Patterns and Predictors of First-Line Taxane Use in Patients with Metastatic Triple-Negative Breast Cancer in US Clinical Practice
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. 1L Taxane Treatment Patterns
2.3. Predictors of 1L mTNBC Taxane Use
3. Discussion
4. Materials and Methods
4.1. Data Source
4.2. Patient Population
4.3. Exposure Definition
4.4. Covariate Definitions
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swain, S. Triple-negative breast cancer: Metastatic risk and role of platinum agents. In Proceedings of the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, USA, 30 May–3 June 2008. [Google Scholar]
- Trivers, K.F.; Lund, M.J.; Porter, P.L.; Liff, J.M.; Flagg, E.W.; Coates, R.J.; Eley, J.W. The epidemiology of triple-negative breast cancer, including race triple-negative breast cancer: Metastatic risk and role of platinum agents. Cancer Causes Control. 2009, 20, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- den Brok, W.D.; Speers, C.H.; Gondara, L.; Baxter, E.; Tyldesley, S.K.; Lohrisch, C.A. Survival with metastatic breast cancer based on initial presentation, do novo versus relapsed. Breast Cancer Res. Treat. 2017, 161, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Bonotto, M.; Gerratana, L.; Poletto, E.; Driol, P.; Giangreco, M.; Russo, S.; Minisini, A.M.; Andretta, C.; Mansutti, M.; Pisa, F.E.; et al. Measures of outcome in metastatic breast cancer: Insights from a real-world scenario. Oncologist. 2014, 19, 608–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinner, K.E.; Walker, M.S.; Haiderali, A.; Schwartzberg, L.S. Understanding real-world treatment and outcomes in patients diagnosed with metastatic triple negative breast cancer (mTNBC). J. Clin. Oncol. 2018, 36 (Suppl. 15), e13116. [Google Scholar] [CrossRef]
- Bajaj, P.S.; Latremouille-Viau, D.; Guerin, A.; Reyes, C.; Stein, A.; Kurian, P.; Cortazar, P. What are the treatment patterns and overall survival in patients with inoperable locally advanced or metastatic triple negative breast cancer in US clinical practice? Ann. Oncol. 2017, 8 (Suppl. 5), 74–108. [Google Scholar] [CrossRef]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann. Oncol. 2018, 29, 634–1657. [Google Scholar] [CrossRef] [PubMed]
- Senkus, E.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rutgers, E.; Zackrisson, S.; Cardoso, F. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v8–v30. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-La Roche, F. Seattle Genetics. A Study Evaluating the Efficacy and Safety of Multiple Immunotherapy-based Treatment Combinations in Patients with Metastatic or Inoperable Locally Advanced Triple-Negative Breast Cancer (Morpheus-TNBC). Available online: https://clinicaltrials.gov/ct2/show/NCT03424005 (accessed on 13 December 2019).
- Novartis Pharmaceuticals. Study of Safety and Efficacy of Novel Immunotherapy Combinations in Patients with Triple Negative Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03742349 (accessed on 13 December 2019).
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Seock-Ah, I.; Wright, G.S.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Adams, S.; Barrios, C.H.; Diéras, V.; Iwata, H.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Winer, E.P.; Patel, S.; et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol. 2021, in press. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. V6.2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 20 November 2020).
