Activity and Safety of NAB-FOLFIRI and NAB-FOLFOX as First-Line Treatment for metastatic Pancreatic Cancer (NabucCO Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
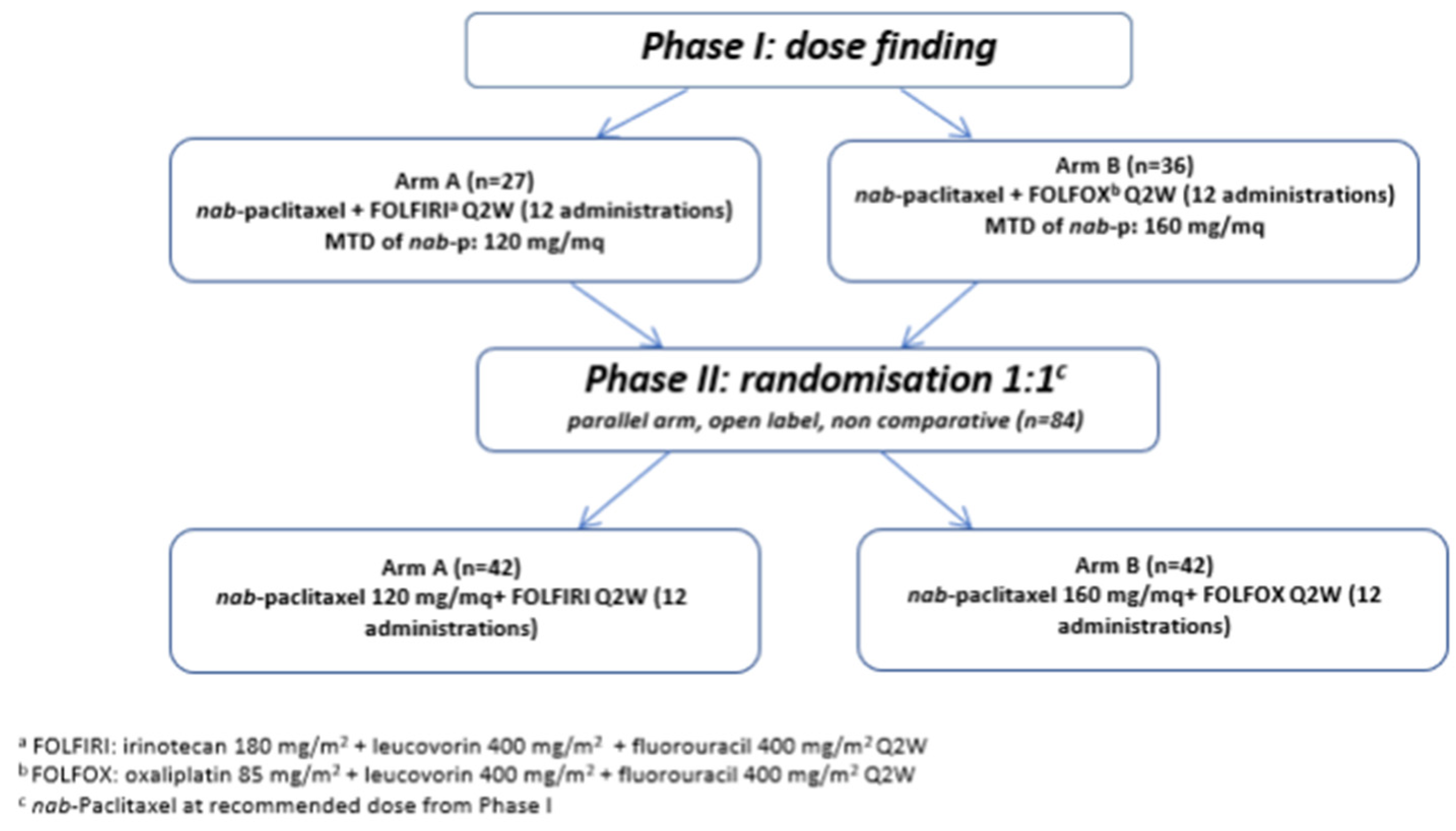
2.1.1. Phase I
2.1.2. Phase II
2.1.3. Study Assessment
3. Results
3.1. Phase I
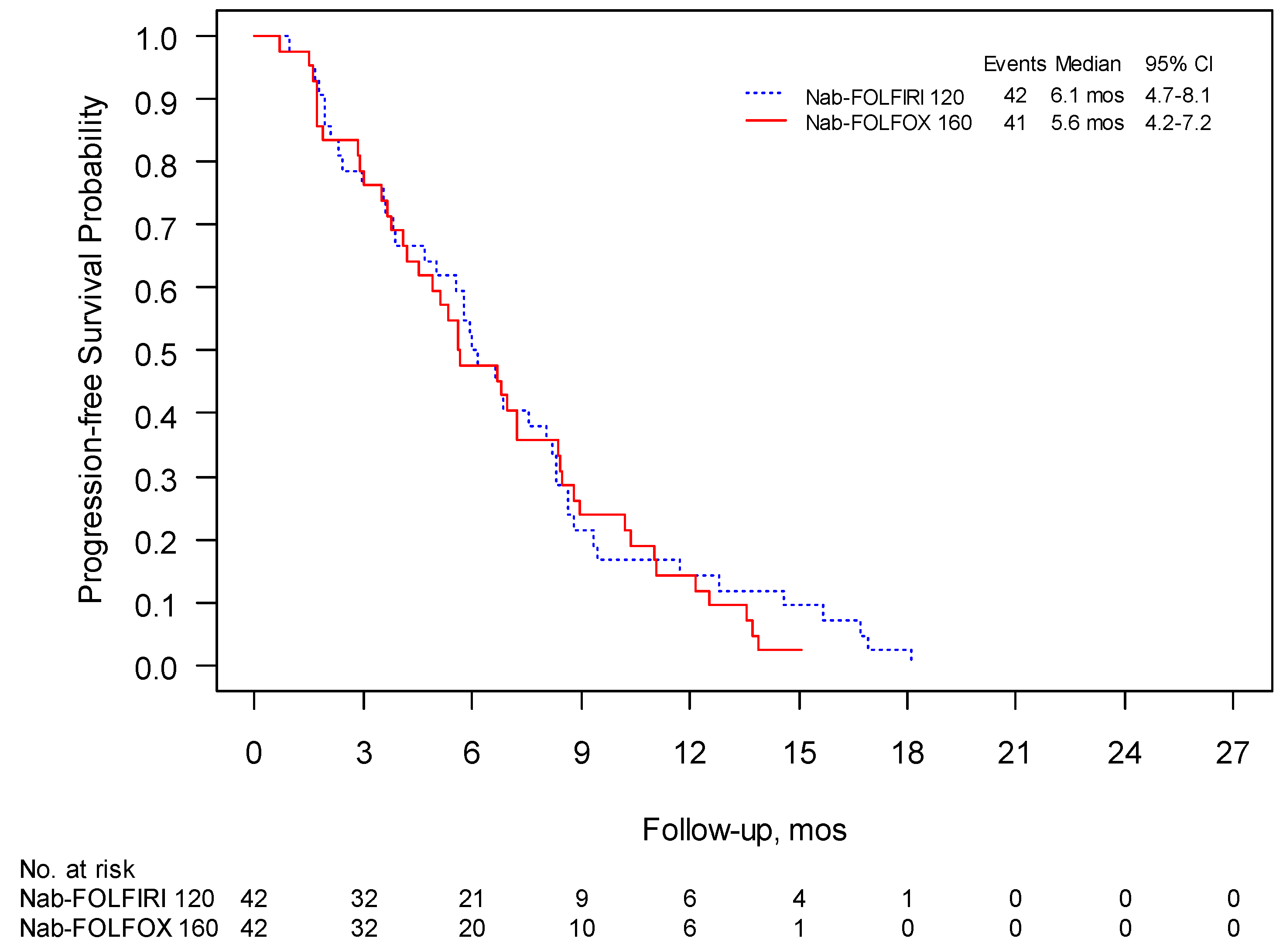
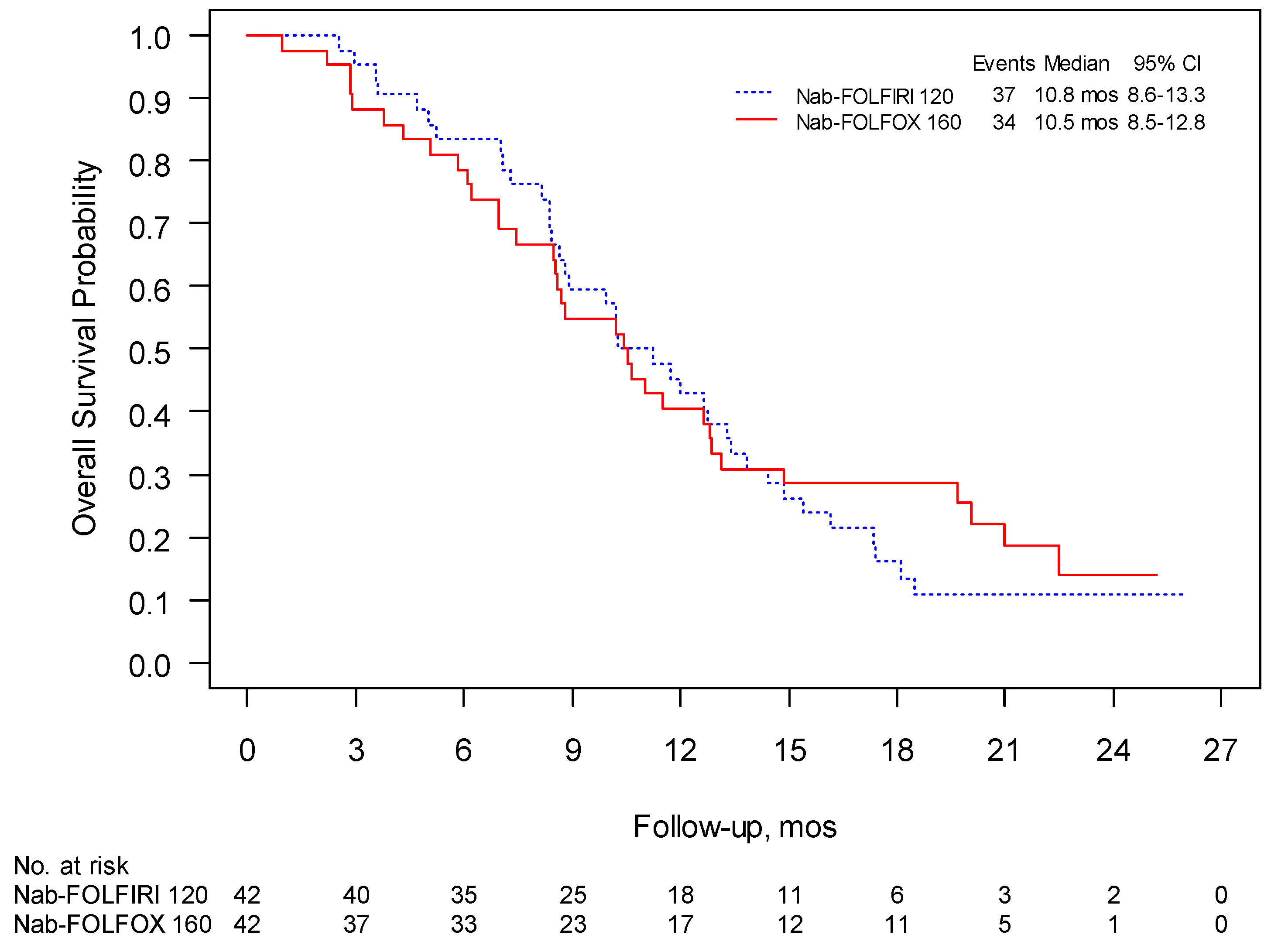
3.2. Phase II
3.3. QoL
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Carioli, G.; Bertuccio, P.; Rosso, T.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2016 with focus on leukaemias. Ann. Oncol. 2016, 27, 725–731. [Google Scholar] [CrossRef]
- Bosetti, C.; Bertuccio, P.; Malvezzi, M.; Levi, F.; Chatenoud, L.; Negri, E.; La Vecchia, C. Cancer mortality in Europe, 2005–2009, and an overview of trends since 1980. Ann. Oncol. 2013, 24, 2657–2671. [Google Scholar] [CrossRef]
- Sohal, D.P.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Philip, P.A.; O’Reilly, E.M.; Uronis, H.E.; Ramanathan, R.K.; Crane, C.H.; Engebretson, A.; et al. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2784–2796. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Smith, C.T.; Cunningham, D.; Starling, N.; Neoptolemos, J.P.; Ghaneh, P. Meta-Analyses of Chemotherapy for Locally Advanced and Metastatic Pancreatic Cancer. J. Clin. Oncol. 2007, 25, 2607–2615. [Google Scholar] [CrossRef]
