Prognostic Radiological Tools for Clinical Stage IA Pure Solid Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
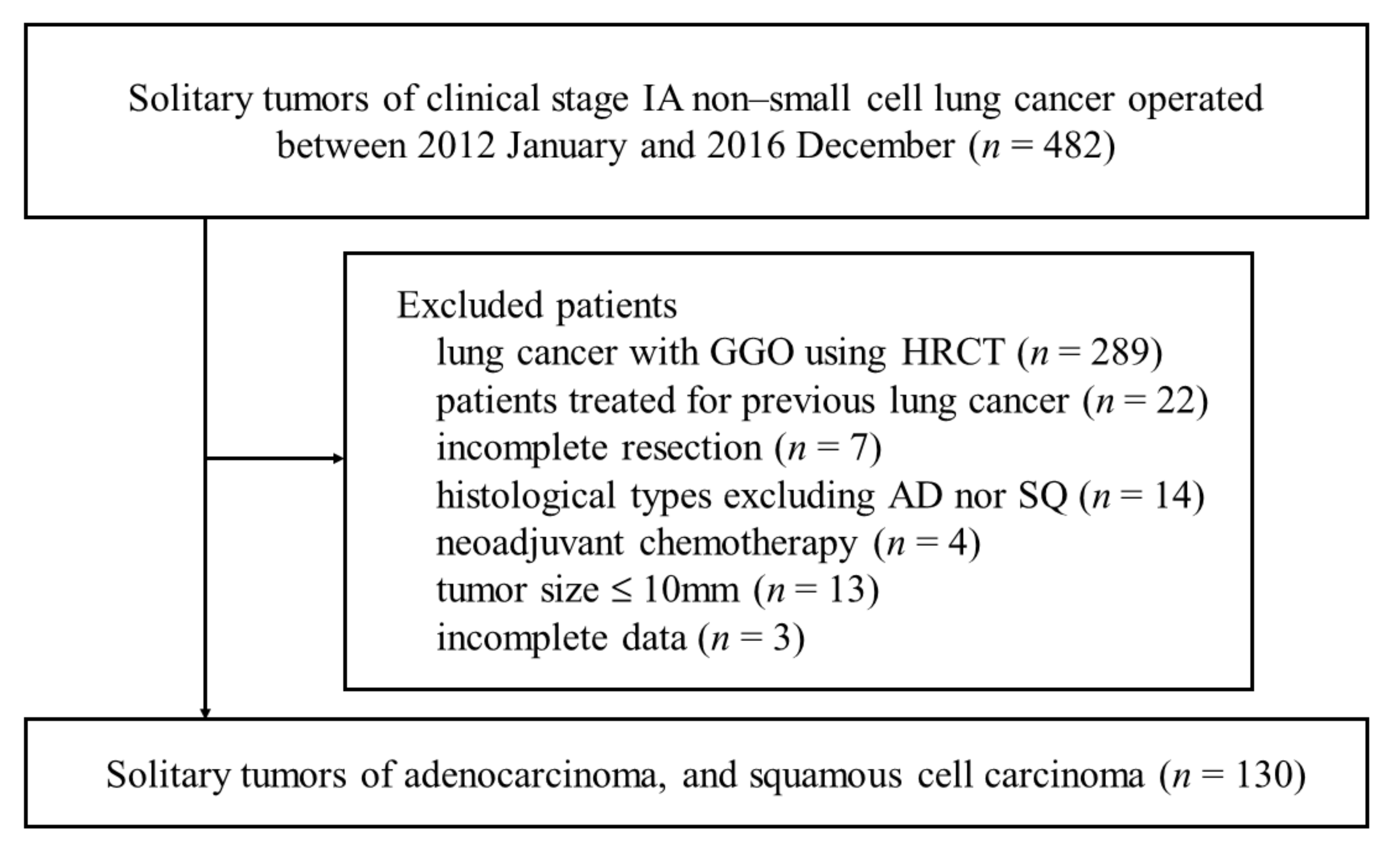
2.1. Patients
2.2. Data
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
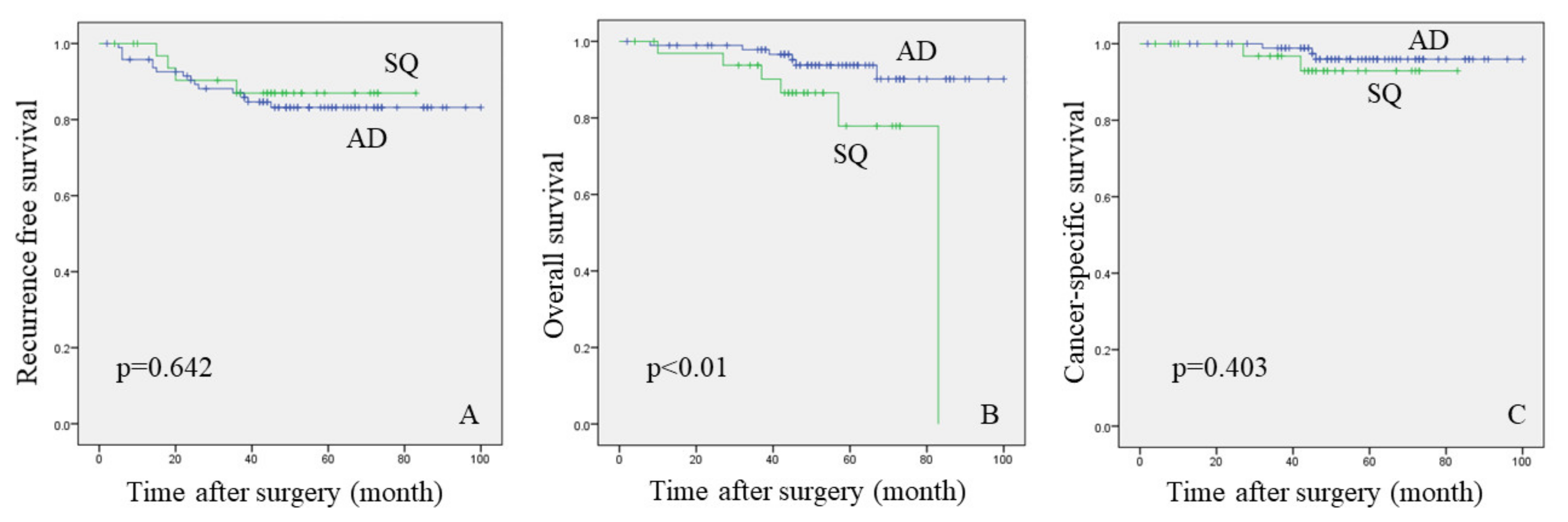
3.2. Analysis between the AD and SQ Groups
3.3. Analysis for the Recurrence
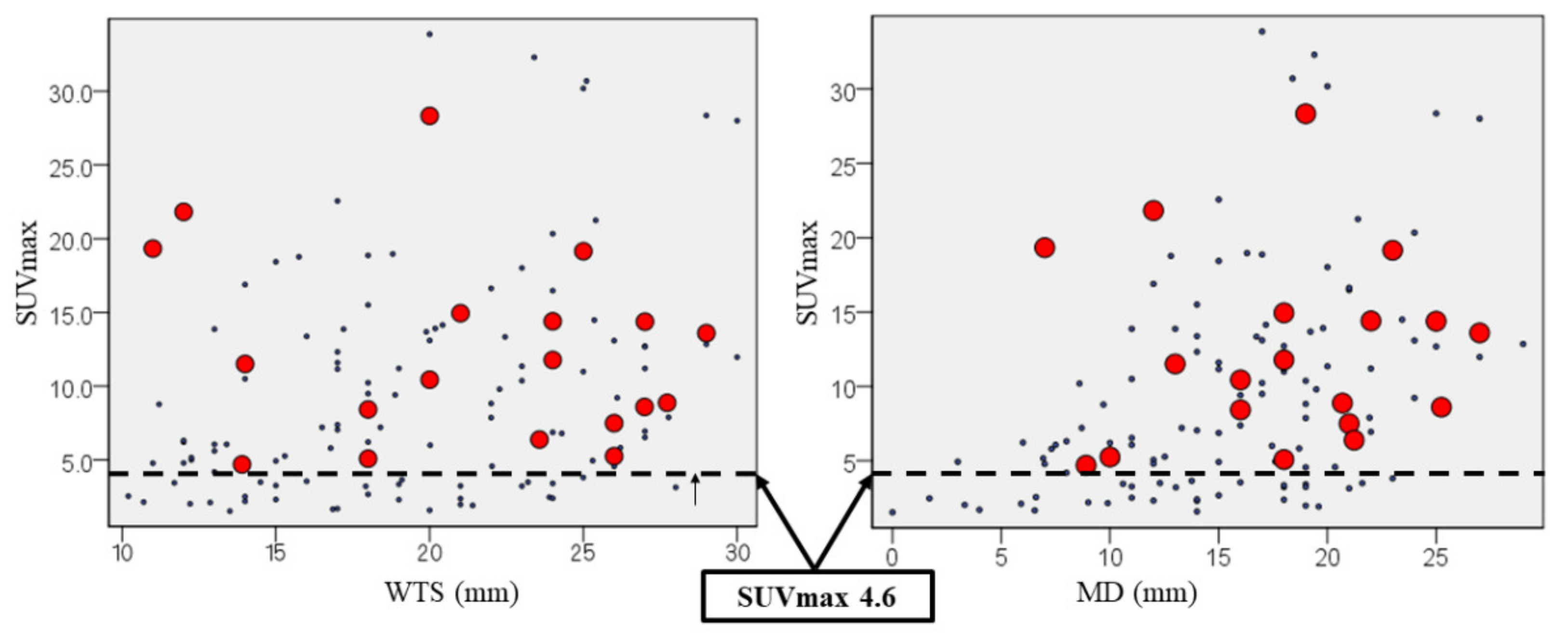
3.4. The Cut-Off Value of Suvmax Associated with Cancer Recurrence
3.5. Analysis for the Lymph Node Metastasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hattori, A.; Matsunaga, T.; Takamochi, K.; Oh, S.; Suzuki, K. Importance of Ground Glass Opacity Component in Clinical Stage IA Radiologic Invasive Lung Cancer. Ann. Thorac. Surg. 2017, 104, 313–320. [Google Scholar] [CrossRef]
- Fu, F.; Zhang, Y.; Wen, Z.; Zheng, D.; Gao, Z.; Han, H.; Deng, L.; Wang, S.; Liu, Q.; Li, Y.; et al. Distinct Prognostic Factors in Patients with Stage I Non-Small Cell Lung Cancer with Radiologic Part-Solid or Solid Lesions. J. Thorac. Oncol. 2019, 14, 2133–2142. [Google Scholar] [CrossRef]
- Hattori, A.; Hirayama, S.; Matsunaga, T.; Hayashi, T.; Takamochi, K.; Oh, S.; Suzuki, K. Distinct Clinicopathologic Characteristics and Prognosis Based on the Presence of Ground Glass Opacity Component in Clinical Stage IA Lung Adenocarcinoma. J. Thorac. Oncol. 2019, 14, 265–275. [Google Scholar] [CrossRef]
- Ye, T.; Deng, L.; Wang, S.; Xiang, J.; Zhang, Y.; Hu, H.; Sun, Y.; Li, Y.; Shen, L.; Xie, L.; et al. Lung Adenocarcinomas Manifesting as Radiological Part-Solid Nodules Define a Special Clinical Subtype. J. Thorac. Oncol. 2019, 14, 617–627. [Google Scholar] [CrossRef]
- Miyoshi, T.; Aokage, K.; Katsumata, S.; Tane, K.; Ishii, G.; Tsuboi, M. Ground-Glass Opacity Is a Strong Prognosticator for Pathologic Stage IA Lung Adenocarcinoma. Ann. Thorac. Surg. 2019, 108, 249–255. [Google Scholar] [CrossRef]