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; Andre, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- Luhn, P.; Chui, S.Y.; Hsieh, A.F.-C.; Jingbo, Y.; Mecke, A.; Bajab, P.S.; Hasnain, W.; Falgas, A.; Ton, T.G.; Kurian, A.W. Comparative effectiveness of first-line nab-paclitaxel versus paclitaxel monotherapy in triple-negative breast cancer. Comp. Eff. Res. 2019, 8, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Krasnojon, D.; Cheporov, S.; Makhson, A.N.; Manikhas, G.M.; Clawson, A.; Bhar, P. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J. Clin. Oncol. 2009, 27, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Pérez-García, J.; Whiting, S.; Wan, Y.; Solem, C.; Tai, M.-H.; Margunato-Debay, S.; Ko AFandi, A.; Botteman, M. Quality-Adjusted Survival With nab-Paclitaxel Versus Standard Paclitaxel in Metastatic Breast Cancer: A Q-TWiST Analysis. Clin. Breast Cancer. 2018, 18, e919–e926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzaro, C.; Bordonaro, R.; Cognetti, F.; Fabi, A.; De Placido, S.; Arpino, G.; Marchetti, P.; Botticelli, A.; Pronzato, P.; Martelli, E. An Italian cost-effectiveness analysis of paclitaxel albumin (nab-paclitaxel) versus conventional paclitaxel for metastatic breast cancer