- Heinemann, V.; Haas, M.; Boeck, S. Systematic treatment of advanced pancreatic cancer. Cancer Treat Rev. 2013, 38, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K.; Desai, N.; Legha, S.; Soon-Shiong, P.; Theriault, R.L.; Rivera, E.; Esmaeli, B.; Ring, S.E.; Bedikian, A.; Hortobagyi, G.N.; et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin. Cancer Res. 2002, 8, 1038–1044. [Google Scholar] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Signorovitch, J.E.; Yang, H.; Patterson-Lomba, O.; Xiang, C.Q.; Ung, B.; Parisi, M.; Marshall, J.L. Comparative Effectiveness of nab-Paclitaxel Plus Gemcitabine vs FOLFIRINOX in Metastatic Pancreatic Cancer: A Retrospective Nationwide Chart Review in the United States. Adv. Ther. 2018, 35, 1564–1577. [Google Scholar] [CrossRef]
- Pusceddu, S.; Ghidini, M.; Torchio, M.; Corti, F.; Tomasello, G.; Niger, M.; Prinzi, N.; Nichetti, F.; Coinu, A.; Di Bartolomeo, M.; et al. Comparative Effectiveness of Gemcitabine plus Nab-Paclitaxel and FOLFIRINOX in the First-Line Setting of Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 484. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v56–v68. [Google Scholar] [CrossRef]
- Lambert, A.; Gavoille, C.; Conroy, T. Current status on the place of FOLFIRINOX in metastatic pancreatic cancer and future directions. Ther. Adv. Gastroenterol. 2017, 10, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Mahaseth, H.; Brutcher, E.; Kauh, J.; Hawk, N.; Kim, S.; Chen, Z.; Kooby, D.A.; Maithel, S.K.; Landry, J.; El-Rayes, B.F. Modified FOLFIRINOX Regimen with Improved Safety and Maintained Efficacy in Pancreatic Adenocarcinoma. Pancreas 2013, 42, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Blazer, M.; Wu, C.; Goldberg, R.M.; Phillips, G.; Schmidt, C.; Muscarella, P.; Wuthrick, E.; Williams, T.M.; Reardon, J.; Ellison, E.C.; et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann. Surg. Oncol. 2014, 22, 1153–1159. [Google Scholar] [CrossRef]