- Miyakawa, A.; Shibamoto, Y.; Kosaki, K.; Hashizume, C. Early response and local control of stage I non-small-cell lung cancer after stereotactic radiotherapy: Difference by histology. Cancer Sci. 2013, 104, 130–134. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Chen, H. A propensity score matching analysis of survival following segmentectomy or wedge resection in early-stage lung invasive adenocarcinoma or squamous cell carcinoma. Oncotarget 2016, 7, 13880–13885. [Google Scholar] [CrossRef][Green Version]
- Ishiguro, F.; Matsuo, K.; Fukui, T.; Mori, S.; Hatooka, S.; Mitsudomi, T. Effect of selective lymph node dissection based on patterns of lobe-specific lymph node metastases on patient outcome in patients with resectable non-small cell lung cancer: A large-scale retrospective cohort study applying a propensity score. J. Thorac. Cardiovasc. Surg. 2010, 139, 1001–1006. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Introduction to the 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J. Thorac. Oncol. 2015, 10, 1240–1242. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Patel, V.K.; Naik, S.K.; Naidich, D.P.; Travis, W.D.; Weingarten, J.A.; Lazzaro, R.; Gutterman, D.D.; Wentowski, C.; Grosu, H.B.; Raoof, S. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: Part 1: Radiologic characteristics and imaging modalities. Chest 2013, 143, 825–839. [Google Scholar] [CrossRef]
- Patel, V.K.; Naik, S.K.; Naidich, D.P.; Travis, W.D.; Weingarten, J.A.; Lazzaro, R.; Gutterman, D.D.; Wentowski, C.; Grosu, H.B.; Raoof, S. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: Part 2: Pretest probability and algorithm. Chest 2013, 143, 840–846. [Google Scholar] [CrossRef]
- Groheux, D.; Quere, G.; Blanc, E.; Lemarignier, C.; Vercellino, L.; de Margerie-Mellon, C.; Merlet, P.; Querellou, S. FDG PET-CT for solitary pulmonary nodule and lung cancer: Literature review. Diagn. Interv. Imaging 2016, 97, 1003–1017. [Google Scholar] [CrossRef]
- Sakakura, N.; Inaba, Y.; Yatabe, Y.; Mizuno, T.; Kuroda, H.; Yoshimura, K.; Sakao, Y. Estimation of the pathological invasive size of pulmonary adenocarcinoma using high-resolution computed tomography of the chest: A consideration based on lung and mediastinal window settings. Lung Cancer 2016, 95, 51–56. [Google Scholar] [CrossRef]
