Abstract
Canada’s vast geography, and centralized delivery of cancer care and clinical trials create barriers for trial participation for patients in remote and rural settings. The development and implementation of a framework that enables safe and regulatory compliant trial participation through local healthcare providers would benefit Canadian patients, clinicians, trial sponsors and the health care system. To address this issue, representatives of Canada’s cancer clinical trial community met to identify key challenges and develop recommendations for remote patient participation in trials. A structured literature review identified remote/rural trial delivery models. A panel of expert stakeholders reviewed the models and participated in a workshop to assess health system readiness, identify needed processes, tools and mechanisms, and develop recommendations for a Canadian framework for decentralized clinical trial conduct. The Canadian Remote Access Framework for clinical Trials (CRAFT) represents a risk-based approach used by site investigators to delegate responsibilities for a given trial to satellite health centres within a hub-and-spoke “trial cluster”. The Framework includes specific recommendations to ensure research experience, capacity, regulatory compliance and patient safety. Canada’s cancer care and telemedicine systems can be leveraged to enable broader access to clinical trials for patients who are geographically remote from cancer centres. CRAFT’s risk-based framework is based on other successful models of remote trial patient management and is in the pilot implementation phase in Canada.
1. Introduction
Clinical trials are vital to improving standards of cancer care, yet participation in clinical trials is low in Canada. For cancer trials, the reported rates of trial participants to new incident cancer cases are 4.7% overall, and as low as 1% in some Canadian provinces, as compared to 14% in the UK [1,2]. This difference may partly be due to issues of access. Canada’s vast geography creates challenges that limit trial participation and the ability of patients to access innovative new therapies and treatment strategies. Over 30% of the population reside outside of large/medium population areas where regional cancer centres are located [3,4]. Additional barriers to clinical trial participation may include lack of awareness of available trial options and patient or clinician biases toward trial participation [5,6]. Given that low patient accrual is a leading reason cited for premature trial closure, the scale of unrealized accrual potential in Canada is enormous [7].
There are strong ethical, scientific, and medical reasons that cancer patients should have access to trials. The ethical principle of respect for persons and equity, including the just distribution of resources and of risk and benefit, requires that people, regardless of where they live, should have the opportunity to participate in clinical trials [8]. It also aligns with the necessary condition for accessibility of services identified within the Canada Health Act [9]. Involving underrepresented populations can improve generalizability of research results and broader access would improve the feasibility of novel therapeutic trials in a country with a low population density, particularly in the study of rare diseases.
Telecommunication advances and health care delivery models have enabled cancer patients to be managed closer to their homes. Telemedicine for patient assessments and extending cancer care delivery to include clinical teams at health care facilities remote from cancer centres have become standard across Canada. In addition, remote trial participation during the COVID-19 pandemic was enabled through evolving Canadian and international regulatory guidances [10,11,12]. Proof-of-concept initiatives that leverage these approaches have shown that patients can participate safely and trial conduct can be maintained with appropriate compliance and oversight [13].
2. Methods
In line with its objective to support the conduct of academic sponsored clinical trials, the Canadian Cancer Clinical Trials Network (3CTN) convened a steering committee of academic, public, government and industry stakeholders with expertise and knowledge of Canada’s clinical trial environment. The committee was charged to review existing models for remote access to trials; assess national health system readiness; identify needs and enabling mechanisms; and to develop recommendations that would serve as a framework for a Canadian approach (refer to Table A1 for a list of steering committee members and Supplementary Materials S1 for committee terms of reference).
Published literature was searched using MESH terms “clinical trials” and “health services accessibility” of Medline and Embase databases to identify existing models that enabled rural and remote patients to participate in trials closer to their homes. Both publications and references were reviewed for relevance. Stakeholder interviews with trial sponsors, ethics board members, regulators, health services providers, patients and clinical trial researchers were conducted to identify relevant initiatives, case studies and existing resources to help plan, assess feasibility and support trial conduct for geographically remote patients.