patients: The COSTANza study. Clinicoecon. Outcomes Res. 2013, 5, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahtani, R.L.; Parisi, M.; Glück, S.; Ni, Q.; Park, S.; Pelletier, C.; Faria, C.; Braiteh, F. Comparative effectiveness of early-line nab-paclitaxel vs. paclitaxel in patients with metastatic breast cancer: A US community-based real-world analysis. Cancer Manag. Res. 2018, 10, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Klabunde, C.N.; Ptoosky, A.L.; Legler, J.M.; Warren, J.L. Development of a comorbidity index using physician claims data. J. Clin. Epidemiol. 2000, 53, 1258–1267. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
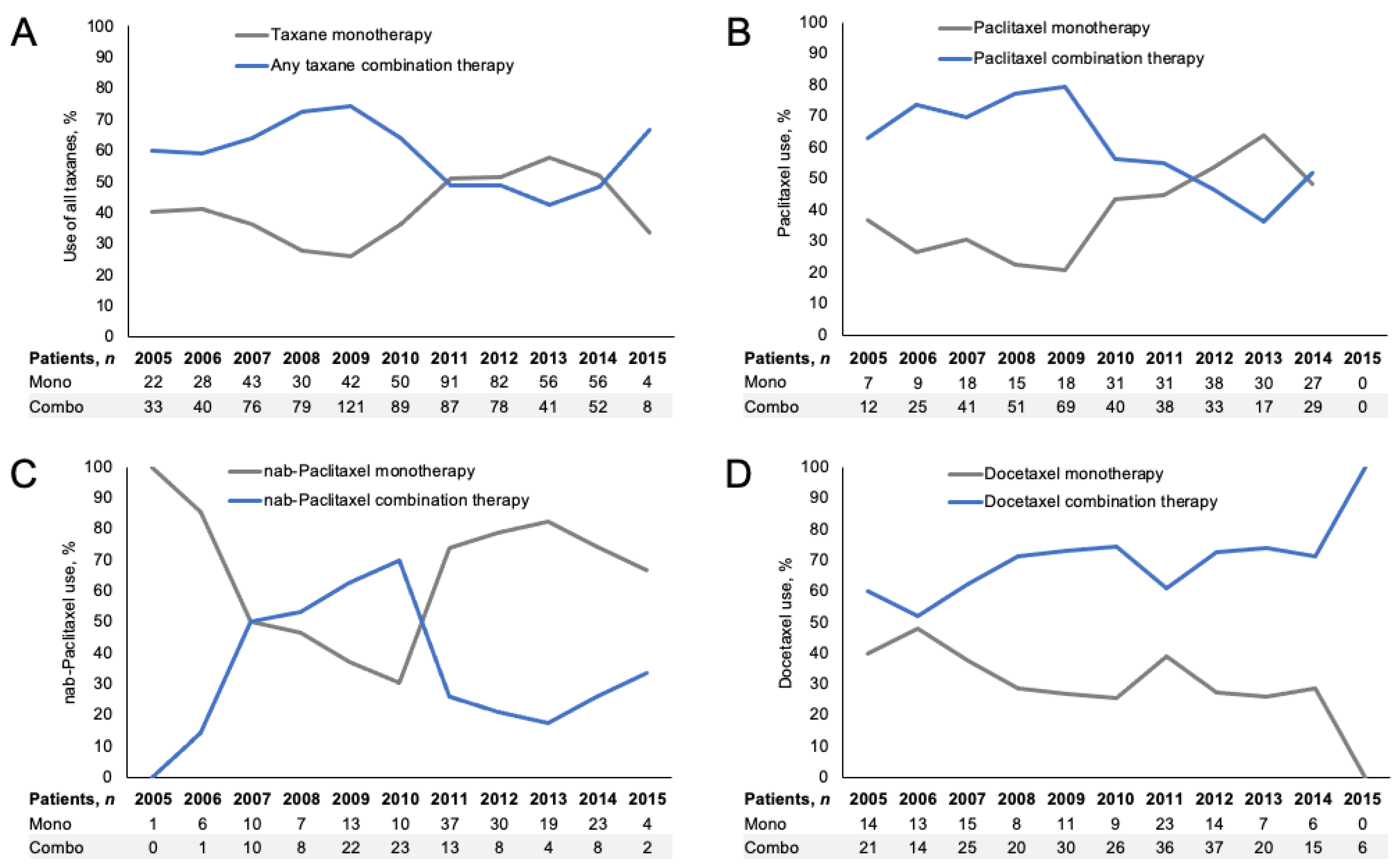
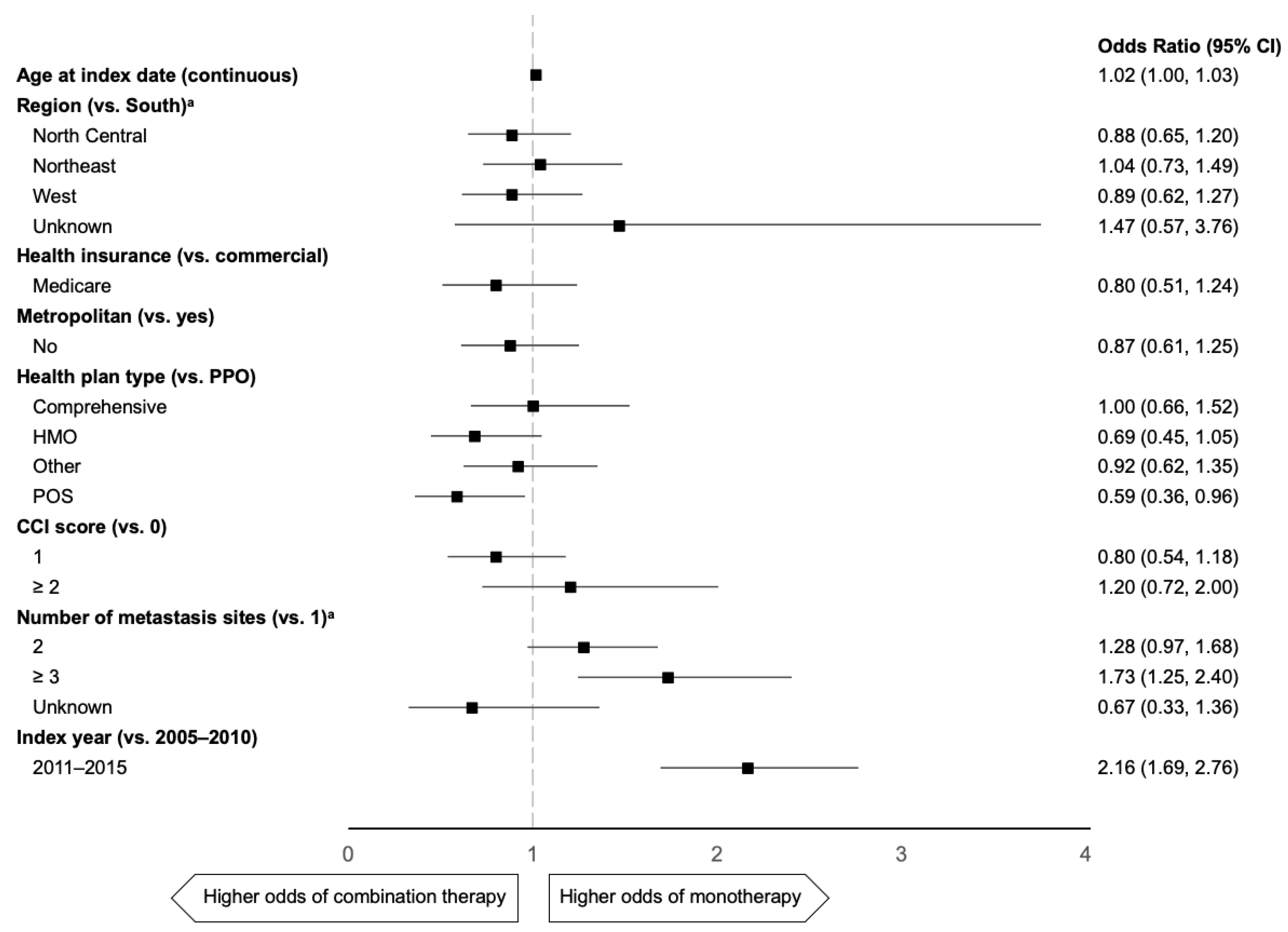
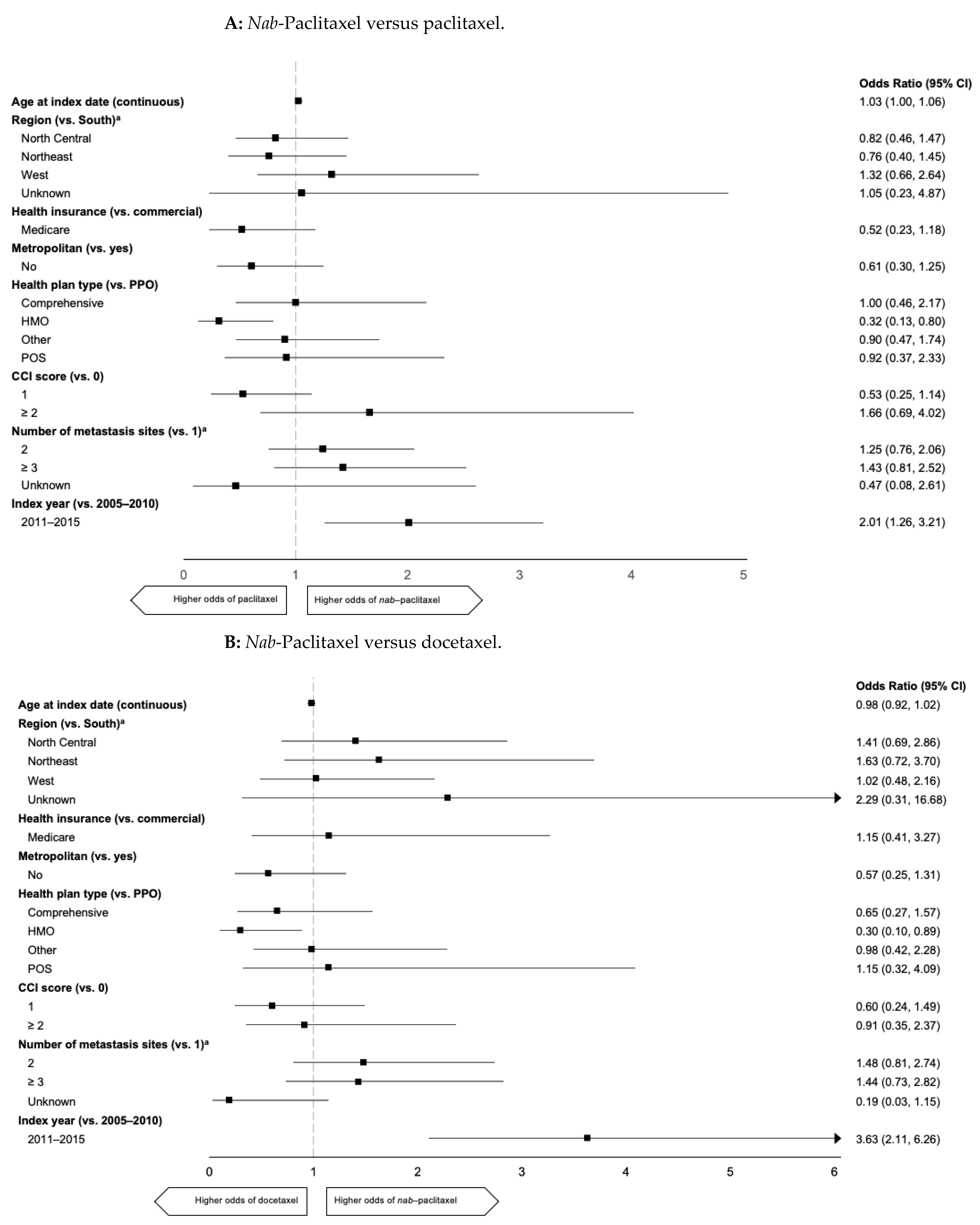
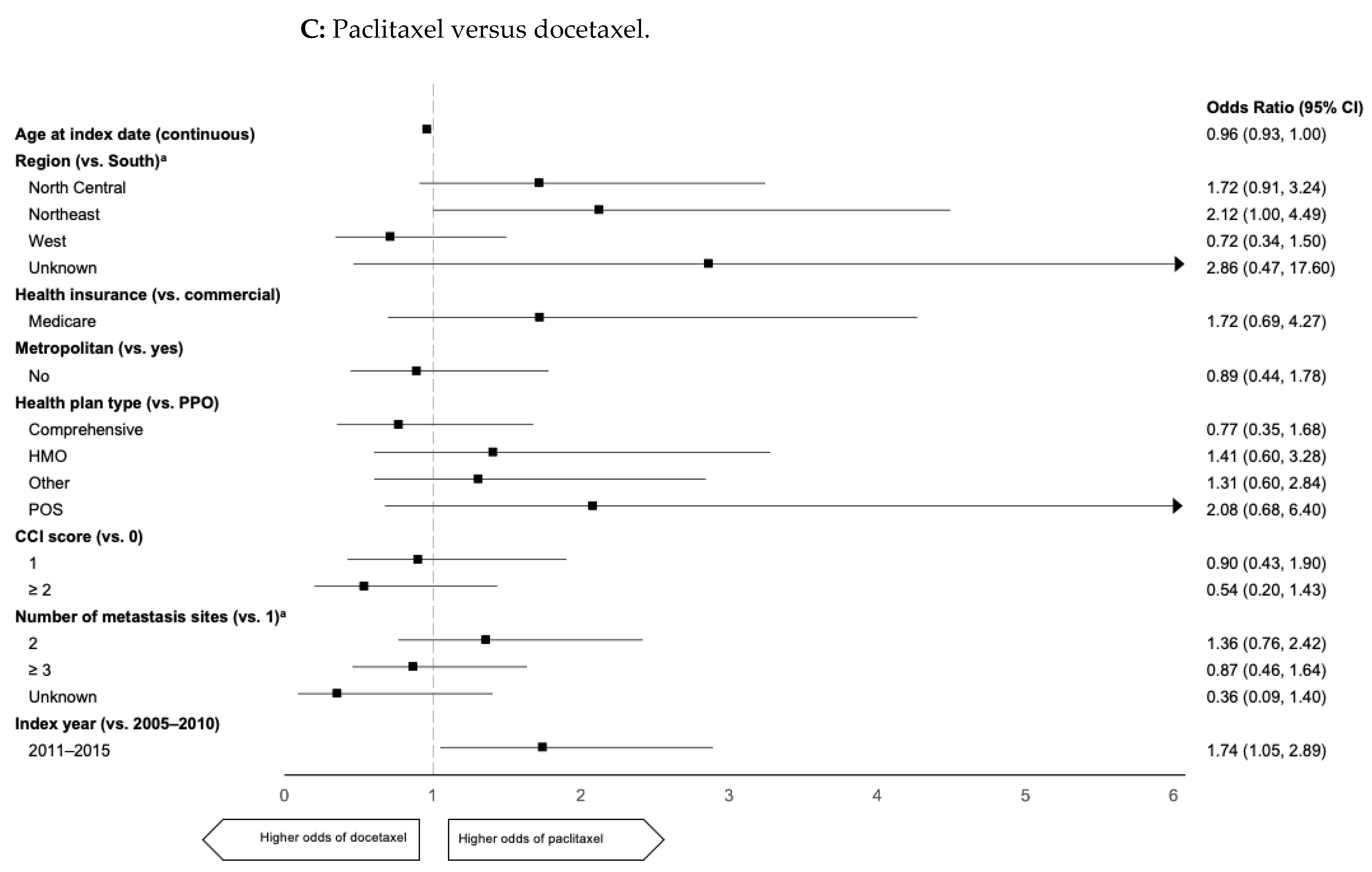
| Characteristic | All Patients (n = 2271) | 1L Paclitaxel (n = 579) | 1L nab-Paclitaxel (n = 259) | 1L Docetaxel (n = 370) |
|---|---|---|---|---|
| Age, median (IQR), years | 58 (51–64) | 58 (51–65) | 59 (53–64) | 59 (53–65) |
| Metropolitan area, n (%) | 1895 (83) | 480 (83) | 226 (87) | 319 (86) |
| US region, n (%) | ||||
| Northeast | 447 (20) | 101 (17) | 50 (19) | 50 (13) |
| North Central | 585 (26) | 165 (29) | 65 (25) | 81 (22) |
