Complex Differential Diagnosis between Primary Breast Cancer and Breast Metastasis from EGFR-Mutated Lung Adenocarcinoma: Case Report and Literature Review
Abstract
:1. Introduction
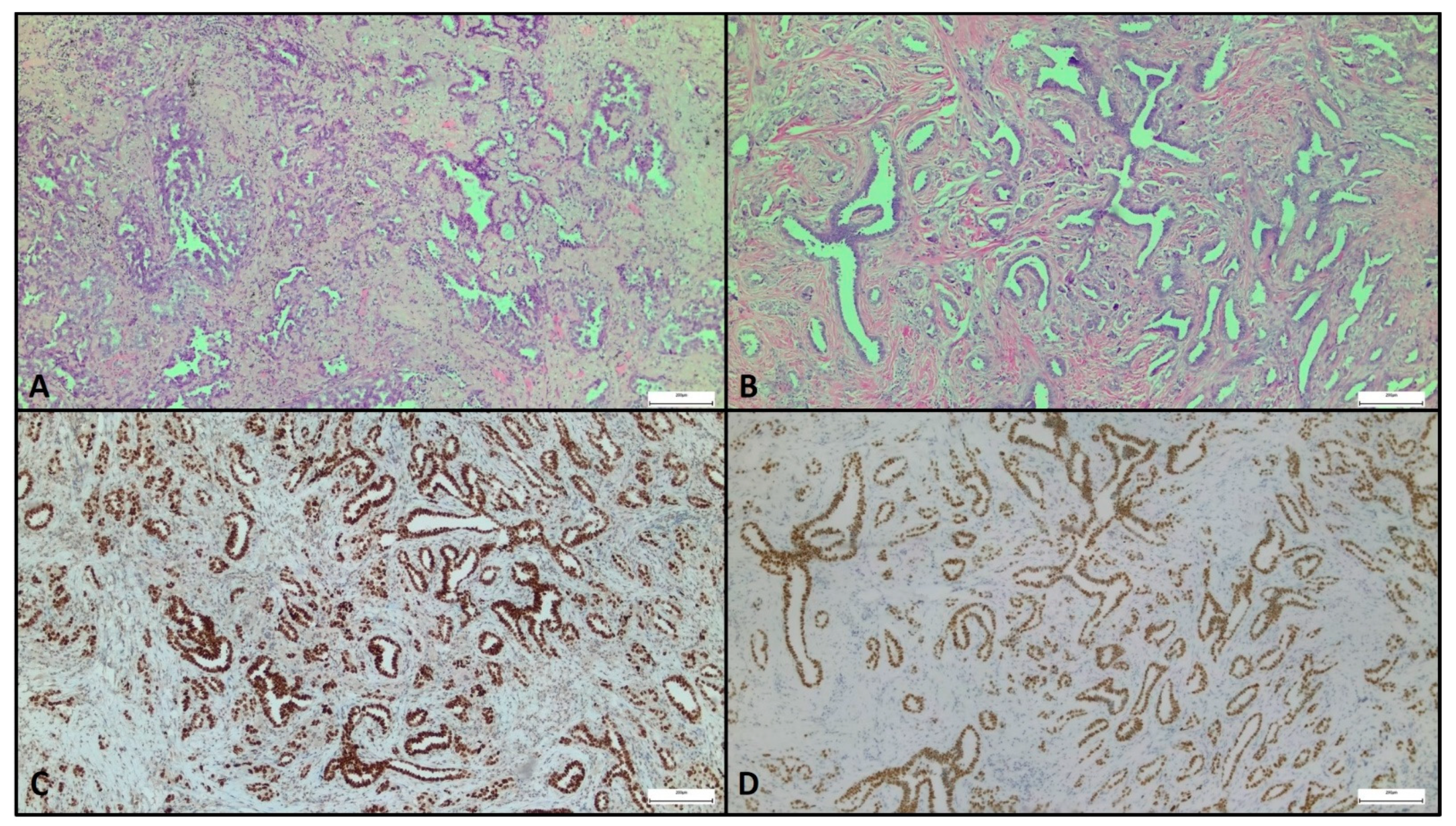
2. Case Report
Pathological Features and Differential Diagnosis
3. Literature Review: Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Georgiannos, S.N.; Chin Aleong, J.; Goode, A.W.; Sheaff, M. Secondary neoplasms of the breast. Cancer 2001, 92, 2259–2266. [Google Scholar] [CrossRef]
- Klingen, T.A.; Klaasen, H.; Aas, H.; Chen, Y.; Akslen, L.A. Secondary breast cancer: A 5-year population-based study with review of the literature. APMIS 2009, 117, 762–767. [Google Scholar] [CrossRef]
- Sousaris, N.; Mendelsohn, G.; Barr, R.G. Lung Cancer Metastatic to Breast. Ultrasound Q. 2013, 29, 205–209. [Google Scholar] [CrossRef]
- Ali, R.H.; Taraboanta, C.; Mohammad, T.; Hayes, M.M.; Ionescu, D.N. Metastatic non-small cell lung carcinoma a mimic of primary breast carcinoma—Case series and literature review. Virchows Arch. 2018, 472, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Sauer, T. Fine-needle aspiration cytology of extra mammary metastatic lesions in the breast: A retrospective study of 36 cases diagnosed during 18 years. Cytojournal 2010, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Fiedler, E.; Holzhausen, H.-J.; Ruschke, K.; Schmoll, H.-J.; Spielmann, R.-P. Metastases to the Breast from Non-mammary Malignancies. Acad. Radiol. 2011, 18, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Alva, S. An Update of Tumor Metastasis to the Breast Data. Arch. Surg. 1999, 134, 450. [Google Scholar] [CrossRef]
- Mirrielees, J.A.; Kapur, J.H.; Szalkucki, L.M.; Harter, J.M.; Salkowski, L.R.; Strigel, R.M.; Traynor, A.M.; Wilke, L.G. Metastasis of primary lung carcinoma to the breast: A systematic review of the literature. J. Surg. Res. 2014, 188, 419–431. [Google Scholar] [CrossRef]
- Fukumoto, K.; Usami, N.; Okasaka, T.; Kawaguchi, K.; Okagawa, T.; Suzuki, H.; Yokoi, K. Late breast metastasis from resected lung cancer diagnosed by epidermal growth factor receptor gene mutation. Lung Cancer 2011, 74, 352–353. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takeyama, Y.; Yoshihara, M.; Kato, T.; Hashimoto, H.; Fukui, Y.; Gonda, H.; Suzuki, R. CBDCA + Pemetrexed + Bevacizumab and Its Maintenance Chemotherapy in a Case of Solitary Breast Metastasis from a Lung Adenocarcinoma Resistant to Gefitinib. Case Rep. Oncol. 2012, 5, 546–553. [Google Scholar] [CrossRef]
- Huang, H.-C.; Hang, J.-F.; Wu, M.-H.; Chou, T.-Y.; Chiu, C.-H. Lung Adenocarcinoma with Ipsilateral Breast Metastasis: A Simple Coincidence? J. Thorac. Oncol. 2013, 8, 974–979. [Google Scholar] [CrossRef] [Green Version]