Results from the publications and interviews informed the approach for a structured stakeholder workshop held at the 2019 Canadian Cancer Research Conference (see Supplementary Materials Table S1). Workshop participants included trial sponsors, experts in telemedicine, clinical trial agreements, regulatory affairs, research ethics and privacy, clinical research professionals, patient partners, as well as representatives from Health Canada. Interactive sessions were designed to obtain recommendations on framework options for remote access, requirements for implementation, and areas requiring clarification within current regulations to foster adoption (see Supplementary Materials Table S2). The draft framework and recommendations were developed by the steering committee and shared with the workshop participants and broader stakeholder community for comments before finalizing.
3. Results
3.1. Results from the Literature Review
Multiple publications recommended trial sponsors and researchers explore the use of technologies and other tools to reduce the effort, time, cost and travel burdens associated with clinical trial participation [6,14]. Models of decentralized trial conduct in Canada and Australia were identified, as were frameworks for delivering standard of care cancer treatment at local community healthcare centres via telemedicine programs [5,15].
Telemedicine and remote care are available in all provinces and can be used to conduct trial specific activities. The Federation of Medical Regulatory Authorities of Canada defines telemedicine as a medical service provided remotely via information and communication technology. “Remotely” is defined as without physical contact regardless of distances [16]. Existing technologies can support consent, document and data collection, training, oversight and compliance processes. Electronic healthcare platforms and technology advancements have enabled timelier, effective patient assessments and data collection. Compliance with privacy regulations and electronic data standards enable extension of the circle of care among health providers at different health care facilities [2,17,18]. Table 1 provides some representative examples of technology-enabled, distributed models of adult and paediatric cancer care from across Canada which can be leveraged to conduct clinical trials.

Table 1.
Canadian examples of oncology networks/distributed models of care.
3.2. Two Case Studies of Remote Trial Participation
Two successful models of remote trial access are the Clinical Oncology Society of Australia’s Australasian Teletrial Model (COSA ATM) and the Pediatric Oncology Group of Ontario (POGO) Satellite Program. Both leverage telemedicine technologies and health care collaborations and provide guidelines to enable participation of remote and rural patients in clinical research [19,20].
3.2.1. COSA Australasian Tele-Trial Model (COSA ATM)
Australia has made a significant investment in developing and implementing a model to address the low population density and vast geography to enable trial participation [21,22]. An implementation pilot project evaluated a hub and spoke model linking cancer centres to remote health care providers participating in a portfolio of industry, cooperative group and investigator-sponsored trials of different designs and interventions [23]. Table 2 provides examples of trials in the COSA ATM pilot.

Table 2.
Examples of COSA ATM portfolio trial diversity [22].
A formal evaluation of trial conduct for centres participating in the randomized Phase III trial, monarchE found that the data produced was acceptable for commercially sponsored research destined for marketing applications and regulators. Furthermore, it showed successful recruitment and management of rural and remote patients to trials closer to their homes and increased clinical trials capability and training of regional sites in Good Clinical Practice (GCP) [13]. These findings are consistent with those from the pilot of the US National Cancer Institute Community Cancer Centers Program, which demonstrated both feasibility and improvement in trial recruitment at community centres on NCI sponsored trials [24].
3.2.2. Pediatric Oncology Group of Ontario (POGO) Satellite Program
Participation in multi-centred trials is a core component of childhood cancer care. Given the small numbers of children with cancer, community hospitals cannot independently obtain and maintain the expertise, capacity or infrastructure for clinical trial activities. Since 1998, POGO’s Provincial Pediatric Oncology Satellite Program has enabled the transfer of certain aspects of a child’s clinical care, including clinical trial research activities, to community hospitals closer to the children’s homes. The POGO model is a networked, shared-care system partnership of Ontario’s five tertiary hospitals and POGO Satellite community hospitals that enables some clinical trial activities to be conducted in community settings. Industry and academic trial sponsors such as the Children’s Oncology Group (COG) and the US National Cancer Institute (NCI) endorse the model for conduct of their trials [20,25].