| South | 842 (37) | 219 (38) | 94 (36) | 166 (45) |
| West | 344 (15) | 83 (14) | 44 (17) | 67 (18) |
| Unknown | 53 (2) | 11 (2) | 6 (2) | 6 (2) |
| Health insurance, n (%) | ||||
| Commercial coverage | 1751 (77) | 442 (76) | 197 (76) | 289 (78) |
| Medicare | 520 (23) | 137 (24) | 62 (24) | 81 (22) |
| Health plan type, n (%) | ||||
| Comprehensive | 280 (12) | 75 (13) | 32 (12) | 52 (14) |
| HMO | 223 (10) | 58 (10) | 12 (5) | 48 (13) |
| PPO | 1347 (59) | 347 (60) | 157 (61) | 199 (54) |
| POS | 166 (7) | 41 (7) | 23 (9) | 28 (8) |
| Other | 255 (11) | 58 (10) | 35 (13) | 43 (12) |
| Charlson Comorbidity Index, n (%) | ||||
| 0 | 1903 (84) | 483 (83) | 224 (87) | 302 (82) |
| 1 | 245 (11) | 70 (12) | 16 (16) | 43 (12) |
| ≥2 | 123 (5) | 26 (5) | 19 (7) | 25 (7) |
| 1L treatment index date, n (%) | ||||
| 2005–2010 | 1142 (50) | 336 (58) | 111 (43) | 206 (56) |
| 2011–2015 | 1129 (50) | 243 (42) | 148 (57) | 164 (44) |
| Site of metastases, n (%) | ||||
| Location | ||||
| Bone | 1089 (48) | 296 (51) | 157 (61) | 174 (47) |
| Brain | 311 (14) | 68 (12) | 33 (13) | 54 (15) |
| Liver | 599 (26) | 174 (30) | 89 (34) | 82 (22) |
| Lung | 743 (33) | 208 (36) | 86 (33) | 111 (30) |
