390/curroncol28050336
APA StyleIwami, K., Watanabe, T., Osuka, K., Ogawa, T., Miyachi, S., & Fujimoto, Y. (2021). Combined Exoscopic and Endoscopic Technique for Craniofacial Resection. Current Oncology, 28(5), 3945-3958. https://doi.org/10.3390/curroncol28050336






