Emerging Concepts in the Surgical Management of Peri-Acetabular Metastatic Bone Disease
Abstract
:1. Introduction
2. Novel Techniques
2.1. Percutaneous Screw Fixation
2.2. Percutaneous Cementoplasty Adjuncts
2.3. Limitations of Percutaneous Techniques
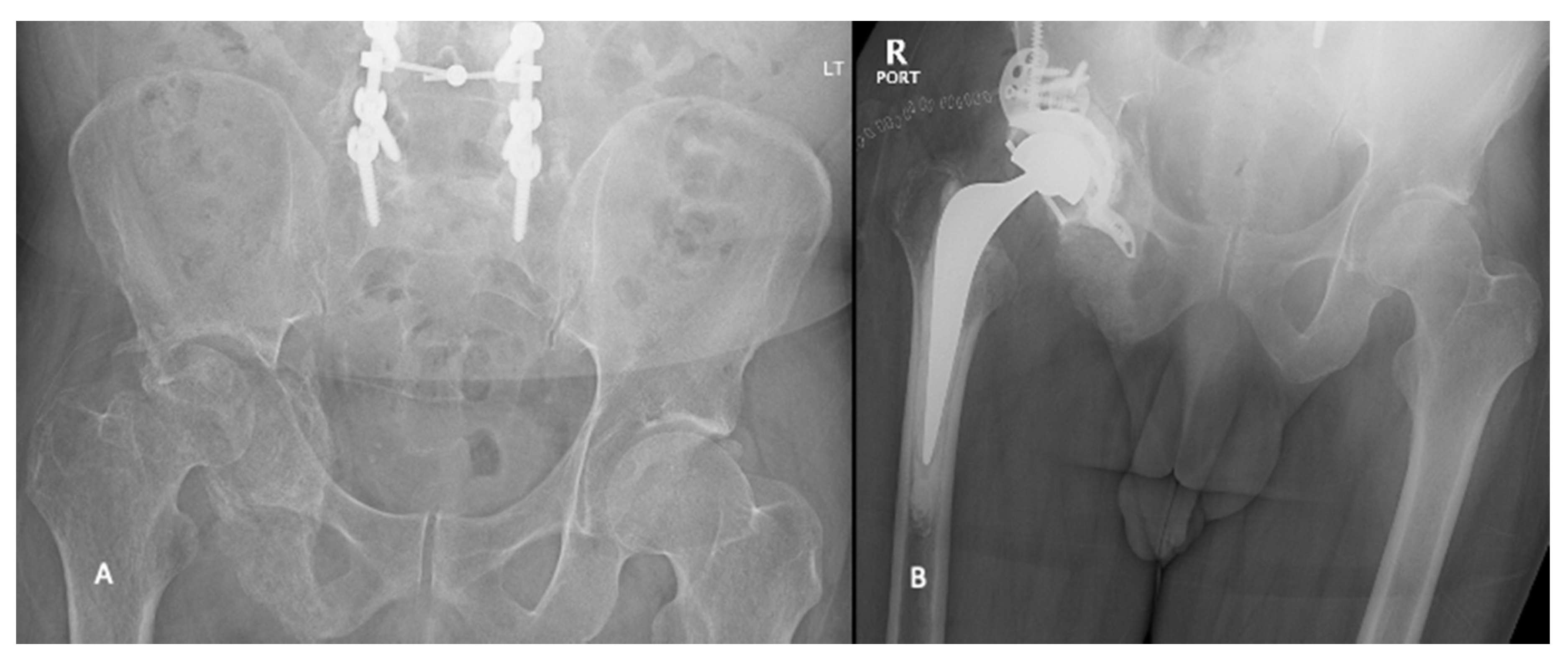
2.4. Harrington Procedure Adjuncts
2.5. Porous Tantalum Implants
2.6. Endoprosthetic Reconstructions
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Kyriakopoulos, C.E.; Chen, Y.-H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbini, E.; Ezzalfani, M.; Dieras, V.; Bachelot, T.; Brain, E.; Debled, M.; Jacot, W.; Mouret-Reynier, M.A.; Goncalves, A.; Dalenc, F. Time Trends of Overall Survival among Metastatic Breast Cancer Patients in the Real-Life ESME Cohort. Eur. J. Cancer 2018, 96, 17–24. [Google Scholar] [CrossRef]
- Fonseca, R.; Abouzaid, S.; Bonafede, M.; Cai, Q.; Parikh, K.; Cosler, L.; Richardson, P. Trends in Overall Survival and Costs of Multiple Myeloma, 2000–2014. Leukemia 2017, 31, 1915–1921. [Google Scholar] [CrossRef] [Green Version]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar]
- Hernandez, R.K.; Wade, S.W.; Reich, A.; Pirolli, M.; Liede, A.; Lyman, G.H. Incidence of Bone Metastases in Patients with Solid Tumors: Analysis of Oncology Electronic Medical Records in the United States. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratasvuori, M.; Wedin, R.; Keller, J.; Nottrott, M.; Zaikova, O.; Bergh, P.; Kalen, A.; Nilsson, J.; Jonsson, H.; Laitinen, M. Insight Opinion to Surgically Treated Metastatic Bone Disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry Report of 1195 Operated Skeletal Metastasis. Surg. Oncol. 