| Other | 1008 (44) | 273 (47) | 107 (41) | 144 (39) |
| Number of unique organ sites of metastases, n (%) | ||||
| 1 | 1103 (49) | 269 (47) | 102 (39) | 207 (56) |
| 2 | 670 (29) | 183 (32) | 103 (40) | 88 (24) |
| ≥3 | 388 (17) | 111 (19) | 50 (19) | 54 (15) |
| Unknown | 110 (5) | 16 (3) | 4 (2) | 21 (6) |
| Treatment Regimen, n (%) | Any Taxane Regimen (n = 1208) | 1L Paclitaxel (n = 579) | 1L nab-Paclitaxel (n = 259) | 1L Docetaxel (n = 370) |
|---|---|---|---|---|
| Monotherapy | 504 (42) | 224 (39) | 160 (62) | 120 (32) |
| Doublet | 566 (47) | 302 (52) | 89 (34) | 175 (47) |
| + platinum | 203 (36) | 137 (45) | 19 (21) | 47 (27) |
| + bevacizumab a | 192 (34) | 122 (40) | 58 (65) | 12 (7) |
| + anthracycline | 14 (2) | 5 (2) | 2 (2) | 7 (4) |
| + other agent | 157 (28) | 38 (13) | 10 (11) | 109 (62) |
| Triplet or more | 138 (11) | 53 (9) | 10 (4) | 75 (20) |
| Schedule for administration | ||||
| qw | 574 (48) | 338 (58) | 175 (67) | 61 (16) |
| q3w | 518 (43) | 195 (34) | 60 (23) | 263 (71) |
| Other | 116 (10) | 46 (8) | 24 (9) | 46 (12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Shaughnessy, J.; Emens, L.A.; Chui, S.Y.; Wang, W.; Russell, K.; Lin, S.-W.; Flores Avile, C.; Luhn, P.; Schneeweiss, A. Patterns and Predictors of First-Line Taxane Use in Patients with Metastatic Triple-Negative Breast Cancer in US Clinical Practice. Curr. Oncol. 2021, 28, 2741-2752. https://doi.org/10.3390/curroncol28040239