- Koh, Y.W.; Lee, S.J.; Park, S.Y. Differential expression and prognostic significance of GLUT1 according to histologic type of non-small-cell lung cancer and its association with volume-dependent parameters. Lung Cancer 2017, 104, 31–37. [Google Scholar] [CrossRef]
- Schuurbiers, O.C.; Meijer, T.W.; Kaanders, J.H.; Looijen-Salamon, M.G.; de Geus-Oei, L.F.; van der Drift, M.A.; van der Heijden, E.H.; Oyen, W.J.; Visser, E.P.; Span, P.N.; et al. Glucose metabolism in NSCLC is histology-specific and diverges the prognostic potential of 18FDG-PET for adenocarcinoma and squamous cell carcinoma. J. Thorac. Oncol. 2014, 9, 1485–1493. [Google Scholar] [CrossRef]
- Meijer, T.W.; Schuurbiers, O.C.; Kaanders, J.H.; Looijen-Salamon, M.G.; de Geus-Oei, L.F.; Verhagen, A.F.; Lok, J.; van der Heijden, H.F.; Rademakers, S.E.; Span, P.N.; et al. Differences in metabolism between adeno—And squamous cell non-small cell lung carcinomas: Spatial distribution and prognostic value of GLUT1 and MCT4. Lung Cancer 2012, 76, 316–323. [Google Scholar] [CrossRef]
- Ito, R.; Iwano, S.; Kishimoto, M.; Ito, S.; Kato, K.; Naganawa, S. Correlation between FDG-PET/CT findings and solid type non-small cell cancer prognostic factors: Are there differences between adenocarcinoma and squamous cell carcinoma? Ann. Nucl. Med. 2015, 29, 897–905. [Google Scholar] [CrossRef]
- Sakurai, H.; Asamura, H.; Watanabe, S.; Suzuki, K.; Tsuchiya, R. Clinicopathologic features of peripheral squamous cell carcinoma of the lung. Ann. Thorac. Surg. 2004, 78, 222–227. [Google Scholar] [CrossRef]
- Ichikawa, T.; Aokage, K.; Miyoshi, T.; Tane, K.; Suzuki, K.; Makinoshima, H.; Tsuboi, M.; Ishii, G. Correlation between maximum standardized uptake values on FDG-PET and microenvironmental factors in patients with clinical stage IA radiologic pure-solid lung adenocarcinoma. Lung Cancer 2019, 136, 57–64. [Google Scholar] [CrossRef]
