Real-World Treatment Patterns, Clinical Outcomes, and Health Care Resource Utilization in Extensive-Stage Small Cell Lung Cancer in Canada
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
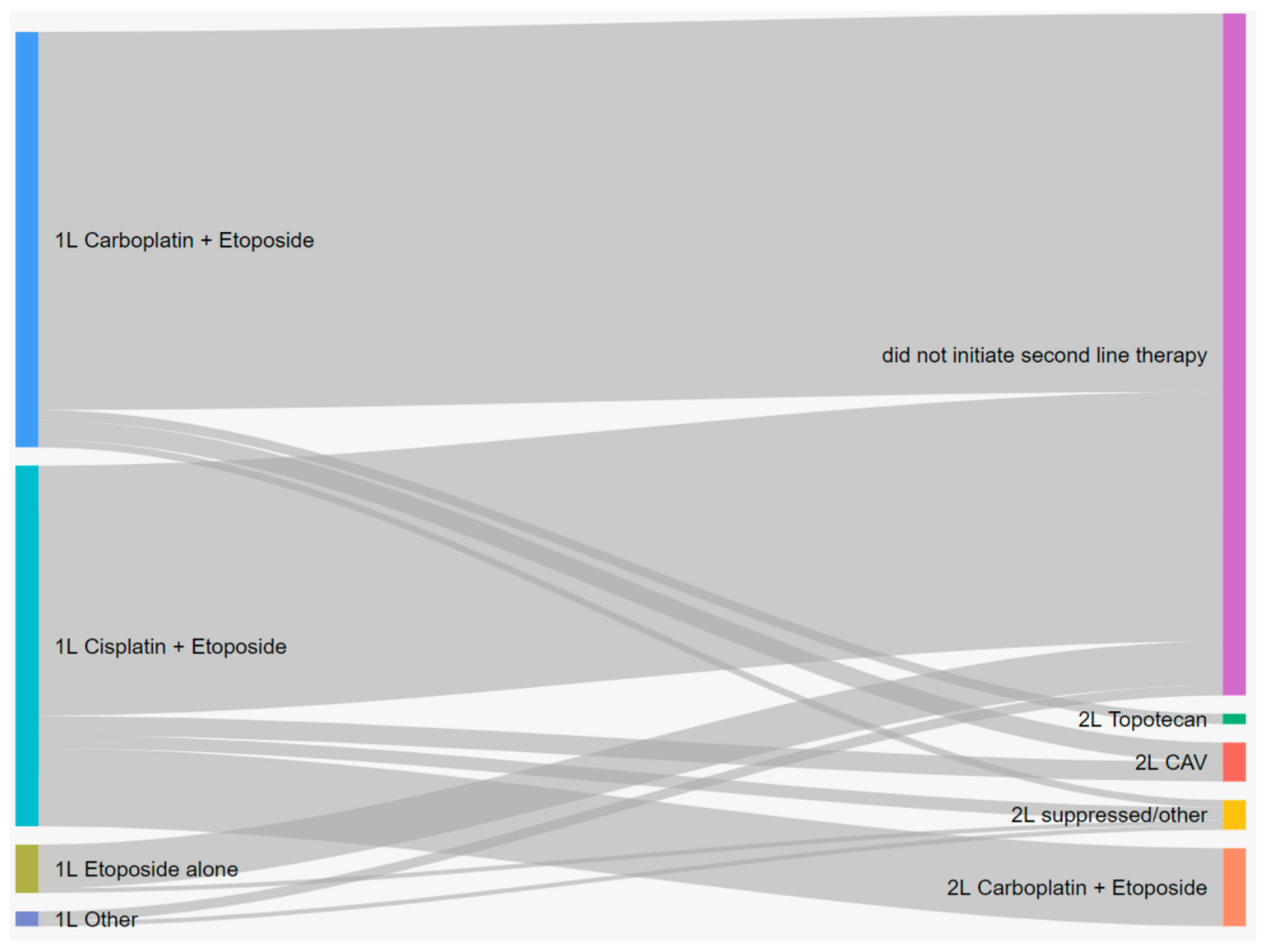
3.2. Treatment Patterns
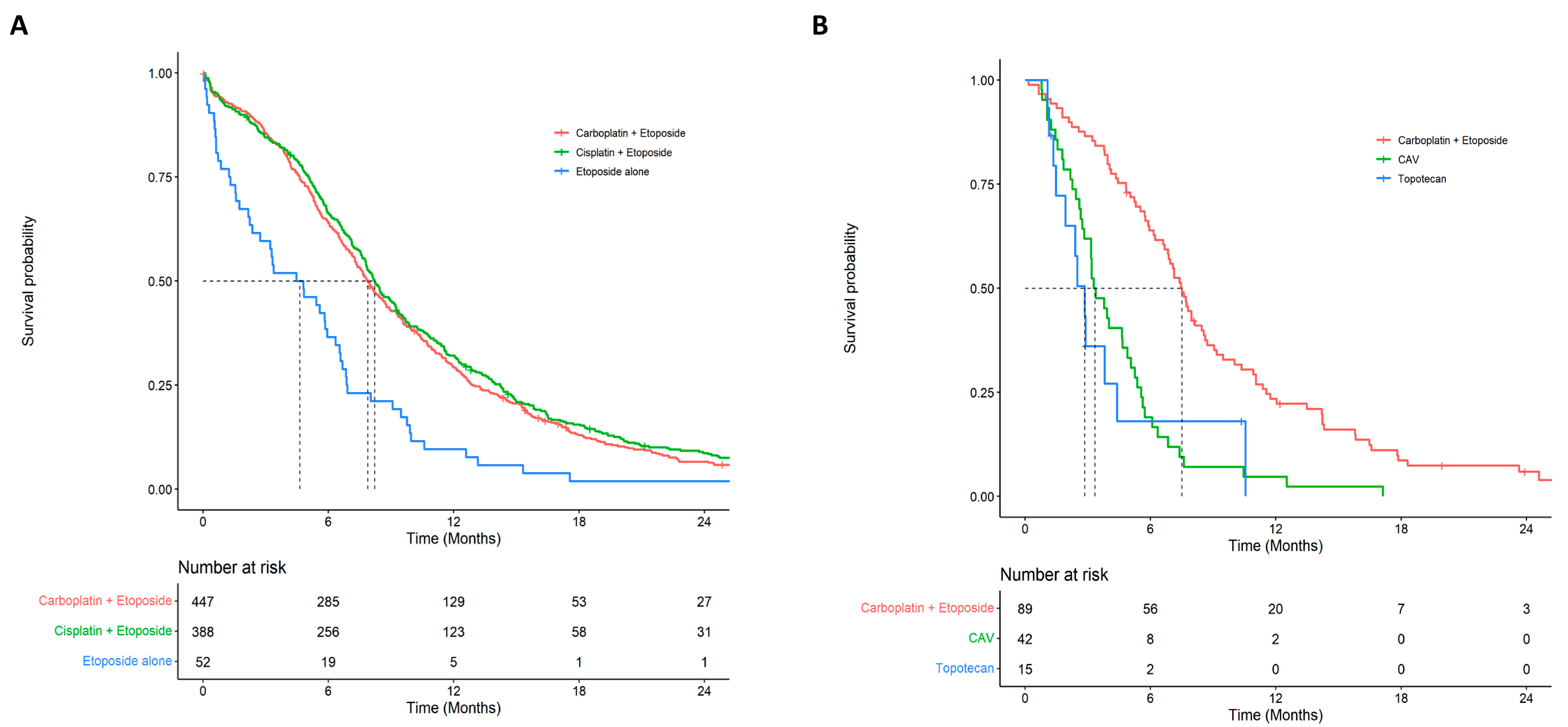
3.3. Clinical Outcomes
3.4. Health Care Resource Utilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M. Projected estimates of cancer in Canada in 2020. Can. Med. Assoc. J. 2020, 192, E199–E205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Meerbeeck, J.P.; Fennell, D.A.; De Ruysscher, D.K. Small-cell lung cancer. Lancet 2011, 378, 1741–1755. [Google Scholar] [CrossRef]
- Statistics, C. Canadian Cancer Society’s Advisory Committee on Cancer Statistics. In Canadian Cancer Statistics; Canadian Cancer Society: Toronto, ON, Canada, 2020. [Google Scholar]
- Asai, N.; Ohkuni, Y.; Kaneko, N.; Yamaguchi, E.; Kubo, A. Relapsed small cell lung cancer: Treatment options and latest developments. Ther. Adv. Med. Oncol. 2014, 6, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povsic, M.; Enstone, A.; Wyn, R.; Kornalska, K.; Penrod, J.R.; Yuan, Y. Real-world effectiveness and tolerability of small-cell lung cancer (SCLC) treatments: A systematic literature review (SLR). PLoS ONE 2019, 14, e0219622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittberg, R.; Green, S.; Aquin, T.; Bucher, O.; Banerji, S.; Dawe, D.E. Effect of Hospitalization During First Chemotherapy and Performance Status on Small-cell Lung Cancer Outcomes. Clin. Lung Cancer 2020, 21, e388–e404. [Google Scholar] [CrossRef] [PubMed]
- Elegbede, A.A.; Gibson, A.J.; Fu, H.; Dean, M.L.; Ezeife, D.A.; Lau, H.; Cheung, W.Y.; Bebb, D.G. Real-World Adherence to Guideline-Recommended Treatment for Small Cell Lung Cancer. Am. J. Clin. Oncol. 2020, 43, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Tendler, S.; Zhan, Y.; Pettersson, A.; Lewensohn, R.; Viktorsson, K.; Fang, F.; De Petris, L. Treatment patterns and survival outcomes for small-cell lung cancer patients—A Swedish single center cohort study. Acta Oncol. 