- Ghorani, E.; Wong, H.H.; Hewitt, C.; Calder, J.; Corrie, P.; Basu, B. Safety and Efficacy of Modified FOLFIRINOX for Advanced Pancreatic Adenocarcinoma: A UK Single-Centre Experience. Oncology 2015, 89, 281–287. [Google Scholar] [CrossRef]
- Li, X.; Guo, C.; Li, Q.; Wei, S.; Zhang, Q.; Chen, Y.; Shen, Y.; Ma, T.; Li, G.; Gao, S.; et al. Association of Modified-FOLFIRINOX-Regimen-Based Neoadjuvant Therapy with Outcomes of Locally Advanced Pancreatic Cancer in Chinese Population. Oncologist 2018, 24, 301-e93. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Zhang, F.; Deng, T.; Zhang, L.; Feng, F.; Wang, F.-H.; Wang, W.; Wang, D.-S.; Luo, H.-Y.; Xu, R.-H.; et al. The efficacy and safety of modified FOLFIRINOX as first-line chemotherapy for Chinese patients with metastatic pancreatic cancer. Cancer Commun. 2019, 39, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Remenar, E.; Van Herpen, C.; Gorlia, T.; Mesia, R.; Degardin, M.; Stewart, J.S.; Jelic, S.; Betka, J.; EORTC 24971/TAX 323 Study Group; et al. Cisplatin, Fluorouracil, and Docetaxel in Unresectable Head and Neck Cancer. N. Engl. J. Med. 2007, 357, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Safran, H.; Charpentier, K.P.; Perez, K.; Mantripragada, K.; Miner, T.; DiPetrillo, T.; Kuritzky, B.; Apor, E.; Bishop, K.; Luppe, D.; et al. FOLFOX+Nab-Paclitaxel (FOLFOX-A) for Advanced Pancreatic Cancer: A Brown University Oncology Research Group Phase I Study. Ann. J. Clin. Oncol. 2016, 39, 619–622. [Google Scholar]