O’Shaughnessy J, Emens LA, Chui SY, Wang W, Russell K, Lin S-W, Flores Avile C, Luhn P, Schneeweiss A. Patterns and Predictors of First-Line Taxane Use in Patients with Metastatic Triple-Negative Breast Cancer in US Clinical Practice. Current Oncology. 2021; 28(4):2741-2752. https://doi.org/10.3390/curroncol28040239
Chicago/Turabian StyleO’Shaughnessy, Joyce, Leisha A. Emens, Stephen Y. Chui, Wei Wang, Kenneth Russell, Shih-Wen Lin, Carlos Flores Avile, Patricia Luhn, and Andreas Schneeweiss. 2021. "Patterns and Predictors of First-Line Taxane Use in Patients with Metastatic Triple-Negative Breast Cancer in US Clinical Practice" Current Oncology 28, no. 4: 2741-2752. https://doi.org/10.3390/curroncol28040239
APA StyleO’Shaughnessy, J., Emens, L. A., Chui, S. Y., Wang, W., Russell, K., Lin, S.-W., Flores Avile, C., Luhn, P., & Schneeweiss, A. (2021). Patterns and Predictors of First-Line Taxane Use in Patients with Metastatic Triple-Negative Breast Cancer in US Clinical Practice. Current Oncology, 28(4), 2741-2752. https://doi.org/10.3390/curroncol28040239