- Bayarri-Lara, C.I.; de Miguel Pérez, D.; Cueto Ladrón de Guevara, A.; Rodriguez Fernández, A.; Puche, J.L.; Sánchez-Palencia Ramos, A.; Ruiz Zafra, J.; Giraldo Ospina, C.F.; Delgado-Rodríguez, M.; Expósito Ruiz, M.; et al. Association of circulating tumour cells with early relapse and 18F-fluorodeoxyglucose positron emission tomography uptake in resected non-small-cell lung cancers. Eur. J. Cardiothorac. Surg. 2017, 52, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Choi, J.Y.; Kim, K.; Kim, J.; Shim, Y.M.; Um, S.W.; Kim, H.; Lee, K.H.; Kim, B.T. Volume-based parameters of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography improve outcome prediction in early-stage non-small cell lung cancer after surgical resection. Ann. Surg. 2013, 257, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Bhatt, M.; Bashirzadeh, F.; Hundloe, J.; Ware, R.; Fielding, D.; Ravi Kumar, A.S. Comparison of objective criteria and expert visual interpretation to classify benign and malignant hilar and mediastinal nodes on 18-F FDG PET/CT. Respirology 2015, 20, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Jeon, K.; Koh, W.J.; Suh, G.Y.; Kim, H.; Kwon, O.J.; Chung, M.P.; Lee, K.S.; Shim, Y.M.; Han, J.; et al. Occult nodal metastasis in patients with non-small cell lung cancer at clinical stage IA by PET/CT. Respirology 2010, 15, 1179–1184. [Google Scholar] [CrossRef]
- Kaseda, K.; Asakura, K.; Kazama, A.; Ozawa, Y. Risk Factors for Predicting Occult Lymph Node Metastasis in Patients with Clinical Stage I Non-small Cell Lung Cancer Staged by Integrated Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. World J. Surg. 2016, 40, 2976–2983. [Google Scholar] [CrossRef]
- Nambu, A.; Kato, S.; Sato, Y.; Okuwaki, H.; Nishikawa, K.; Saito, A.; Matsumoto, K.; Ichikawa, T.; Araki, T. Relationship between maximum standardized uptake value (SUVmax) of lung cancer and lymph node metastasis on FDG-PET. Ann. Nucl. Med. 2009, 23, 269–275. [Google Scholar] [CrossRef]