3.3. Recommended Model
Similarities between Canadian and Australian population distributions, national cancer centre networks, regulations and health system funding arrangements suggest the COSA ATM “hub and spoke” of lead cancer centre linked with community health care facilities and personnel is a feasible option for Canada. The model is conceptually straightforward, applicable to a wide variety of trials and scalable across regions and trial populations. When tested, it showed that patient recruitment, retention and national trial capacity was enhanced. With agreement of a study sponsor and participating institutions, a regional or metropolitan centre serving as the primary site holds overall responsibility for supervision and coordination for a hub-and-spoke “trial cluster” comprised of one or more local satellite site(s) (see Figure 1).
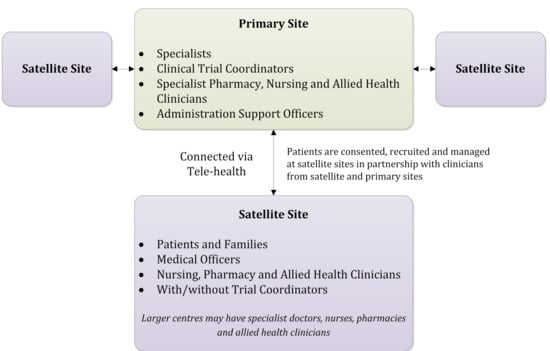
Figure 1.
A trial cluster from the Australasian tele-trial model. Adapted from Clinical Oncology Society of Australia, Australasian Tele-Trial Model: A National Guide for Implementation. 2016.
Satellite sites are delegated responsibilities and collaborate on activities such as recruitment, consent, treatment and/or follow-up using a risk-based approach that considers trial complexity, patient safety, site research capacity and professional competencies. Trial procedures can be streamlined and in-person patient visits can be conducted at the cancer centre, local health centre, via telemedicine, or through compliant remote data collection platforms. Protocol-specific and core research training (e.g., GCP, TCPS 2) needs are determined based on defined research roles and provided to those personnel with identified research responsibilities. Training requirements would not necessarily formally extend to allied healthcare or administrative personnel performing duties that fall within professional education, accreditation or standards of care.
3.4. Canadian Remote Access Framework for Clinical Trials (CRAFT) Recommendations
The CRAFT framework provides recommendations in eight areas, summarized in Table 3, to facilitate remote trial conduct: infrastructure, funding, trial planning and conduct, ethics, regulatory, legislative and legal requirements, indemnity and insurance, engagement and communication.

Table 3.
CRAFT Recommendations.
The CRAFT steering committee recommends the following be conducted to evaluate the remote access framework and guide its implementation in Canadian settings.
- Conduct pilot studies to evaluate the model and share findings. Pilot implementation projects should build upon existing regional networks of shared clinical care with personnel that are supportive of improving trial participation. Pilot clusters would extend care delivery to include trial delivery, leveraging existing regional patterns of care and telemedicine capacity across site networks. The cluster could begin by participating in a trial of interventions of lower risk and complexity, such as a trial assessing different standard of care treatments or supportive care measures. Such a trial experience would establish the cluster, and the processes to ensure trial oversight and conduct (e.g., site contracts, REB, training, delegation of responsibilities).
- Identify agreement terms among investigators, sub-investigators, healthcare providers (or their representatives), insurers and sponsors that can address research responsibilities, professional liability, and indemnity. Ideally, a core set of template documents could be developed to be used by those interested in implementing the model and framework.
- Identify feasible and cost-effective options for establishing linkages between centres that consider professional capacity, existing workflows, and scheduling requirements. Research activities should be distributed to efficiently and effectively ensure optimal engagement of personnel and resources.
- Continue to consult with Health Canada to ensure recognition and support of models of trial conduct within a cluster across regulations, guidance documents, review of clinical trial applications and site/sponsor inspections.