- Liam, C.-K.; Pang, Y.-K.; Poh, M.-E.; Kow, K.-S.; Wong, C.-K.; Varughese, R. Advanced right lung adenocarcinoma with ipsilateral breast metastasis. Respirol. Case Rep. 2013, 1, 20–22. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Bong, J.G.; Oh, H.K.; Park, S.H.; Kang, S.M.; Bae, S.H. Metachronous isolated breast metastasis from pulmonary adenocarcinoma with micropapillary component causing diagnostic challenges. BMC Cancer 2014, 14, 736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dansin, E.; Carnot, A.; Servent, V.; Daussay, D.; Robin, Y.-M.; Surmei-Pintilie, E.; Lauridant, G.; Descarpentries, C.; Révillion, F.; Delattre, C. EGFR-Mutated Breast Metastasis of Lung Adenocarcinoma: A Case Report. Case Rep. Oncol. 2015, 8, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Harvey, S. Incidental lung cancer found on screening breast MRI with eventual lymphatic metastasis to the breast. Breast Dis. 2015, 35, 207–210. [Google Scholar] [CrossRef]
- Lin, Q.; Cai, G.; Yang, K.-Y.; Yang, L.; Chen, C.-S.; Li, Y.-P. Case report: Small cell transformation and metastasis to the breast in a patient with lung adenocarcinoma following maintenance treatment with epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer 2016, 16, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninan, J.; Naik, V.; George, G.M. ‘Inflammatory breast cancer’ due to metastatic adenocarcinoma of lung. BMJ Case Rep. 2016, bcr2016215857. [Google Scholar] [CrossRef]
- Ota, T.; Hasegawa, Y.; Okimura, A.; Sakashita, K.; Sunami, T.; Yukimoto, K.; Sawada, R.; Sakamoto, K.; Fukuoka, M. Breast metastasis from EGFR-mutated lung adenocarcinoma: A case report and review of the literature. Clin. Case Rep. 2018, 6, 1510–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, S.-L.; Shen, H.-H.; Niu, F.-T.; Niu, Y. Breast metastasis from lung cancer: A report of two cases and literature review. Cancer Biol. Med. 2014, 11, 208–215. [Google Scholar] [PubMed]
- Lee, J.H.; Kim, S.H.; Kang, B.J.; Cha, E.S.; Kim, H.S.; Choi, J.J. Metastases to the breast from extramammary malignancies-sonographic features. J. Clin. Ultrasound 2011, 39, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Muttarak, M.; Ho, L.W. Nonmammary malignancies of the breast: Ultrasound, CT, and MRI. Semin. Ultrasound CT MRI 2000, 21, 375–394. [Google Scholar] [CrossRef]
- Mun, S.H.; Ko, E.Y.; Han, B.-K.; Shin, J.H.; Kim, S.J.; Cho, E.Y. Breast Metastases from Extramammary Malignancies: Typical and Atypical Ultrasound Features. Korean J. Radiol. 2014, 15, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.H.S. The histological diagnosis of metastases to the breast from extramammary malignancies. J. Clin. Pathol. 2006, 60, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Gown, A.M.; Fulton, R.S.; Kandalaft, P.L. Markers of metastatic carcinoma of breast origin. Histopathology 2016, 68, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.S.; Hodi, Z.; Soomro, I.; Sovani, V.; Abbas, A.; Rakha, E.; Ellis, I.O. Histological clues to the diagnosis of metastasis to the breast from extramammary malignancies. Histopathology 2020, 77, 303–313. [Google Scholar] [CrossRef]