- Taira, N.; Atsumi, E.; Nakachi, S.; Takamatsu, R.; Yohena, T.; Kawasaki, H.; Kawabata, T.; Yoshimi, N. Comparison of GLUT-1, SGLT-1, and SGLT-2 expression in false-negative and true-positive lymph nodes during the (18)F-FDG PET/CT mediastinal nodal staging of non-small cell lung cancer. Lung Cancer 2018, 123, 30–35. [Google Scholar] [CrossRef]


| Variables | Overall (n = 130) | AD Group (n = 96) | SQ Group (n = 34) | p-Value |
|---|---|---|---|---|
| N (%), Median or Average ± SD, Range | N (%), Median or Average ± SD, Range | N (%), Median or Average ± SD, Range | ||
| Age (year old) | 69 ± 8.6 (43–86) | 68 ± 8.9 (43–84) | 75 ± 6.1 (57–86) | <0.01 a |
| Sex (male) | 69 (53.1%) | 39 (40.6%) | 30 (88.2%) | <0.01 b |
| Smoking index | 625 ± 736 (0–3600) | 7 ± 663 (0–2950) | 1030 ± 700 (0–3600) | <0.01 a |
| Spirometry test | ||||
| %VC | 98 ± 13.8 (67.8–149) | 99.6 ± 13.7 (68.9–149) | 95 ± 13.6 (67.8–120.7) | 0.058 a |
| FEV1.0% | 76.5 ± 8.5 (46–98) | 77.3 ± 9.1 (46–98) | 74 ± 6.5 (59.5–88.8) | 0.128 a |
| Findings on CT | ||||
| whole tumor size (mm) | 19.9 ± 5.2 (11–30) | 20 ± 5.1 (11–30) | 18.9 ± 5.5 (11–30) | 0.484 a |
| mediastinal diameter (mm) | 15.7 ± 5.9 (0–29) | 15.4 ± 6 (0–27) | 16.4 ± 5.4 (7–29) | 0.398 d |
| TDR (%) | 15.8 ± 19 (0–100) | 17.2 ± 20.6 (0–100) | 14.3 ± 12 (0–47.3) | 0.048 a |
| SUVmax | 7.7 ± 7.2 (1.5–33.9) | 6.4 ± 6.2 (1.5–33.9) | 14 ± 7.6 (3.6–30.7) | <0.01 a |
| Lobectomy/sublobar resection | 95/35 (73.1/26.9%) | 70/26 (72.9/26.1%) | 25/9 (73.5/26.5%) | 0.945 b |
| Mediastinal lymph node dissection | 90 (69.2%) | 68 (70.8%) | 22 (64.7%) | 0.506 b |
| Pathological invasive size (mm) | 17 ± 7.2 (1–44) | 16 ± 6.9 (1–40) | 18 ± 8 (4–44) | 0.349 a |
| Lymphovascular invasion | 100 (76.9%) | 74 (77.1%) | 26 (76.5%) | 0.942 b |
| Pleural invasion (pl2) | 10 (7.7%) | 9 (9.4%) | 1 (2.9%) | 0.208 c |
| Histological low grade | 32 (24.6%) | 26 (27.1%) | 6 (17.6%) | 0.272 b |
| Pathological lymph node metastasis | 19 (14.6%) | 13 (13.5%) | 6 (17.6%) | 0.571 b |