4. Discussion
The CRAFT initiative aims to develop a model for Canada that would address the geographic barriers to trial participation. Although development of the framework was initiated in 2019, prior to the COVID-19 pandemic, transformational changes in healthcare services since have helped address access restrictions imposed by the pandemic and allowed many of the key concepts of decentralized trial conduct to be implemented. For example, there is now more widespread use of distributed care models and virtual platforms for healthcare and for trial conduct, along with new regulatory measures that enable leveraging virtual care models for decentralized clinical trial (DCT) activities [26,27]. Telemedicine for virtual consults, direct-to-patient shipping of oral drugs, use of local laboratories and imaging services to limit visits to cancer centres have been implemented to ensure that patient participation on trial protocols could continue [10]. Such activities can and should continue beyond the pandemic to become routine means for trial participation.
The POGO and Australian experiences and the approaches adopted to ensure continued trial participation during the pandemic showed that remote or decentralized participation in clinical trials is feasible and preferable for many patients, health care providers and trial sponsors. The CRAFT model provides practical recommendations that if implemented, would ensure successful trial conduct. Fundamental to adoption would be recognition by sponsors, regulators, ethics boards and participating health care institutions of a “cluster” as an organizational unit for trial conduct. Trial complexity, capacity and capabilities within the cluster will determine the scope of delegated activities. Access to and sharing of trial participants’ health information as part of the “the circle of care” within the cluster is also a key consideration. The extent of training required by remote practitioners and staff should be based on whether the proposed trial activities fall within the scope of routine standard practice or are research-specific. Additional communication, oversight, SOPs and training within the cluster and provision of resources to support the activities of personnel at participating sites are needed to assure patient safety, research quality and regulatory compliance.
Decentralized trial activities require added commitments for tools, infrastructure, communication frameworks and funds along with added time investments for site personnel. New agreements, training, study drug distribution and handling, new workflows for trial management, oversight and monitoring are implementation costs to be factored. Costs may be mitigated by improved recruitment and retention, reduced patient travel, better patient quality of life, and broader health system and economic benefits of a decentralized approach to trial delivery. Additional benefits may include more rapid trial completion, trial results that are more representative of outcomes of the intended “real world” population, and a broader health care workforce with expertise in clinical research. Higher trial accrual rates would improve trial feasibility, particularly for rare diseases. Improved overall participation rates across all trials would serve to reduce the disparity in outcomes for geographically dispersed populations.
There is a unique and timely opportunity to implement, evaluate and improve CRAFT across all regions of Canada. 3CTN plans for demonstration of CRAFT proof of concept pilot among member cancer centres. The pilot will be enabled by the CRAFT framework and the 3CTN collaborative network of clinicians, patient representatives, industry sponsors, Health Canada and other stakeholders. Scaled implementation of CRAFT across the Canadian clinical trial system will be informed by evaluation of pilot outcomes, further knowledge exchange and would also benefit from health system policy development that recognizes and enables equitable access to clinical trials as a fundamental component of standard of care delivery.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/curroncol28050329/s1, Materials S1: CRAFT Steering Committee Terms of Reference, Table S1: CRAFT Workshop Agenda, Table S2: Remote Clinical Trial Conduct—Framework Considerations for discussion at CRAFT Workshop.
Author Contributions
Conceptualization, S.S. and J.E.D.; methodology, S.S., G.B., K.B.-R., K.D., B.J.E., D.K.L., J.L., H.L., J.P., A.S., P.S. and J.E.D.; writing—original draft preparation, S.S. and J.E.D.; writing—review and editing, S.S., G.B., K.B.-R., K.D., B.J.E., D.K.L., J.L., H.L., J.P., A.S., P.S. and J.E.D.; project administration, S.S.; funding acquisition, S.S. and J.E.D. All authors have read and agreed to the published version of the manuscript.
Funding
Support for CRAFT and production of this publication has been made possible through collaboration and financial support from the Canadian Partnership against Cancer Corporation and Health Canada, as well as the Ontario Institute for Cancer Research, funded by the Government of Ontario. The views and opinions expressed in this article are those of the authors and do not necessarily reflect government policy or the position of the Canadian Partnership against Cancer or the Ontario Institute for Cancer Research.