- Striebel, J.M.; Dacic, S.; Yousem, S.A. Gross Cystic Disease Fluid Protein—(GCDFP-15). Am. J. Surg. Pathol. 2008, 32, 426–432. [Google Scholar] [CrossRef]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Rys, J.; Czapiewski, P.; Wazny, K.; Langfort, R.; Waloszczyk, P.; Biernat, W.; Lasota, J.; et al. GATA3: A Multispecific But Potentially Useful Marker in Surgical Pathology. Am. J. Surg. Pathol. 2014, 38, 13–22. [Google Scholar] [CrossRef]
- Suzuki, A.; Shijubo, N.; Yamada, G.; Ichimiya, S.; Satoh, M.; Abe, S.; Sato, N. Napsin A is useful to distinguish primary lung adenocarcinoma from adenocarcinomas of other organs. Pathol. Res. Pract. 2005, 201, 579–586. [Google Scholar] [CrossRef]
- Vitkovski, T.; Chaudhary, S.; Sison, C.; Nasim, M.; Esposito, M.J.; Bhuiya, T. Aberrant Expression of Napsin A in Breast Carcinoma With Apocrine Features. Int. J. Surg. Pathol. 2016, 24, 377–381. [Google Scholar] [CrossRef]
- Kaufmann, O.; Dietel, M. Thyroid transcription factor-1 is the superior immunohistochemical marker for pulmonary adenocarcinomas and large cell carcinomas compared to surfactant proteins A and B. Histopathology 2000, 36, 8–16. [Google Scholar] [CrossRef]
- Klingen, T.A.; Chen, Y.; Gundersen, M.D.; Aas, H.; Westre, B.; Sauer, T. Thyroid transcription factor-1 positive primary breast cancer: A case report with review of the literature. Diagn. Pathol. 2010, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Robens, J.; Goldstein, L.; Gown, A.M.; Schnitt, S.J. Thyroid Transcription Factor-1 Expression in Breast Carcinomas. Am. J. Surg. Pathol. 2010, 34, 1881–1885. [Google Scholar] [CrossRef]
- Nadji, M.; Gomez-Fernandez, C.; Ganjei-Azar, P.; Morales, A.R. Immunohistochemistry of Estrogen and Progesterone Receptors Reconsidered. Am. J. Clin. Pathol. 2005, 123, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Fernandez, C.; Mejias, A.; Walker, G.; Nadji, M. Immunohistochemical Expression of Estrogen Receptor in Adenocarcinomas of the Lung. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Mazières, J.; Rouquette, I.; Lepage, B.; Milia, J.; Brouchet, L.; Guibert, N.; Beau-Faller, M.; Validire, P.; Hofman, P.; Fouret, P. Specificities of Lung Adenocarcinoma in Women Who Have Never Smoked. J. Thorac. Oncol. 2013, 8, 923–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, H.K.; Shin, B.K. Expression of female sex hormone receptors and its relation to clinicopathological characteristics and prognosis of lung adenocarcinoma. J. Pathol. Transl. Med. 2020, 54, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-T.; Chang, Y.-L.; Shih, J.-Y.; Lee, Y.-C. The significance of estrogen receptor β in 301 surgically treated non–small cell lung cancers. J. Thorac. Cardiovasc. Surg. 2005, 130, 979–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef]