2013, 22, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kirkinis, M.N.; Lyne, C.J.; Wilson, M.D.; Choong, P.F.M. Metastatic Bone Disease: A Review of Survival, Prognostic Factors and Outcomes Following Surgical Treatment of the Appendicular Skeleton. Eur. J. Surg. Oncol. (EJSO) 2016, 42, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Picci, P.; Manfrini, M.; Fabbri, N.; Gambarotti, M.; Vanel, D. Atlas of Musculoskeletal Tumors and Tumorlike Lesions: The Rizzoli Case Archive; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Ji, T.; Guo, W.; Yang, R.-L.; Tang, S.; Sun, X. Clinical Outcome and Quality of Life after Surgery for Peri-Acetabular Metastases. J. Bone Joint Surg. Br. 2011, 93, 1104–1110. [Google Scholar] [CrossRef]
- Harrington, K.D. The Management of Acetabular Insufficiency Secondary to Metastatic Malignant Disease. JBJS 1981, 63, 653–664. [Google Scholar] [CrossRef]
- Kask, G.; Nieminen, J.; van Iterson, V.; Naboistsikov, M.; Pakarinen, T.-K.; Laitinen, M.K. Modified Harrington’s Procedure for Periacetabular Metastases in 89 Cases: A Reliable Method for Cancer Patients with Good Functional Outcome, Especially with Long Expected Survival. Acta Orthop. 2020, 91, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Harty, J.A.; Brennan, D.; Eustace, S.; O’Byrne, J. Percutaneous Cementoplasty of Acetabular Bony Metastasis. Surgeon 2003, 1, 48–50. [Google Scholar] [CrossRef]