| Adjuvant chemotherapy | 10 (7.7%) | 7 (7.3%) | 3 (8.8%) | 0.528 c |
| Cancer recurrence | 19 (14.6%) | 15 (15.8%) | 4 (11.8%) | 0.408 c |
| Variables | Recurrence Group (N = 19) | Non-Recurrence Group (N = 111) | p-Value |
|---|---|---|---|
| N (%), Median ± SD, Range | N (%), Median ± SD, Range | ||
| Age (year old) | 67 ± 7.8 (53–81) | 69 ± 8.8 (43–86) | 0.649 a |
| Sex (male) | 11 (57.9%) | 66 (59.5%) | 0.898 b |
| Smoking index | 720 ± 764 (0–2580) | 620 ± 735 (0–3600) | 0.730 a |
| Findings on CT | |||
| whole tumor size (mm) | 23.6 ± 5.6 (11–29) | 19 ± 5.1 (11–30) | 0.127 a |
| mediastinal diameter (mm) | 18 ± 5.7 (7–27) | 16 ± 5.8 (0–29) | 0.066 c |
| tumor disappearance ratio (%) | 9.9 ± 15.3 (0–61.5) | 16.7 ± 19.5 (0–100) | 0.082 a |
| SUVmax | 11.5 ± 6.3 (4.7–28.3) | 6.9 ± 7.3 (1.5–33.9) | 0.016 a |
| Spirometry test | |||
| %VC | 100 ± 16.3 (67.8–127.5) | 98 ± 13.5 (68.9–149) | 0.642 a |
| FEV1.0% | 77.4 ± 8.1 (54.8–91.5) | 76.4 ± 8.7 (46–98) | 0.767 a |
| CEA (ng/mL) | 3.2 ± 9.3 (0.8–35.3) | 3.3 ± 3.4 (0.5–20.1) | 0.395 a |
| CYFRA (ng/mL) | 1.4 ± 0.7 (0.6–2.8) | 1.3 ± 0.7 (0.4–3.9) | 0.806 a |
| Lobectomy/sublobar resection | 15/4 (78.9/21.1%) | 80/31 (72.1/27.9%) | 0.532 b |
| Mediastinal lymph node dissection | 11 (57.9%) | 79 (71.2%) | 0.247 b |
| Squamous cell carcinoma | 4 (21.1%) | 30 (27%) | 0.408 d |
| Pathological whole size (mm) | 21 ± 7.8 (4–40) | 18 ± 6.2 (8–44) | 0.034 a |
| Pleural invasion (pl2) | 2 (10.5%) | 8 (7.2%) | 0.446 d |
| Histological low grade | 8 (42.1%) | 24 (21.6%) | 0.056 d |
| Lymph node metastasis | 7 (36.8%) | 12 (10.8%) | 0.007 d |
| Adjuvant chemotherapy | 3 (15.8%) | 7 (6.3%) | 0.140 d |
| Variables | LNM Group (N = 15) | Non-LNM Group (N = 68) | p-Value |
|---|---|---|---|
| N (%), Median or Average ± SD, Range | N (%), Median or Average ± SD, Range | ||
| Age (year old) | 66 ± 8.5 (47–79) | 68 ± 7.7 (43–80) | 0.424 a |
| Sex (male) | 6 (40%) | 38 (55.9%) | 0.265 b |
| Smoking index | 0 ± 586 (0–1920) | 645 ± 684 (0–3000) | 0.080 a |
| Findings on CT | |||