- Teng, Y.H.-F.; Tan, W.-J.; Thike, A.-A.; Cheok, P.-Y.; Tse, G.M.-K.; Wong, N.-S.; Yip, G.W.-C.; Bay, B.-H.; Tan, P.-H. Mutations in the epidermal growth factor receptor (EGFR) gene in triple negative breast cancer: Possible implications for targeted therapy. Breast Cancer Res. 2011, 13, R35. [Google Scholar] [CrossRef] [Green Version]
- Vora, H.H.; Patel, N.A.; Thakore, P.M.; Shukla, S.N. Immunohistochemical Localization of Wild-type EGFR, E746-A750 Frame Deletion in Exon 19, and L858R Point Mutation in Exon 21 in Triple-negative Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 653–660. [Google Scholar] [CrossRef]
- Williams, S.A.; Ehlers, R.A.; Hunt, K.K.; Yi, M.; Kuerer, H.M.; Singletary, S.E.; Ross, M.I.; Feig, B.W.; Fraser Symmans, W.; Meric-Bernstam, F. Metastases to the breast from nonbreast solid neoplasms. Cancer 2007, 110, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Ben Hassouna, J.; Slimene, M.; Bouzaiene, H.; Khomsi, F.; Chargui, R.; Kochbati, L.; Mtaallah, M.H.; Gamoudi, A.; Benna, F. Cancer du sein secondaire après traitement pour maladie de Hodgkin. À propos de sept cas. Gynécologie Obs. Fertil. 2007, 35, 536–540. [Google Scholar] [CrossRef] [PubMed]
- DeLair, D.F.; Corben, A.D.; Catalano, J.P.; Vallejo, C.E.; Brogi, E.; Tan, L.K. Non-mammary metastases to the breast and axilla: A study of 85 cases. Mod. Pathol. 2013, 26, 343–349. [Google Scholar] [CrossRef]
- Rodriguez-Lara, V.; Hernandez-Martinez, J.-M.; Arrieta, O. Influence of estrogen in non-small cell lung cancer and its clinical implications. J. Thorac. Dis. 2018, 10, 482–497. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung cancer in never smokers—A different disease. Nat. Rev. Cancer 2007, 7, 778–790. [Google Scholar] [CrossRef]
- Nose, N.; Sugio, K.; Oyama, T.; Nozoe, T.; Uramoto, H.; Iwata, T.; Onitsuka, T.; Yasumoto, K. Association Between Estrogen Receptor-β Expression and Epidermal Growth Factor Receptor Mutation in the Postoperative Prognosis of Adenocarcinoma of the Lung. J. Clin. Oncol. 2009, 27, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Garbán, D.C.; Chen, H.-W.; Fishbein, M.C.; Goodglick, L.; Pietras, R.J. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 2007, 72, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Shen, H.; Guo, R.; Sun, J.; Gao, W.; Shu, Y. Combine therapy of gefitinib and fulvestrant enhances antitumor effects on NSCLC cell lines with acquired resistance to gefitinib. Biomed. Pharmacother. 2012, 66, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Siegfried, J.M.; Stabile, L.P.; Young, P.A.; Marquez-Garban, D.C.; Park, D.J.; Patel, R.; Hu, E.H.; Sadeghi, S.; Parikh, R.J.; et al. Randomized phase II study of fulvestrant and erlotinib compared with erlotinib alone in patients with advanced or metastatic non-small cell lung cancer. Lung Cancer 2018, 123, 91–98. [Google Scholar] [CrossRef]