- Garnon, J.; Jennings, J.W. Percutaneous Consolidation for Extraspinal Osteolytic Lesions: To Cementoplasty and Beyond. J. Vasc. Interv. Radiol. 2020, 31, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzo, L.; Maccauro, G.; Rossi, B.; Messuti, L.; Maffulli, N.; Logroscino, C.A. Quality of Life in Patients Following Percutaneous PMMA Acetabuloplasty for Acetabular Metastasis Due to Carcinoma. Acta Orthopædica Belg. 2009, 75, 484. [Google Scholar]
- Issack, P.S.; Kotwal, S.Y.; Lane, J.M. Management of Metastatic Bone Disease of the Acetabulum. JAAOS-J. Am. Acad. Orthop. Surg. 2013, 21, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Couraud, G.; Gaston, A.-P.; Thuillier, T.; Eymard, F.; Hourdille, A.; Chevalier, X.-J.; Boussion, H.; Guignard, S. Evaluation of Short-Term Efficacy of Extraspinal Cementoplasty for Bone Metastasis: A Monocenter Study of 31 Patients. J. Bone Oncol. 2018, 13, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Pugash, R.; David, E.; Yee, A.; Sinclair, E.; Myers, J.; Chow, E. Percutaneous Cementoplasty of Lytic Metastasis in Left Acetabulum. Curr. Oncol. 2007, 14, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Starr, A.J.; Reinert, C.M.; Jones, A.L. Percutaneous Fixation of the Columns of the Acetabulum: A New Technique. J. Orthop. Trauma 1998, 12, 51–58. [Google Scholar] [CrossRef]
- Bishop, J.A.; Routt Jr, M.L.C. Osseous Fixation Pathways in Pelvic and Acetabular Fracture Surgery: Osteology, Radiology, and Clinical Applications. J. Trauma Acute Care Surg. 2012, 72, 1502–1509. [Google Scholar] [CrossRef]