2020, 59, 388–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer-van der Welle, C.M.; Schramel, F.; van Leeuwen, A.S.; Groen, H.J.M.; van de Garde, E.M.W. Real-world treatment patterns and outcomes of patients with extensive disease small cell lung cancer. Eur. J. Cancer Care 2020, 29, e13250. [Google Scholar] [CrossRef] [PubMed]
- Steffens, C.-C.; Elender, C.; Hutzschenreuter, U.; Dille, S.; Binninger, A.; Spring, L.; Jänicke, M.; Marschner, N. Treatment and outcome of 432 patients with extensive-stage small cell lung cancer in first, second and third line—Results from the prospective German TLK cohort study. Lung Cancer 2019, 130, 216–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, J.; Dawe, D.E.; Pond, G.R.; Ellis, P.M. The effect of age on referral to an oncologist and receipt of chemotherapy among small cell lung cancer patients in Ontario, Canada. J. Geriatr. Oncol. 2019, 10, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kong, S.; Cheung, W.Y.; Bouchard-Fortier, A.; Dort, J.C.; Quan, H.; Buie, E.M.; McKinnon, G.; Quan, M.L. Development and validation of case-finding algorithms for recurrence of breast cancer using routinely collected administrative data. BMC Cancer 2019, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Normand, S.T.; Landrum, M.B.; Guadagnoli, E.; Ayanian, J.Z.; Ryan, T.J.; Cleary, P.D.; McNeil, B.J. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: A matched analysis using propensity scores. J. Clin. Epidemiol. 2001, 54, 387–398. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroglu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Facchinetti, F.; Di Maio, M.; Tiseo, M. Adding PD-1/PD-L1 Inhibitors to Chemotherapy for the First-Line Treatment of Extensive Stage Small Cell Lung Cancer (SCLC): A Meta-Analysis of Randomized Trials. Cancers 2020, 12, 2645. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Torri, V.; Michetti, G.; Lo Dico, M.; La Verde, N.; Aglione, S.; Mancuso, A.; Gallerani, E.; Galetta, D.; Martelli, O.; et al. Outcomes of small-cell lung cancer patients treated with second-line chemotherapy: A multi-institutional retrospective analysis. Lung Cancer 2011, 72, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, M.; Fukui, T.; Kusuhara, S.; Hiyoshi, Y.; Ishihara, M.; Kasajima, M.; Nakahara, Y.; Otani, S.; Igawa, S.; Yokoba, M.; et al. Prognostic significance of the 8th edition of the TNM classification for patients with extensive disease small cell lung cancer. Cancer Manag. Res. 2018, 10, 6039–6047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Overall | Chemotherapy ** | No Chemotherapy *** | p-Value | SMD |
|---|---|---|---|---|---|
| (n = 1465) | (n = 803) | (n = 662) | |||
| Demographics | |||||
| Age, years (mean (SD)) | 69.07 (9.65) | 67.08 (9.15) | 71.50 (9.70) | <0.001 | 0.469 |
| <60 years (%) | 274 (18.8) | 181 (22.5) | 93 (14.0) | <0.001 | 0.221 |
| ≥60 years (%) | 1191 (81.3) | 622 (77.5) | 569 (86.0) | ||
| Male (%) | 741 (50.6) | 414 (51.6) | 327 (49.4) | 0.441 | 0.043 |
| Socioeconomic Status | |||||
| Urban residence (%) | 1138 (77.7) | 634 (79.0) | 504 (76.1) | 0.22 | 0.068 |
| Neighbourhood annual household income in Canadian dollars (mean (SD)) | 36,073.50 (13,518.67) | 36,571.78 (13,359.62) | 35,469.09 (13,694.91) | 0.12 | 0.082 |
| Categories of neighbourhood annual household income in Canadian dollars (%) | 0.051 | 0.146 | |||
| 0–25,000 | 131 (8.9) | 62 (7.7) | 69 (10.4) | ||
| 25,000–35,000 | 703 (48.0) | 372 (46.3) | 331 (50.0) | ||
| 35,000–45,000 | 413 (28.2) | 238 (29.6) | 175 (26.4) | ||
| >45,000 | 218 (14.9) | 131 (16.3) | 87 (13.1) | ||
| Proportion of neighbourhood residents who achieved a high school education or greater (mean (SD)) | 0.74 (0.11) | 0.74 (0.11) | 0.73 (0.11) | 0.09 | 0.089 |
| Categories of neighbourhood education (%) | 0.333 | 0.097 | |||
| 0.00–0.60 | 166 (11.3) | 88 (11.0) | 78 (11.8) | ||
| 0.60–0.70 | 328 (22.4) | 174 (21.7) | 154 (23.3) | ||
| 0.70–0.80 | 510 (34.8) | 272 (33.9) | 238 (36.0) | ||
| >0.80 | 461 (31.5) | 269 (33.5) | 192 (29.0) | ||
| Comorbidity | |||||
| Charlson comorbidity index (%) | <0.001 | 0.306 | |||
| 0 | 601 (41.0) | 369 (46.0) | 232 (35.0) | ||
| 1 | 432 (29.5) | 238 (29.6) | 194 (29.3) | ||
| 2 | 225 (15.4) | 110 (13.7) | 115 (17.4) | ||
