The Screening and COnsensus Based on Practices and Evidence (SCOPE) Program–Results of a Survey on Daily Practice Patterns for Patients with mCRC
Abstract
:1. Introduction
2. Materials and Methods
- Case 1: A fit and active 54-year-old male with a left-sided, KRAS-wildtype colon adenocarcinoma
- Case 2: A 68-year-old female with a KRAS-mutated left-sided colon adenocarcinoma, comorbidities, and previous tolerability issues
- Case 3: An 82-year-old male with a KRAS-wildtype right-sided colon adenocarcinoma who had comorbidities, limited support, and difficult hospital accessibility
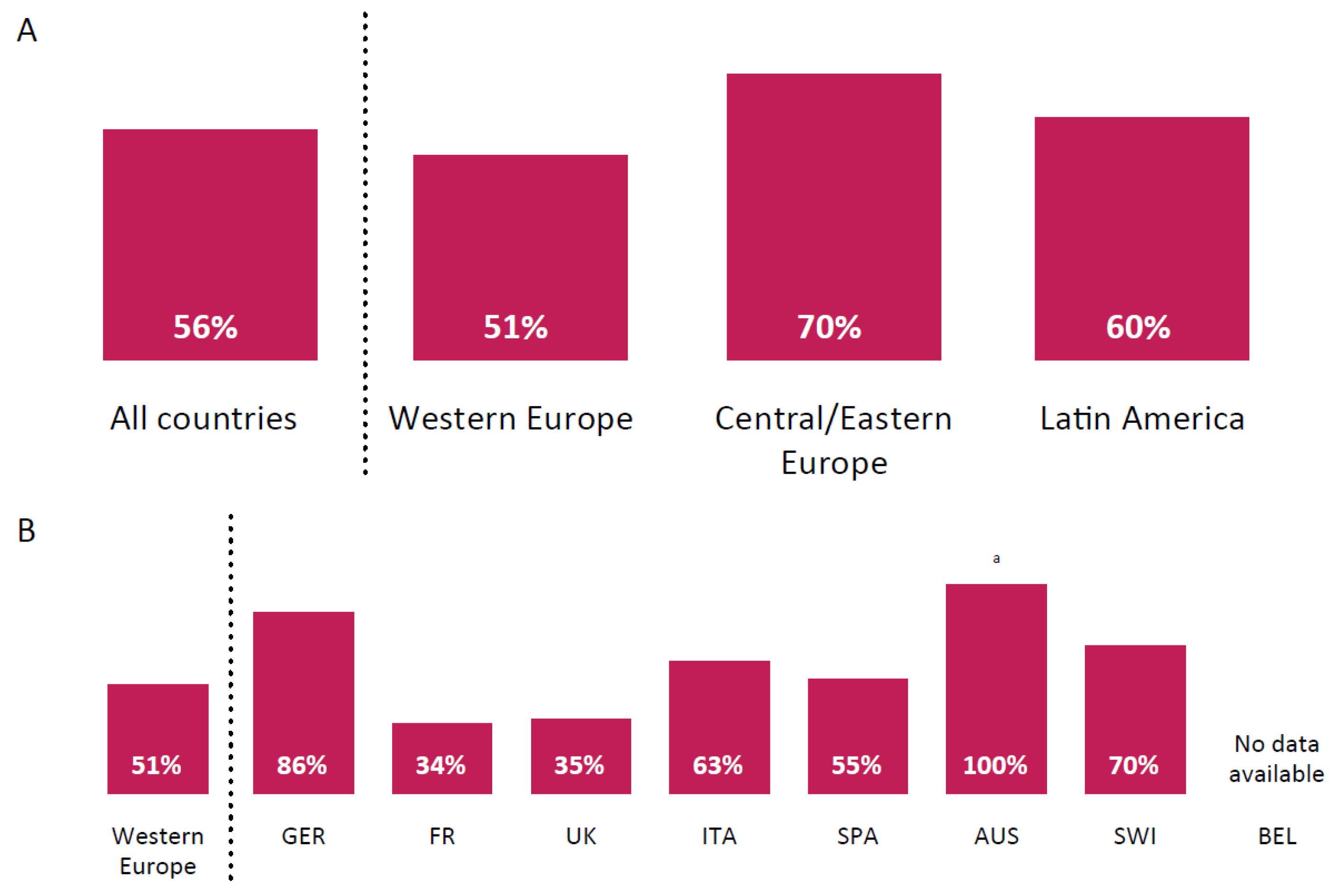
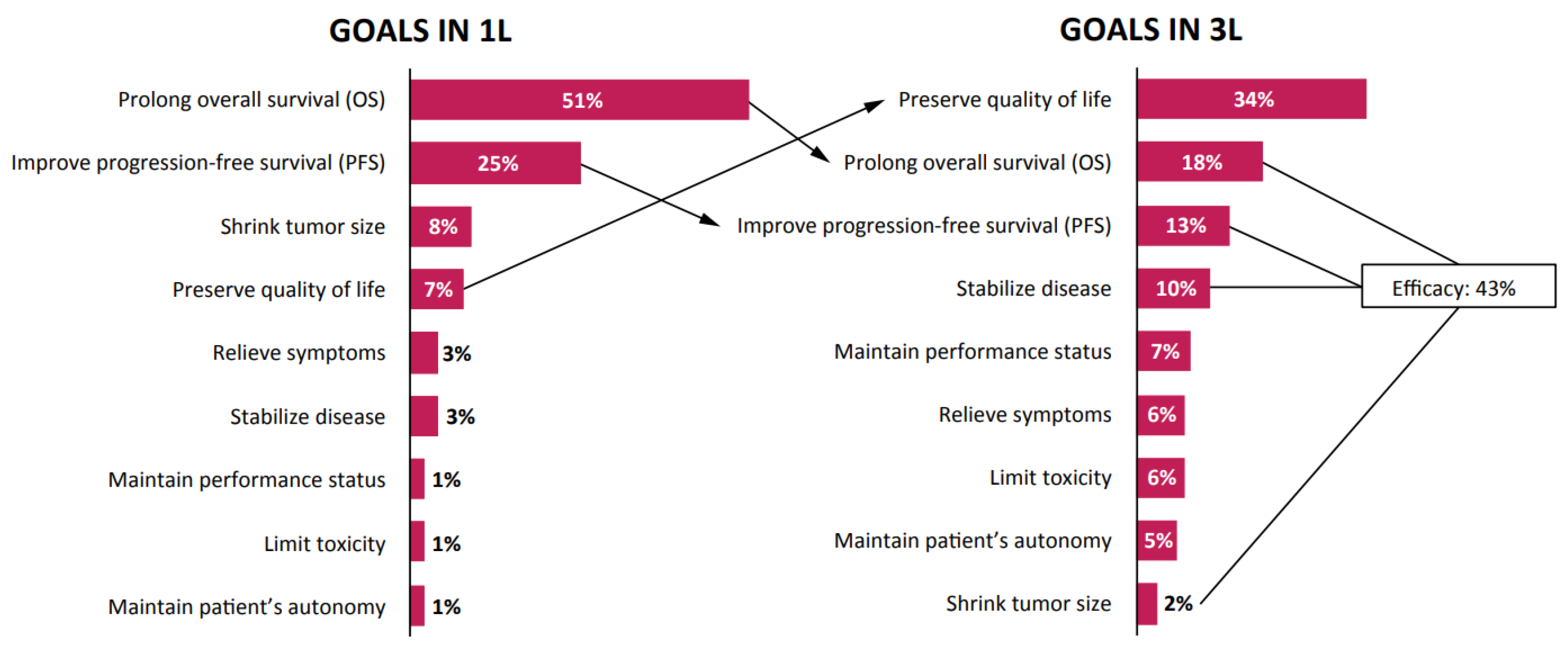
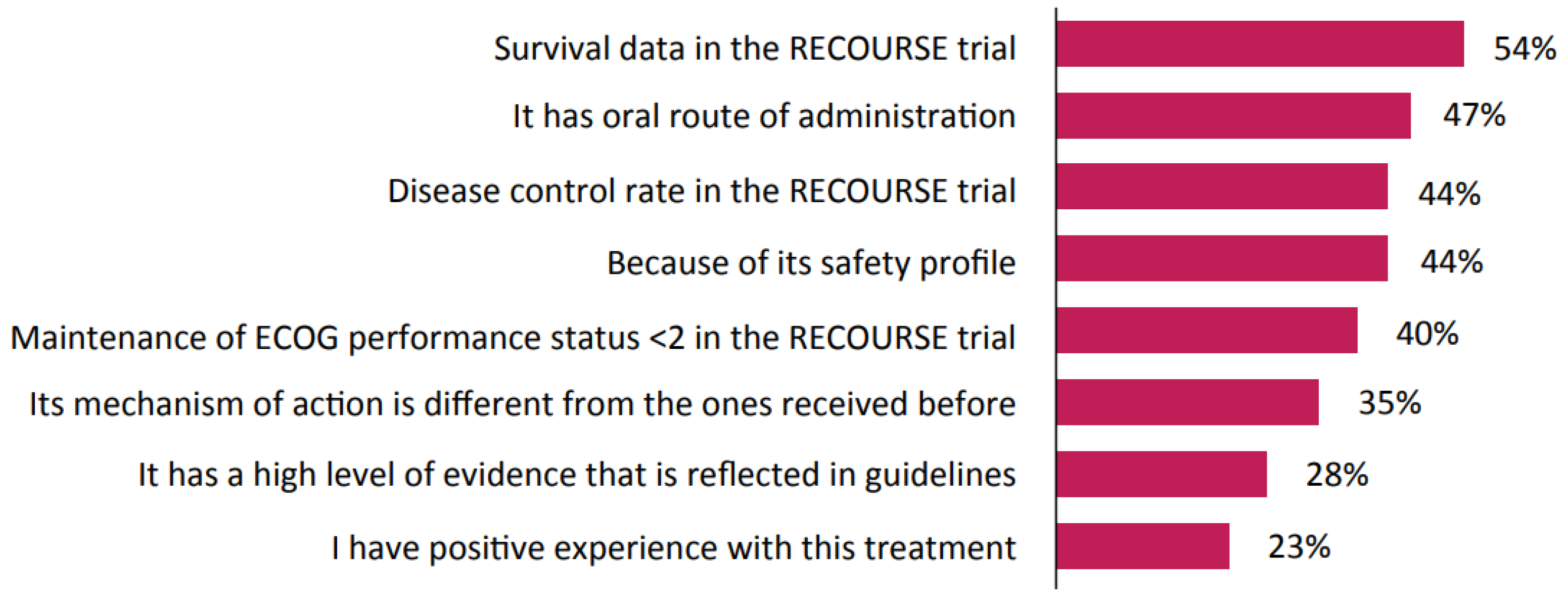
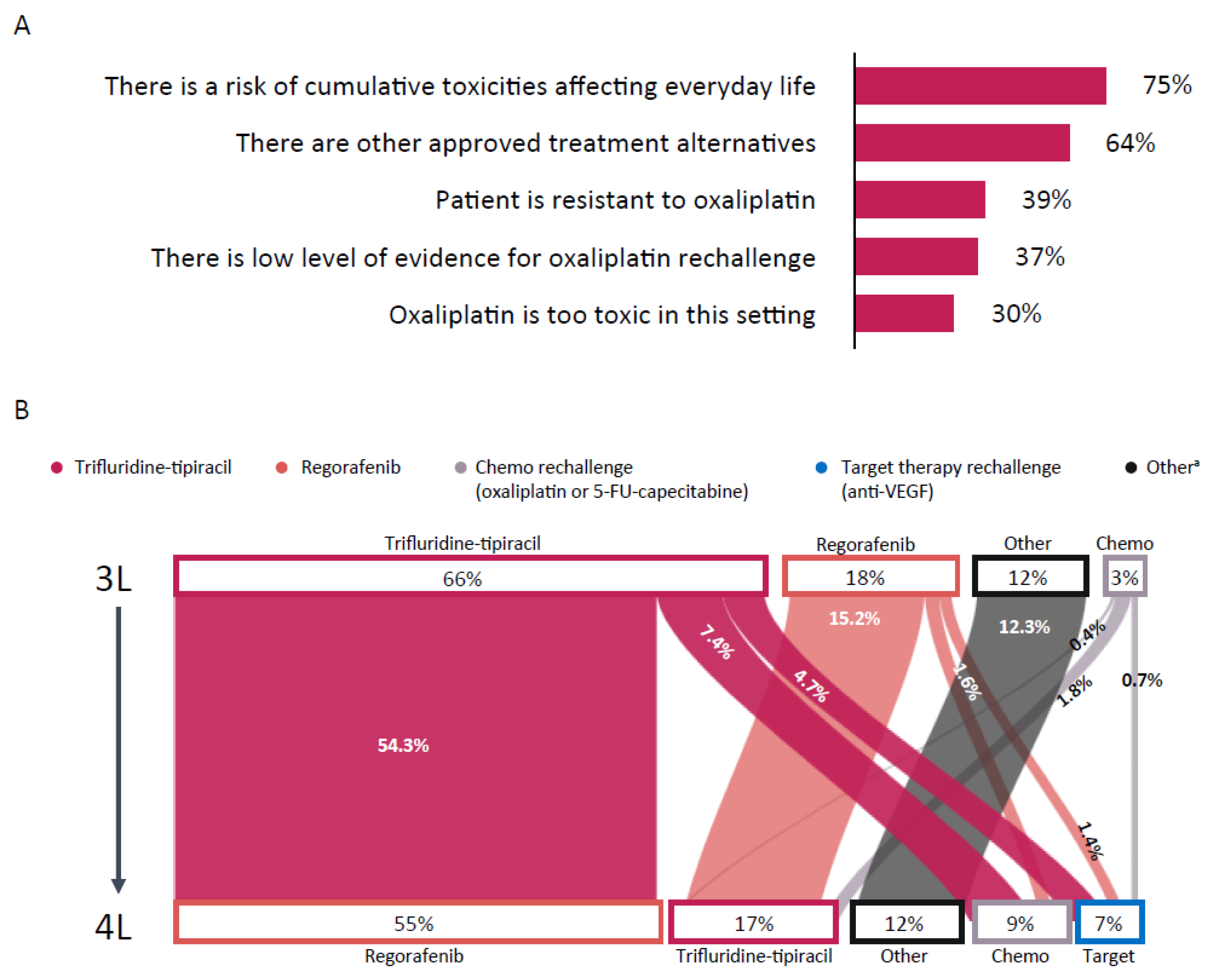
3. Results
3.1. Practice Patterns
3.2. Patient Cases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Salazar, R.; Tabernero, J. Molecular subtypes and the evolution of treatment decisions in metastatic colorectal cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabbinavar, F.F.; Hambleton, J.; Mass, R.D.; Hurwitz, H.J.; Bergsland, E.; Sarkar, S. Combined analysis of efficacy: The addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J. Clin. Oncol. 2005, 23, 3706–3712. [Google Scholar] [CrossRef] [PubMed]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Mahoney, M.R.; O’Neil, B.H.; Shaw, J.E.; Polite, B.N.; Hochster, H.S.; Atkins, J.N.; et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J. Clin. Oncol. 2014, 32. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Colon Cancer. Version 4.2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 26 January 2021).
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Fernández-Montes, A.; Grávalos, C.; Pericay, C.; Safont, M.J.; Benavides, M.; Élez, E.; García-Alfonso, P.; García-Paredes, B.; Carrato, A.; Aranda, E. Current options for third-line and beyond treatment of metastatic colorectal cancer. Spanish TTD Group expert opinion. Clin. Colorectal Cancer 2020, 19, 165–177. [Google Scholar] [CrossRef]
- World Health Organization. Medicines Reimbursement Policies in Europe. 2018. Available online: https://www.euro.who.int/en/health-topics/Health-systems/health-technologies-and-medicines/publications/2018/medicines-reimbursement-policies-in-europe (accessed on 26 January 2021).