- Kazemi, N.; Archdeacon, M.T. Immediate Full Weightbearing after Percutaneous Fixation of Anterior Column Acetabulum Fractures. J. Orthop. Trauma 2012, 26, 73–79. [Google Scholar] [CrossRef]
- Mouhsine, E.; Garofalo, R.; Borens, O.; Wettstein, M.; Blanc, C.-H.; Fischer, J.-F.; Moretti, B.; Leyvraz, P.-F. Percutaneous Retrograde Screwing for Stabilisation of Acetabular Fractures. Injury 2005, 36, 1330–1336. [Google Scholar] [CrossRef]
- Yang, R.; Goch, A.; Murphy, D.; Wang, J.; Charubhumi, V.; Fox, J.; Sen, M.; Hoang, B.; Geller, D. A Novel Tripod Percutaneous Reconstruction Technique in Periacetabular Lesions Caused by Metastatic Cancer. JBJS 2020, 102, 592–599. [Google Scholar] [CrossRef]
- Roux, C.; Tselikas, L.; Yevich, S.; Sandes Solha, R.; Hakime, A.; Teriitehau, C.; Gravel, G.; De Baere, T.; Deschamps, F. Fluoroscopy and Cone-Beam CT–Guided Fixation by Internal Cemented Screw for Pathologic Pelvic Fractures. Radiology 2019, 290, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone Cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lea, W.B.; Neilson, J.C.; King, D.M.; Tutton, S.M. Minimally Invasive Stabilization Using Screws and Cement for Pelvic Metastases: Technical Considerations for the Pelvic “Screw and Glue” Technique. In Proceedings of the Seminars in Interventional Radiology; Thieme Medical Publishers: New York, NY, USA, 2019; Volume 36, pp. 229–240. [Google Scholar]
- Hesler, M.-C.; Buy, X.; Catena, V.; Brouste, V.; Kind, M.; Palussière, J.; Crombé, A. Assessment of Risk Factors for Occurrence or Worsening of Acetabular Fracture Following Percutaneous Cementoplasty of Acetabulum Malignancies. Eur. J. Radiol. 2019, 120, 108694. [Google Scholar] [CrossRef]