| 3 | 105 (7.2) | 52 (6.5) | 53 (8.0) | ||
| ≥4 | 102 (7.0) | 34 (4.2) | 68 (10.3) | ||
| Chronic obstructive pulmonary disease (%) | 532 (36.3) | 265 (33.0) | 267 (40.3) | 0.004 | 0.153 |
| Diabetes (%) | 325 (22.2) | 169 (21.0) | 156 (23.6) | 0.275 | 0.061 |
| Cardiovascular disease (%) | 271 (18.5) | 119 (14.8) | 152 (23.0) | <0.001 | 0.209 |
| Renal disease (%) | 66 (4.5) | 24 (3.0) | 42 (6.3) | 0.003 | 0.16 |
| Liver disease (%) | 56 (3.8) | 26 (3.2) | 30 (4.5) | 0.251 | 0.067 |
| Connective tissue disease (%) | 27 (1.8) | 16 (2.0) | 11 (1.7) | 0.784 | 0.025 |
| Indicators of health | |||||
| Prior cancer | 123 (8.4) | 59 (7.3) | 64 (9.7) | 0.134 | 0.083 |
| No. of hospitalizations within 1 year prior to diagnosis * (%) | <0.001 | 0.326 | |||
| 0 | 1222 (83.4) | 712 (88.7) | 510 (77.0) | ||
| 1 | 153 (10.4) | 64 (8.0) | 89 (13.4) | ||
| 2 | 53 (3.6) | 15 (1.9) | 38 (5.7) | ||
| ≥3 | 37 (2.5) | 12 (1.5) | 25 (3.8) | ||
| No. of ambulatory care encounters within the year prior to diagnosis * (mean (SD)) | 4.27 (9.90) | 3.58 (7.88) | 5.10 (11.84) | 0.004 | 0.15 |
| No. of health practitioner encounters within the year prior to diagnosis * (mean (SD)) | 13.46 (14.73) | 11.89 (10.93) | 15.35 (18.14) | <0.001 | 0.231 |
| Metastatic Sites | |||||
| Number of metastatic sites at diagnosis | 0.269 | 0.133 | |||
| 1 | 548 (37.4) | 285 (35.5) | 263 (39.7) | ||
| 2 | 452 (30.9) | 265 (33.0) | 187 (28.2) | ||
| 3 | 251 (17.1) | 141 (17.6) | 110 (16.6) | ||
| 4 | 126 (8.6) | 70 (8.7) | 56 (8.5) | ||
| ≥5 | 86 (5.9) | 41 (5.1) | 45 (6.8) | ||
| Missing | 2 (0.1) | 1 (0.1) | 1 (0.2) | ||
| Sites of metastasis at diagnosis | |||||
| Hepatic | 695 (47.4) | 363 (45.2) | 332 (50.2) | 0.067 | 0.099 |
| Pleura | 674 (46.0) | 336 (41.8) | 338 (51.1) | 0.001 | 0.186 |
| Osseous | 445 (30.4) | 260 (32.4) | 185 (27.9) | 0.075 | 0.097 |
| Lymph nodes | 282 (19.2) | 171 (21.3) | 111 (16.8) | 0.034 | 0.116 |
| Brain | 266 (18.2) | 148 (18.4) | 118 (17.8) | 0.817 | 0.016 |
| Adrenals | 262 (17.9) | 151 (18.8) | 111 (16.8) | 0.345 | 0.053 |
| Pulmonary | 199 (13.6) | 119 (14.8) | 80 (12.1) | 0.149 | 0.08 |