- Tejpar, S.; Bertagnolli, M.; Bosman, F.; Lenz, H.J.; Garraway, L.; Waldman, F.; Warren, R.; Bild, A.; Collins-Brennan, D.; Hahn, H.; et al. Prognostic and predictive biomarkers in resected colon cancer: Current status and future perspectives for integrating genomics into biomarker discovery. Oncologist 2010, 15, 390–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, M.; Kafatos, G.; Taylor, A.; Gastanaga, V.M.; Oliner, K.S.; Hechmati, G.; Terwey, J.H.; van Krieken, J.H. Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: A pooled analysis of randomised controlled trials. Eur. J. Cancer 2015, 51, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Boeckx, N.; Koukakis, R.; Op de Beeck, K.; Rolfo, C.; Van Camp, G.; Siena, S.; Tabernero, J.; Douillard, J.Y.; André, T.; Peeters, M. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: Results from two randomized first-line panitumumab studies. Ann. Oncol. 2017, 28, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Tomasello, G.; Borgonovo, K.; Ghidini, M.; Turati, L.; Dallera, P.; Passalacqua, R.; Sgroi, G.; Barni, S. Prognostic survival associated with left-sided vs right-sided colon cancer: A systematic review and meta-analysis. JAMA Oncol. 2017, 3, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.Y.; Diakos, C.I.; Engel, A.; Chan, D.L.H.; Pavlakis, N.; Gill, A.; Clarke, S.J. Tumor sidedness is not an independent prognostic marker of colorectal cancer patients undergoing curative resection: A retrospective cohort study. PLoS ONE 2019, 14, e0218207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, D.; Lueza, B.; Douillard, J.Y.; Peeters, M.; Lenz, H.J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.P.; Tabernero, J.; et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef] [PubMed]
- Baran, B.; Ozupek, N.M.; Tetik, N.Y.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference between left-sided and right-sided colorectal cancer: A focused review of literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.P.; Miller-Sonet, E.; Nipp, R.D.; Kamal, A.H.; Love, S.; Rocque, G.B. Importance of quality-of-life priorities and preferences surrounding treatment decision making in patients with cancer and oncology clinicians. Cancer 2020, 126, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
| Characteristic, % | N = 629 |
|---|---|
| Age, yr | |
| <35 | 28 |
| 35–55 | 58 |
| >55 | 13 |
| Medical specialty | |
| Medical oncologist | 69 |
| Radio-oncologist | 14 |
| Gastroenterologist | 16 |
| Surgeon | 1 |
| Type of practice setting a | |
| Private office | 8 |
| University hospital | 47 |
| Anticancer center | 21 |
| General/Public hospital | 18 |
| Private hospital/clinic | 7 |
| Patients with mCRC/month | |
| ≥40 | 19 |
| 30–39 | 10 |
| 20–29 | 22 |
| 10–19 | 31 |
| <10 | 18 |
| Number of participants per country | |
| Argentina | 47 (7.5) |
| Austria | 24 (3.8) |
| Belgium | 6 (1.0) |
| Croatia | 29 (4.6) |
| France | 130 (20.7) |
| Germany | 58 (9.2) |
| Hungary | 74 (11.8) |
| Italy | 53 (8.4) |
| Slovenia | 12 (1.9) |
| Spain | 31 (4.9) |
| Switzerland | 40 (6.5) |
| United Kingdom | 125 (19.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prager, G.; Köhne, C.-H.; O’Connor, J.M.; Rivera, F.; Santini, D.; Wasan, H.; Phelip, J.M. The Screening and COnsensus Based on Practices and Evidence (SCOPE) Program–Results of a Survey on Daily Practice Patterns for Patients with mCRC. Curr. Oncol. 2021, 28, 2097-2106. https://doi.org/10.3390/curroncol28030194
Prager G, Köhne C-H, O’Connor JM, Rivera F, Santini D, Wasan H, Phelip JM. The Screening and COnsensus Based on Practices and Evidence (SCOPE) Program–Results of a Survey on Daily Practice Patterns for Patients with mCRC. Current Oncology. 2021; 28(3):2097-2106. https://doi.org/10.3390/curroncol28030194
Chicago/Turabian StylePrager, Gerald, Claus-Henning Köhne, Juan Manuel O’Connor, Fernando Rivera, Daniele Santini, Harpreet Wasan, and Jean Marc Phelip. 2021. "The Screening and COnsensus Based on Practices and Evidence (SCOPE) Program–Results of a Survey on Daily Practice Patterns for Patients with mCRC" Current Oncology 28, no. 3: 2097-2106. https://doi.org/10.3390/curroncol28030194
APA StylePrager, G., Köhne, C.-H., O’Connor, J. M., Rivera, F., Santini, D., Wasan, H., & Phelip, J. M. (2021). The Screening and COnsensus Based on Practices and Evidence (SCOPE) Program–Results of a Survey on Daily Practice Patterns for Patients with mCRC. Current Oncology, 28(3), 2097-2106. https://doi.org/10.3390/curroncol28030194