- Kurup, A.N.; Morris, J.M.; Schmit, G.D.; Atwell, T.D.; Schmitz, J.J.; Rose, P.S.; Callstrom, M.R. Balloon-Assisted Osteoplasty of Periacetabular Tumors Following Percutaneous Cryoablation. J. Vasc. Interv. Radiol. 2015, 26, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Moynagh, M.R.; Kurup, A.N.; Callstrom, M.R. Thermal Ablation of Bone Metastases. Semin. Interv. Radiol. 2018, 35, 299–308. [Google Scholar] [CrossRef]
- Lee, F.Y.; Latich, I.; Toombs, C.; Mungur, A.; Conway, D.; Alder, K.; Ibe, I.; Lindskog, D.; Friedlaender, G. Minimally Invasive Image-Guided Ablation, Osteoplasty, Reinforcement, and Internal Fixation (AORIF) for Osteolytic Lesions in the Pelvis and Periarticular Regions of Weight-Bearing Bones. J. Vasc. Interv. Radiol. 2020, 31, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Bagsby, D.T.; Wurtz, L.D. Effectiveness of Constrained Liner Use During Harrington Hip Reconstruction in Oncology Patient. J. Arthroplast. 2017, 32, 1250–1254. [Google Scholar] [CrossRef]
- Hoskins, W.; Bingham, R.; Hatton, A.; de Steiger, R.N. Standard, Large-Head, Dual-Mobility, or Constrained-Liner Revision Total Hip Arthroplasty for a Diagnosis of Dislocation: An Analysis of 1275 Revision Total Hip Replacements. JBJS 2020, 102, 2060–2067. [Google Scholar] [CrossRef]
- Wegrzyn, J.; Malatray, M.; Al-Qahtani, T.; Pibarot, V.; Confavreux, C.; Freyer, G. Total Hip Arthroplasty for Periacetabular Metastatic Disease. An Original Technique of Reconstruction According to the Harrington Classification. J. Arthroplast. 2018, 33, 2546–2555. [Google Scholar] [CrossRef]