| Peritoneum | 57 (3.9) | 38 (4.7) | 19 (2.9) | 0.089 | 0.097 |
| Bone marrow | 33 (2.3) | 12 (1.5) | 21 (3.2) | 0.048 | 0.111 |
| Variable | Estimate (%) | Time on Therapy | Median Survival | 2-Year Survival | 5-Year Survival |
|---|---|---|---|---|---|
| n = 1941 | Median (KM) | (95% CI) | (95% CI) | (95% CI) | |
| 1L Chemotherapy | 903 (46.5) | ||||
| Carboplatin + Etoposide | 447 (49.5) | 15.0 | 7.89 (7.33–8.65) | 0.066 (0.046–0.095) | 0.022 (0.010–0.050) |
| Cisplatin + Etoposide | 388 (43.0) | 13.1 | 8.22 (7.76–9.14) | 0.087 (0.063–0.121) | 0.041 (0.024–0.071) |
| Etoposide alone | 52 (5.8) | 9.7 | 4.64 (2.37–6.54) | 0.019 (0.003–0.134) | NA |
| Other/Suppressed | 16 (1.8) | - | - | - | - |
| 2L Chemotherapy | 169 (8.7) | ||||
| Carboplatin + Etoposide | 89 (52.7) | 13.3 | 7.50 (6.67–8.61) | 0.059 (0.025–0.143) | 0.020 (0.003–0.123) |
| CAV | 42 (24.9) | 7.4 | 3.34 (2.83–5.06) | NA | NA |
| Topotecan | 15 (8.9) | 9.0 | 2.86 (1.94–NA) | NA | NA |
| Other/Suppressed | 23 (13.6) | - | - | - | - |
| 3L Chemotherapy | 28 (1.4) | ||||
| CAV | 10 (38.5) | 7.1 | 2.89 (1.32–NA) | NA | NA |
| Topotecan | 10 (38.5) | 13.6 | 3.83 (2.24–NA) | NA | NA |
| Other/Suppressed | 8 (23.0) | - | - | - | - |
| First-Line Therapies | Etoposide Alone | Other | Platinum + Etoposide | |||||
|---|---|---|---|---|---|---|---|---|
| Construct | Outcome | Year 1 (n = 52) | Year 1 (n = 16) | Year 1 (n = 835) | Year 2 (n = 253) | Year 3 (n = 58) | Year 4 (n = 27) | Year 5 (n = 17) |
| Hospitalizations | No. of Hospitalizations | 1.06 | 1.50 | 1.49 | 0.98 | 0.60 | 0.44 | 0.88 |
| No. of Days Hospitalized | 15.42 | 16.75 | 16.94 | 11.57 | 9.57 | 6.26 | 10.35 | |
| Ambulatory Care Services | No. of Encounters | 5.75 | 6.69 | 7.54 | 4.49 | 4.74 | 3.78 | 4.59 |
| No. of Emergency Encounters | 1.60 | 3.75 | 2.98 | 1.67 | 1.34 | 1.11 | 1.47 | |
| No. of Non-Emergency Encounters | 4.15 | 2.94 | 4.56 | 2.82 | 3.40 | 2.67 | 3.12 | |
| Cancer Physician Visits | No. of Visits | 5.02 | 8.50 | 11.00 | 5.18 | 4.50 | 3.48 | 3.29 |
| No. of Medical Oncologist Visits | 4.10 | 5.69 | 7.98 | 4.01 | 3.81 | 3.30 | 3.18 | |