| whole tumor size (mm) | 21 ± 5.5 (11–28) | 20 ± 4.8 (12–30) | 0.526 a |
| mediastinal diameter (mm) | 16.9 ± 6 (8–25) | 16 ± 5.7 (0–27) | 0.567 c |
| tumor disappearance ratio (%) | 14.3 ± 15 (5.3–56.8) | 15.4 ± 20.4 (0–100) | 0.939 a |
| SUVmax | 8.9 ± 6.9 (2.4–30.2) | 7.7 ± 7 (1.5–33.9) | 0.558 a |
| SUVmax ≥4.6 | 14 (93.3%) | 49 (72.1%) | 0.072 d |
| CEA (ng/mL) | 3.3 ± 4.7 (0.8–20.1) | 3.3 ± 3.9 (0.6–25.9) | 0.892 a |
| CYFRA (ng/mL) | 1.2 ± 0.6 (0.4–3) | 0.8 ± 0.5 (0.5–2.3) | 0.041 a |
| Pathological whole size (mm) | 21 ± 9.2 (8–44) | 19 ± 5.6 (8–40) | 0.726 a |
| Pathological invasive size (mm) | 20 ± 9.4 (8–44) | 17.5 ± 6.4 (1–40) | 0.529 a |
| Squamous cell carcinoma | 3 (20%) | 17 (25%) | 0.485 d |
| Total number of excised lymph nodes | 15 ± 5.8 (7–26) | 13 ± 6.5 (5–32) | 0.803 |
| Lymphvascular invasion | 15 (100%) | 50 (73.5%) | 0.017 d |
| Pleural invasion (pl2) | 2 (13.3%) | 7 (10.3%) | 0.513 d |
| Histological low grade | 5 (33.3%) | 13 (19.1%) | 0.191 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakada, T.; Takahashi, Y.; Sakakura, N.; Iwata, H.; Ohtsuka, T.; Kuroda, H. Prognostic Radiological Tools for Clinical Stage IA Pure Solid Lung Cancer. Curr. Oncol. 2021, 28, 3846-3856. https://doi.org/10.3390/curroncol28050328
Nakada T, Takahashi Y, Sakakura N, Iwata H, Ohtsuka T, Kuroda H. Prognostic Radiological Tools for Clinical Stage IA Pure Solid Lung Cancer. Current Oncology. 2021; 28(5):3846-3856. https://doi.org/10.3390/curroncol28050328
Chicago/Turabian StyleNakada, Takeo, Yusuke Takahashi, Noriaki Sakakura, Hiroshi Iwata, Takashi Ohtsuka, and Hiroaki Kuroda. 2021. "Prognostic Radiological Tools for Clinical Stage IA Pure Solid Lung Cancer" Current Oncology 28, no. 5: 3846-3856. https://doi.org/10.3390/curroncol28050328
APA StyleNakada, T., Takahashi, Y., Sakakura, N., Iwata, H., Ohtsuka, T., & Kuroda, H. (2021). Prognostic Radiological Tools for Clinical Stage IA Pure Solid Lung Cancer. Current Oncology, 28(5), 3846-3856. https://doi.org/10.3390/curroncol28050328