- Tsagozis, P.; Wedin, R.; Brosjö, O.; Bauer, H. Reconstruction of Metastatic Acetabular Defects Using a Modified Harrington Procedure. Acta Orthop. 2015, 86, 690–694. [Google Scholar] [CrossRef]
- Hoell, S.; Dedy, N.; Gosheger, G.; Dieckmann, R.; Daniilidis, K.; Hardes, J. The Burch-Schneider Cage for Reconstruction after Metastatic Destruction of the Acetabulum: Outcome and Complications. Arch. Orthop. Trauma Surg. 2012, 132, 405–410. [Google Scholar] [CrossRef]
- Rowell, P.; Lowe, M.; Sommerville, S.; Dickinson, I. Is an Acetabular Cage and Cement Fixation Sufficiently Durable for the Treatment of Destructive Acetabular Metastases? Clin. Orthop. Relat. Res. 2019, 477, 1459–1465. [Google Scholar] [CrossRef]
- Plummer, D.; Passen, E.; Alexander, J.; Vajapey, S.; Frantz, T.; Niedermeier, S.; Pettit, R.; Scharschmidt, T. Rapid Return to Function and Stability with Dual Mobility Components Cemented into an Acetabular Reconstructive Cage for Large Osseous Defects in the Setting of Periacetabular Metastatic Disease. J. Surg. Oncol. 2019, 119, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Della Valle, C.J.; Jacobs, J.J. Applications of Porous Tantalum in Total Hip Arthroplasty. JAAOS-J. Am. Acad. Orthop. Surg. 2006, 14, 646–655. [Google Scholar] [CrossRef]