| No. of Radiation Oncologist Visits | 0.65 | 1.38 | 2.02 | 0.77 | 0.66 | <10 | <10 | |
| No. of General/Family Practitioner Visits | <10 | 0.81 | 0.54 | 0.17 | <10 | <10 | <10 | |
| No. of Other Cancer Physician Visits | 0.19 | 0.63 | 0.47 | 0.23 | <10 | <10 | <10 | |
| Non-Cancer Practitioner Visits | No. of Encounters | 28.54 | 26.06 | 26.51 | 20.77 | 19.81 | 17.33 | 18.00 |
| No. of Claims | 50.17 | 52.81 | 49.79 | 38.45 | 35.17 | 32.48 | 40.24 | |
| Radiation Therapy | No. of Days of Therapy | 1.50 | 6.50 | 10.15 | 3.11 | 1.24 | 0.44 | <10 |
| Chemotherapy Cycles | No. of Cycles | 3.52 | 8.69 | 9.73 | 2.47 | 1.52 | 0.59 | <10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Sullivan, D.E.; Cheung, W.Y.; Syed, I.A.; Moldaver, D.; Shanahan, M.K.; Bebb, D.G.; Sit, C.; Brenner, D.R.; Boyne, D.J. Real-World Treatment Patterns, Clinical Outcomes, and Health Care Resource Utilization in Extensive-Stage Small Cell Lung Cancer in Canada. Curr. Oncol. 2021, 28, 3091-3103. https://doi.org/10.3390/curroncol28040270
O’Sullivan DE, Cheung WY, Syed IA, Moldaver D, Shanahan MK, Bebb DG, Sit C, Brenner DR, Boyne DJ. Real-World Treatment Patterns, Clinical Outcomes, and Health Care Resource Utilization in Extensive-Stage Small Cell Lung Cancer in Canada. Current Oncology. 2021; 28(4):3091-3103. https://doi.org/10.3390/curroncol28040270
Chicago/Turabian StyleO’Sullivan, Dylan E., Winson Y. Cheung, Iqra A. Syed, Daniel Moldaver, Mary Kate Shanahan, D. Gwyn Bebb, Christina Sit, Darren R. Brenner, and Devon J. Boyne. 2021. "Real-World Treatment Patterns, Clinical Outcomes, and Health Care Resource Utilization in Extensive-Stage Small Cell Lung Cancer in Canada" Current Oncology 28, no. 4: 3091-3103. https://doi.org/10.3390/curroncol28040270
APA StyleO’Sullivan, D. E., Cheung, W. Y., Syed, I. A., Moldaver, D., Shanahan, M. K., Bebb, D. G., Sit, C., Brenner, D. R., & Boyne, D. J. (2021). Real-World Treatment Patterns, Clinical Outcomes, and Health Care Resource Utilization in Extensive-Stage Small Cell Lung Cancer in Canada. Current Oncology, 28(4), 3091-3103. https://doi.org/10.3390/curroncol28040270