Acknowledgments
CRAFT was developed with input from trial sponsors, experts in telemedicine delivery, clinical trial agreements, regulatory affairs, research ethics and privacy, clinical research professionals and patients from cancer centres and satellite sites as well as representatives from Health Canada. The CRAFT project team would like to extend a sincere thank you for support of this initiative to Sabe Sabesan and fellow contributors to the development of the Clinical Oncology Society of Australia, Australasian Tele-Trial Model, as well as the Pediatric Oncology Group of Ontario for sharing accrued experiences in operationalising the POGO Satellite Program. 3CTN would like to thank Greg Williams of Williams Advisory Services for his contribution in developing the CRAFT position paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
CRAFT steering and writing committee.
Table A1.
CRAFT steering and writing committee.
| Member | Affiliation(s) | Title(s) |
|---|---|---|
| Stephen Sundquist * (Chair) | 3CTN | Executive Director |
| Gerry Batist | Quebec-Clinical Research Organization in Cancer | Scientific Director |
| Kathy Brodeur-Robb * | C-17 | Executive Director |
| Janet Dancey * | 3CTN; Canadian Cancer Trials Group | Scientific Director; Executive Director |
| Kathryn Dyck | CancerCare Manitoba | Clinical Trials Manager |
| Bernie Eigl | BC Cancer | Provincial Director—Clinical Trials |
| David K. Lee | Health Canada—Health Products and Food Branch | Chief Regulatory Officer |
| Jacqueline Limoges * | Ontario Cancer Research Ethics Board | Chair |
| Jim Pankovich | Bold Therapeutics Inc | Executive Vice President, Clinical Development |
| Anna Sadura | Canadian Cancer Trials Group | Manager, Trial Management Group (retired) |
| Patrick Sullivan | Team Finn Foundation; 3CTN; Canadian Cancer Trials Group | Childhood Cancer Research Advocacy; Patient Representative; Research Advisor |
| Holly Longstaff * (Writing Committee only) | Provincial Health Services Authority | Director, Privacy and Access, PHSA Research and New Initiatives Research & Academic Services |
| *Writing Committee Member |
References
- Canadian Partnership Against Cancer. The 2018 Cancer System Performance Report; Canadian Partnership Against Cancer: Toronto, ON, Canada, 2018. [Google Scholar]
- Sinha, G. United Kingdom Becomes the Cancer Clinical Trials Recruitment Capital of the World. J. Natl. Cancer Inst. 2007, 99, 420–422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Statistics Canada. Population Centre. 2017. Available online: https://www12.statcan.gc.ca/census-recensement/2016/ref/dict/geo049a-eng.cfm (accessed on 13 January 2020).
- Canadian Organization of Medical Physicists. Canadian Cancer Centres. Available online: https://www.comp-ocpm.ca/english/career-education/career-resources/canadian-cancer-centres.html (accessed on 14 February 2020).
- Unger, J.M.; Cook, E.; Tai, E.; Bleyer, A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 185–198. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society Cancer Action Network. Barriers to Patient Enrollment in Therapeutic Clinical Trials for Cancer: A Landscape Report; American Cancer Society Cancer Action Network: Washington, DC, USA, 2018. [Google Scholar]
- Schroen, A.T.; Petroni, G.R.; Wang, H.; Gray, R.; Wang, X.F.; Cronin, W.; Sargent, D.; Benedetti, J.; Wickerham, D.L.; Djulbegovic, B.; et al. Preliminary evaluation of factors associated with premature trial closure and feasibility of accrual benchmarks in phase III oncology trials. Clin. Trials 2010, 7, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. TCPS 2 (2018)—Chapter 1: Ethics Framework. Government of Canada; 2019. Available online: https://ethics.gc.ca/eng/tcps2-eptc2_2018_chapter1-chapitre1.html#b (accessed on 8 January 2020).