- Mazieres, J.; Barlesi, F.; Rouquette, I.; Molinier, O.; Besse, B.; Monnet, I.; Audigier-Valette, C.; Toffart, A.C.; Renault, P.A.; Fraboulet, S.; et al. Randomized Phase II Trial Evaluating Treatment with EGFR-TKI Associated with Antiestrogen in Women with Nonsquamous Advanced-Stage NSCLC: IFCT-1003 LADIE Trial. Clin. Cancer Res. 2020, 26, 3172–3181. [Google Scholar] [CrossRef] [Green Version]
| Case (Author, Year) | Age, Sex | Smoke | Primary Lung Cancer | Initial Stage | PE before BM | BM Suspicion | Size BM | Metachronous (Time) | Ipslateral | EGFR Mut | IHC Marker | Axillary LN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fukumoto 2011 [9] | 65, W | N | Left Lower Lobe | IIIA | No | Imaging | Tiny nodule | 6 years later | Yes | Ex 19 (L and B) | TTF-1+ ER- (B) | No |
| Sato 2012 [10] | 57, W | N | Right Upper Lobe | IV | Yes | Clinical | Inflammatory breast tumor | 12 months after | Yes | Ex 19 del (L) T790M (B) | CK7+ TTF-1+ ER-PgR- HER2- (B) | Yes |
| Huang 2013 [11] | 70, W | NA | Left Upper Lobe | IV | Yes | Clinical | Fixed hard mass | 3 months later | Yes | Ex 21 L858R mut (L) | TTF-1+ ER- PgR- GCDFP-15- (B) | Yes |
| Liam 2013 [12] | 70, W | N | Right Lower Lobe | IV | Yes | Clinical | Inflammatory breast tumor | 18 months later | Yes | Ex 20 ins (L) | TTF-1+ (L) TTF-1+ ER- PR- HER2- (B) | Yes |
| Jeong 2014 [13] | 47, W | N | Left Upper lobe | IB | No | Imaging | 1.3 cm | 3 years later | Yes | Ex 19 del (c.2239_2247del9) (L and B) | ER- PR- HER2- GCDFP-15- ALK- TTF-1+ CK-7+ Napsin A+ (B) | No |
| Mirrielees 2014 [8] | 58, W | F | Left Lung | IIIA | No | Clinical | 1.3 cm | 3 years later | Yes | Ex 19 non specified mut (L) | ER+ (20%) PR- HER2- TTF-1+ (B) | Yes |
| Dansin 2015 [14] | 52, W | N | Left Upper Lobe | IV | Yes | Clinical | 26 mm | Synchronous | Yes | Ex 19 del (L and B) | ER- PR- HER2- TTF1+ GATA-3- PAX8- (B) | Yes |
| Lee 2015 [15] | 49, W | N | Right Upper Lobe | IIIA | Yes | Imaging | Inflammatory breast tumor | 4 years later | Yes | NA (L) | CK7+ TTF-1+ CK20+ (L) | No |
| Lin 2016 [16] | 49, M | C | Left Upper Lobe | IV | Yes | Clinical | 5 cm | 18 months later | No | Ex 21 L858R mut (L and B) | Chromogranin A+ Synaptophysin+ CD56+ TTF-1+ ER- GCDFP-15- HER2- (B) | No |
| Ninan 2016 [17] | 67, W | NA | Right Lung | IIIB | NA | Clinical | Inflammatory breast tumor | NA | Yes | NA (L) | TTF-1+ CK7+ GATA-3- GCDFP-15- (B) | No |
| Ota 2018 [18] | 69, W | N | Left Lower Lobe | IV | Yes | Clinical | Inflammatory breast tumor | 12 months later | Yes | Ex 21 L858R mut (L and B) | ER- PR- HER2- (B) | Yes |