- Houdek, M.T.; Ferguson, P.C.; Abdel, M.P.; Griffin, A.M.; Hevesi, M.; Perry, K.I.; Rose, P.S.; Wunder, J.S.; Lewallen, D.G. Comparison of Porous Tantalum Acetabular Implants and Harrington Reconstruction for Metastatic Disease of the Acetabulum. J. Bone Jt. Surg. 2020, 102, 1239. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Abdel, M.P.; Perry, K.I.; Salduz, A.; Rose, P.S.; Sim, F.H.; Lewallen, D.G. Outcome of Patients Treated With Porous Tantalum Acetabular Implants for Neoplastic Periacetabular Lesions. JAAOS-J. Am. Acad. Orthop. Surg. 2020, 28, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Bus, M.P.A.; Campanacci, D.A.; Albergo, J.I.; Leithner, A.; van de Sande, M.A.J.; Gaston, C.L.; Caff, G.; Mettelsiefen, J.; Capanna, R.; Tunn, P.-U.; et al. Conventional Primary Central Chondrosarcoma of the Pelvis: Prognostic Factors and Outcome of Surgical Treatment in 162 Patients. JBJS 2018, 100, 316–325. [Google Scholar] [CrossRef]
- Erol, B.; Sofulu, O.; Sirin, E.; Saglam, F.; Buyuktopcu, O. Reconstruction after Periacetabular Tumor Resection with Lumic® Endoprosthesis: What Are the Midterm Results? J. Surg. Oncol. 2021, 123, 532–543. [Google Scholar] [CrossRef]
- Wei, R.; Lim, C.Y.; Yang, Y.; Tang, X.; Yan, T.; Yang, R.; Guo, W. Surgical Treatment and Proposed Modified Classification for Harrington Class III Periacetabular Metastases. Orthop. Surg. 2021, 13, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.S.; Salib, C.G.; Rose, P.S.; Sim, F.H.; Lewallen, D.G.; Abdel, M.P. Reconstruction of the Hip after Resection of Periacetabular Oncological Lesions. Bone Jt. J. 2018, 100-B, 22–30. [Google Scholar] [CrossRef] [PubMed]
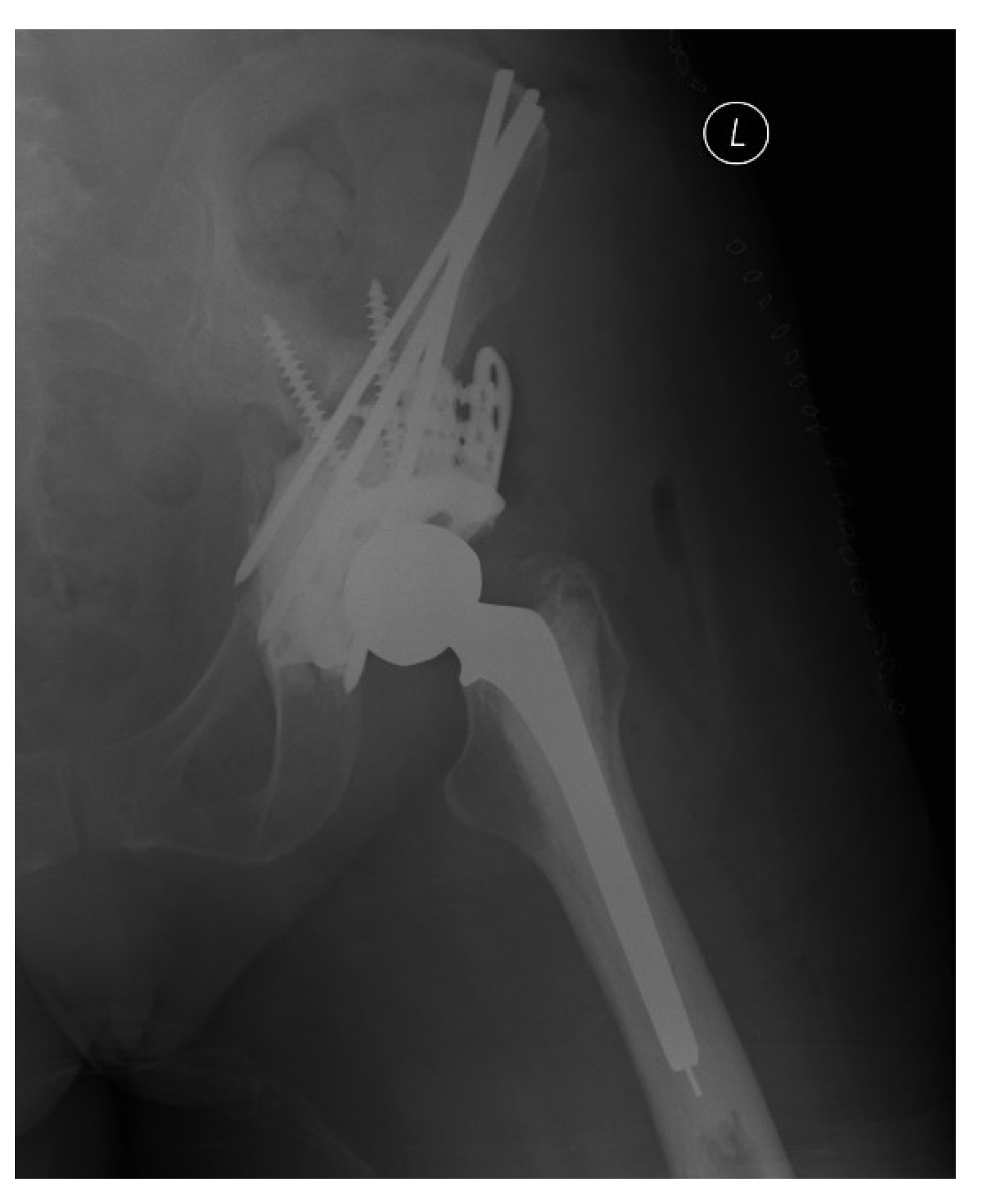
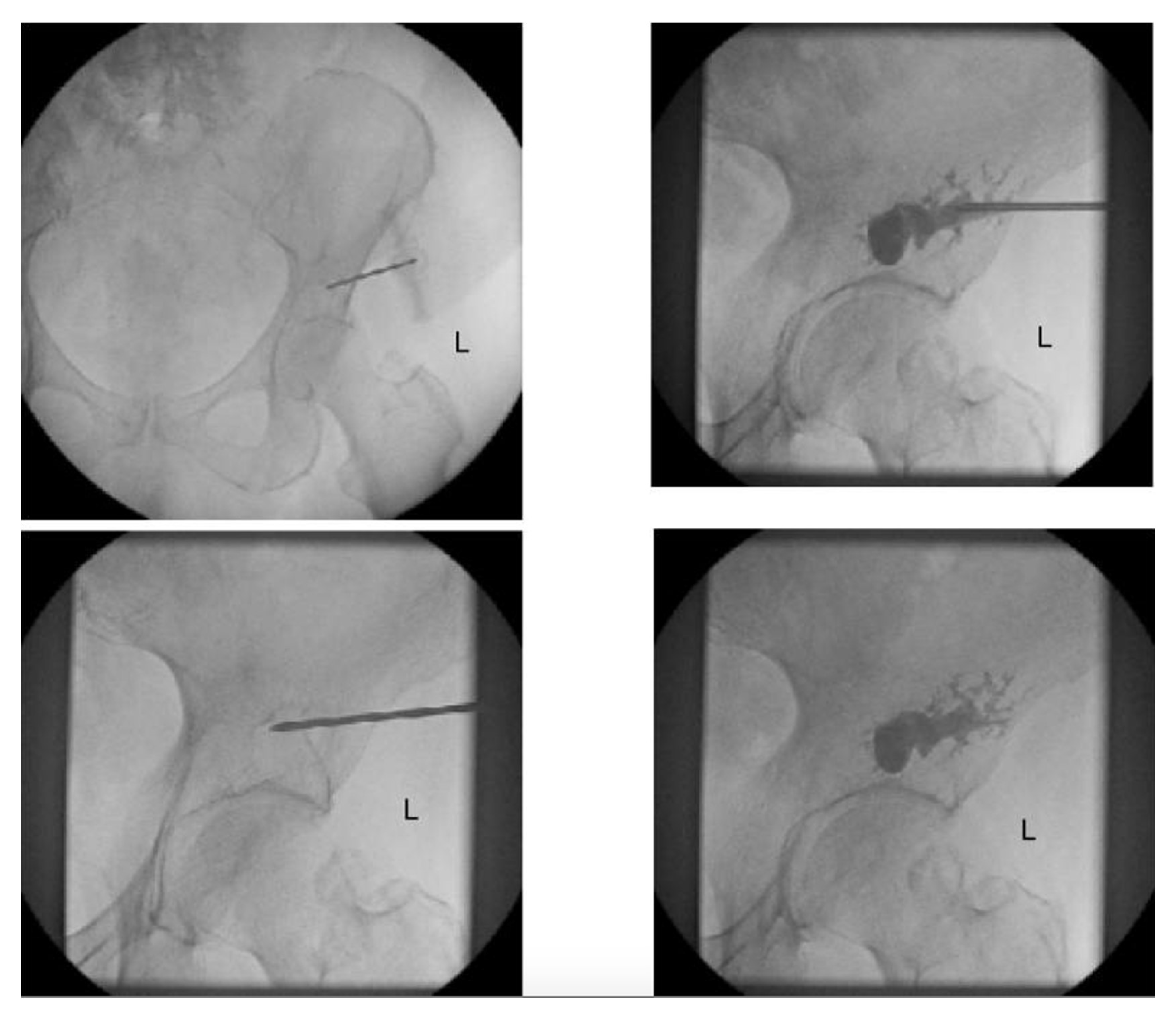