- Government of Canada. Canada Health Act. In R.S.C.; Government of Canada; 1985. [Google Scholar]
- Health Canada. Management of Clinical Trials during the COVID-19 Pandemic: Notice to Clinical Trial Sponsors. 2021. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/management-clinical-trials-during-covid-19-pandemic.html (accessed on 27 May 2020).
- U.S. Food and Drug Administration. Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health Emergency; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020. [Google Scholar]
- European Medicines Agency. Guidance on the Management of Clinical Trials During the COVID-19 (Coronavirus) Pandemic; European Medicines Agency: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Queensland Health. Queensland Health Teletrials Pilot Analysis Report; Queensland Health: Brisbane, Australia, 2019. [Google Scholar]
- Clinical Trials Transformation Initiative. Decentralized Clinical Trials 2. 2018. Available online: https://www.ctti-clinicaltrials.org/sites/www.ctti-clinicaltrials.org/files/dct_recommendations_final.pdf (accessed on 10 February 2020).
- Unger, J.M.; Vaidya, R.; Hershman, D.L.; Minasian, L.M.; Fleury, M.E. Systematic Review and Meta-Analysis of the Magnitude of Structural, Clinical, and Physician and Patient Barriers to Cancer Clinical Trial Participation. J. Natl. Cancer Inst. 2019, 111, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Federation of Medical Regulatory Authorities of Canada. FMRAC Framework on Telemedicine. Available online: http://fmrac.ca/fmrac-framework-on-telemedicine/ (accessed on 1 October 2019).
- Humer, M.F.; Campling, B.G. The Role of Telemedicine in Providing Thoracic Oncology Care to Remote Areas of British Columbia. Curr. Oncol. Rep. 2017, 19, 52. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Sabesan, S.; Zalcberg, J. Telehealth models could be extended to conducting clinical trials-a teletrial approach. Eur. J. Cancer Care 2016, 27, e12587. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Greenberg, M.; Malkin, D.; Portwine, C.; Johnston, D.; Silva, M.; Zelcer, S.; Sonshine, S.; Manzo, J.; Bennett, C.; et al. Pediatric oncology clinical trial participation where the geography is vast: Development of a clinical research system for tertiary and satellite centers in Ontario, Canada. Pediatr. Blood Cancer 2017, 65, e26901. [Google Scholar] [CrossRef] [PubMed]
- Clinical Oncology Society of Australia. Australasian Tele-Trial Model: A National Guide for Implementation; Clinical Oncology Society of Australia: Sydney, Australia, 2016. [Google Scholar]
- Sabesan, S.; Zalcberg, J.; Underhill, C.; Zielinski, R.; Burbury, K.; Ansari, Z.; Rainey, N.; Collins, I.; Osbourne, R.; Sanmugarajah, J.; et al. Implementation of the Australasian Teletrial Model: Lessons from practice. Asia Pac. J. Clin. Oncol. 2019, 15 (Suppl. 8), 3–14. [Google Scholar]
- Clinical Oncology Society of Australia. Pilot Implementation of the Australasian Tele-Trial Model Project Completion Report; Clinical Oncology Society of Australia: Sydney, Australia, 2021. [Google Scholar]
- Hirsch, B.R.; Locke, S.; Abernethy, A.P. Experience of the National Cancer Institute Community Cancer Centers Program on Community-Based Cancer Clinical Trials Activity. J. Oncol. Pract. 2016, 12, e350–e358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pediatric Oncology Group of Ontario. Satellite Manual. 1997. Available online: https://www.pogo.ca/satellite-manual/ (accessed on 1 August 2019).
- U.S. Food and Drug Administration. Advancing Oncology Decentralized Trials. 2021. Available online: https://www.fda.gov/about-fda/oncology-center-excellence/advancing-oncology-decentralized-trials (accessed on 27 May 2021).
- Clinical Trials and Translational Research Advisory Committee. Strategic Planning Working Group Report. 2020. Available online: https://deainfo.nci.nih.gov/advisory/ctac/1120/Loehrer1.pdf (accessed on 27 May 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).