| Current case | 63, W | N | Right Upper Lobe | IIB | Yes | Imaging | 7 mm | 5 years later | Yes | Exon 19 del (c.2236_2250del) (L and B) | TTF-1+ ER+ (75%) PR+ (2%) HER2- (L) TTF-1+ ER+ (95%) PR+ (1%), HER2 (2+), GATA-3- (B) | No |
| IHC or Molecular Characteristic | Breast Cancer (Prevalence) | Lung Adenocarcinoma (Prevalence) |
|---|---|---|
| GCDFP-15 | 60% | 5.2–15% |
| GATA-3 | 67–95% | 8% |
| Napsin A | 14.6% | 84% |
| TTF-1 | 2.4–2.8% | 70–80% |
| ER | 80% | 7.6–27.2% |
| PR | 60% | 1.6–54.8% |
| EGFR-activating mutations | 3–11% (TNBC) | 30.3% |
| Case | Breast Surgery | EGFR-TKI | CT | IT |
|---|---|---|---|---|
| Fukumoto 2011 | Partial mastectomy | No | No | No |
| Sato 2012 | No | No | CBDCA + PMX + Bevacizumab | No |
| Huang 2013 | No | Erlotinib | No | No |
| Liam 2013 | No | No | No | No |
| Jeong 2014 | Lumpectomy and sentinel LN biopsy | Gefitinib | No | No |
| Mirrielees 2014 | No | Erlotinib | CBDCA + PMX | No |
| Dansin 2015 | No | Afatinib | 5-FU + Epi + CTX | No |
| Lee 2015 | No | Afatinib | No | Nivo + Ipi |
| Lin 2016 | No | No | CDDP + VP16 | No |
| Ninan 2016 | No | No | No | No |
| Ota 2018 | No | Erlotinib | CBDCA + Paclitaxel + Bevacizumab | No |
| Current case | Quadrantectomy | Osimertinib | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenza, C.; Porta, F.M.; Rappa, A.; Guerini-Rocco, E.; Viale, G.; Barberis, M.; de Marinis, F.; Curigliano, G.; Catania, C. Complex Differential Diagnosis between Primary Breast Cancer and Breast Metastasis from EGFR-Mutated Lung Adenocarcinoma: Case Report and Literature Review. Curr. Oncol. 2021, 28, 3384-3392. https://doi.org/10.3390/curroncol28050292
Valenza C, Porta FM, Rappa A, Guerini-Rocco E, Viale G, Barberis M, de Marinis F, Curigliano G, Catania C. Complex Differential Diagnosis between Primary Breast Cancer and Breast Metastasis from EGFR-Mutated Lung Adenocarcinoma: Case Report and Literature Review. Current Oncology. 2021; 28(5):3384-3392. https://doi.org/10.3390/curroncol28050292
Chicago/Turabian StyleValenza, Carmine, Francesca Maria Porta, Alessandra Rappa, Elena Guerini-Rocco, Giuseppe Viale, Massimo Barberis, Filippo de Marinis, Giuseppe Curigliano, and Chiara Catania. 2021. "Complex Differential Diagnosis between Primary Breast Cancer and Breast Metastasis from EGFR-Mutated Lung Adenocarcinoma: Case Report and Literature Review" Current Oncology 28, no. 5: 3384-3392. https://doi.org/10.3390/curroncol28050292
APA StyleValenza, C., Porta, F. M., Rappa, A., Guerini-Rocco, E., Viale, G., Barberis, M., de Marinis, F., Curigliano, G., & Catania, C. (2021). Complex Differential Diagnosis between Primary Breast Cancer and Breast Metastasis from EGFR-Mutated Lung Adenocarcinoma: Case Report and Literature Review. Current Oncology, 28(5), 3384-3392. https://doi.org/10.3390/curroncol28050292