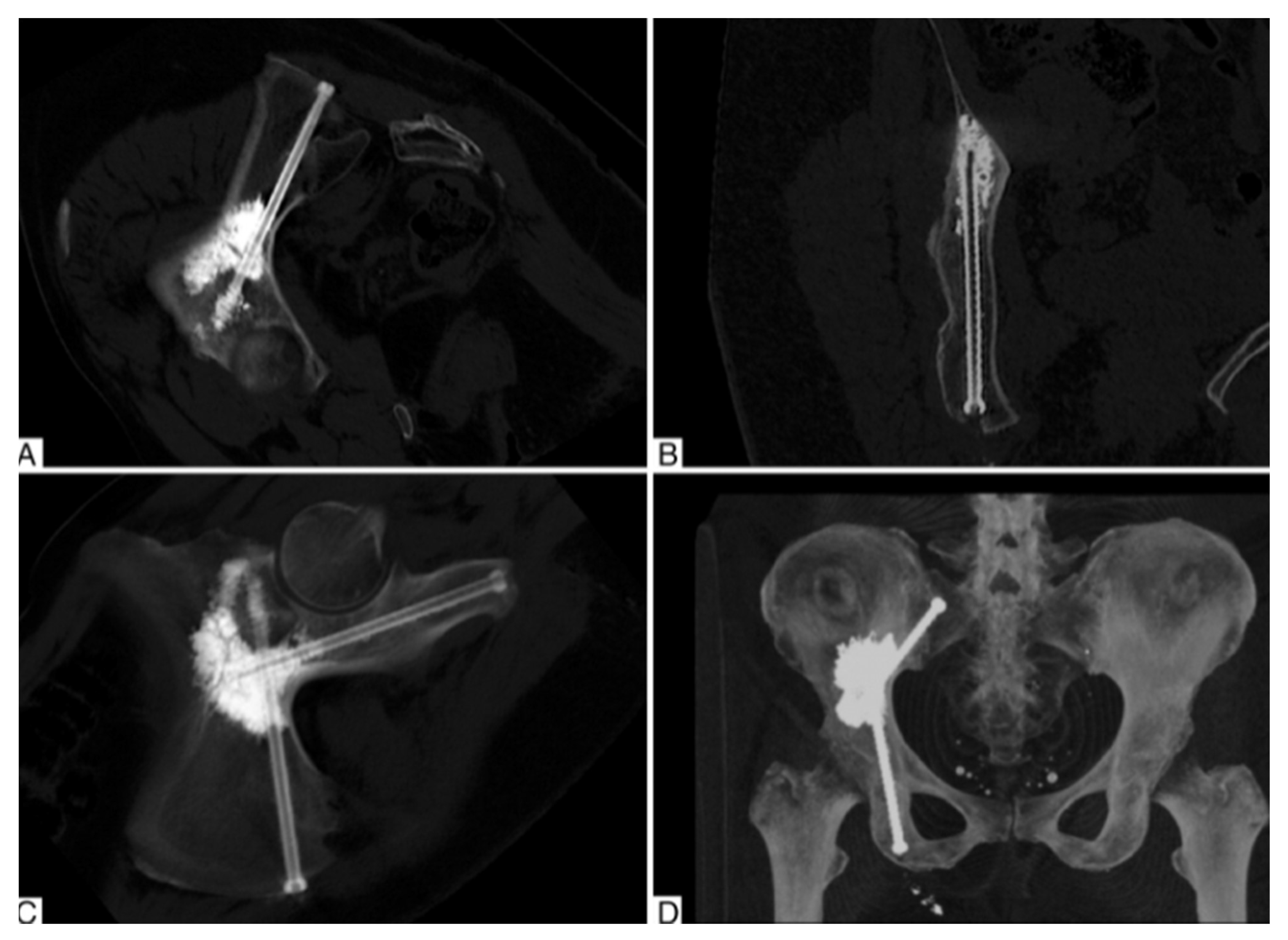
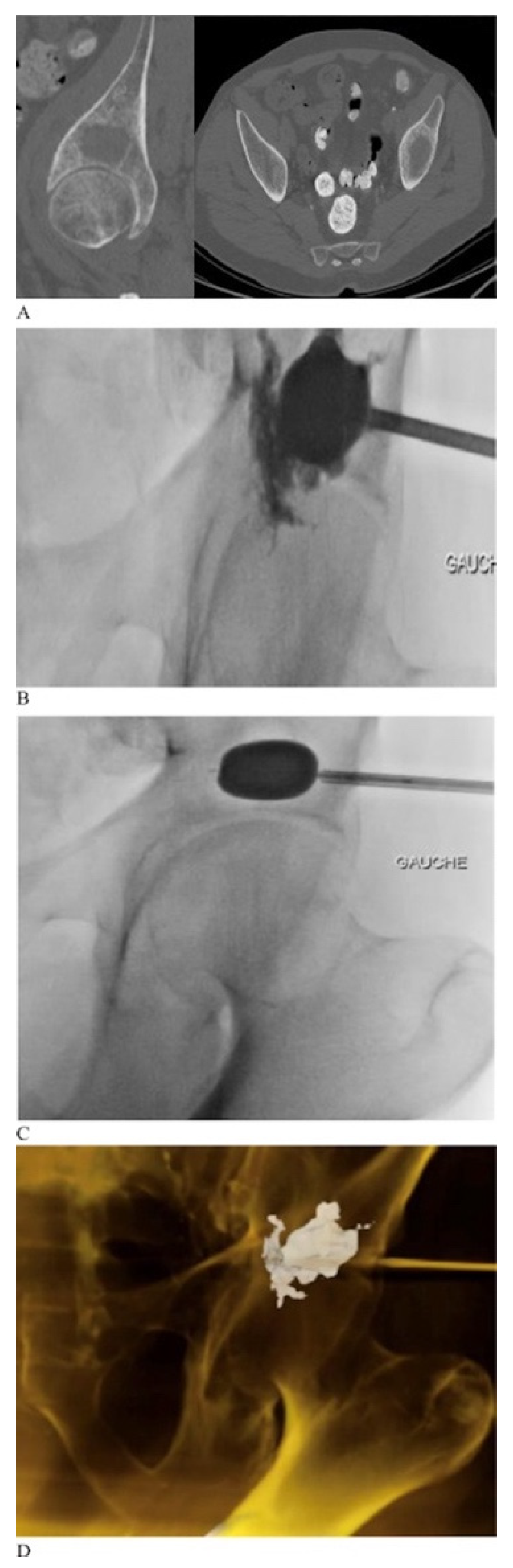
| Group | Description |
|---|---|
| I | Lateral cortices and superior/medial walls intact |
| II | Deficient medial wall |
| III | Acetabular dome defect |
| IV | Isolated lesion that could be resected with curative intent |
| Surgical Procedures | Utility | Drawbacks |
|---|---|---|
| Cementoplasty |
|
|
| Percutaneous Screw Fixation |
|
|
| Percutaneous Screws + Cementoplasty |
|
|
| Harrington Procedure |
|
|
| Acetabular Cages |
|
|
| Porous Tantalum Implants |
|
|
| Endoprosthetic Reconstructions |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazendam, A.; Axelrod, D.; Wilson, D.; Ghert, M. Emerging Concepts in the Surgical Management of Peri-Acetabular Metastatic Bone Disease. Curr. Oncol. 2021, 28, 2731-2740. https://doi.org/10.3390/curroncol28040238
Gazendam A, Axelrod D, Wilson D, Ghert M. Emerging Concepts in the Surgical Management of Peri-Acetabular Metastatic Bone Disease. Current Oncology. 2021; 28(4):2731-2740. https://doi.org/10.3390/curroncol28040238
Chicago/Turabian StyleGazendam, Aaron, Daniel Axelrod, David Wilson, and Michelle Ghert. 2021. "Emerging Concepts in the Surgical Management of Peri-Acetabular Metastatic Bone Disease" Current Oncology 28, no. 4: 2731-2740. https://doi.org/10.3390/curroncol28040238
APA StyleGazendam, A., Axelrod, D., Wilson, D., & Ghert, M. (2021). Emerging Concepts in the Surgical Management of Peri-Acetabular Metastatic Bone Disease. Current Oncology, 28(4), 2731-2740. https://doi.org/10.3390/curroncol28040238