- Braiteh, F.S.; Patel, M.B.; Parisi, M.; Ni, Q.; Park, S.; Faria, C. Comparative effectiveness and resource utilization of nab-paclitaxel plus gemcitabine vs FOLFIRINOX or gemcitabine for the first-line treatment of metastatic pancreatic adenocarcinoma in a US community setting. Cancer Manag. Res. 2017, 9, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, S.; Voutsadakis, I.A. FOLFIRINOX Chemotherapy in Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis of Retrospective and Phase II Studies. J. Clin. Med. 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Abrams, T.A.; Meyer, G.; Meyerhardt, J.A.; Wolpin, B.M.; Schrag, D.; Fuchs, C.S. Patterns of Chemotherapy Use in a U.S.-Based Cohort of Patients with Metastatic Pancreatic Cancer. Oncologist 2017, 22, 925–933. [Google Scholar] [CrossRef]
| Patient’s Characteristics | Nab-FOLFIRI (Arm A) | Nab-FOLFOX (Arm B) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Male | 15 | 56 | 14 | 39 |
| Female | ||||
| Age, years | ||||
| Median | 62 | 60 | ||
| Range | (38–75) | (42–74) | ||
| ECOG | ||||
| 0 | 19 | 70 | 23 | 64 |
| 1 | 8 | 30 | 13 | 36 |
| Prior adjuvant therapy | ||||
| Yes | 2 | 7 | 4 | 11 |
| No | 25 | 93 | 32 | 89 |
| Prior surgery on primary | ||||
| Yes | 7 | 26 | 9 | 25 |
| No | 20 | 74 | 27 | 75 |
| Tumor localization | ||||
| Head | 14 | 52 | 15 | 42 |
| Neck–body | 13 | 48 | 21 | 58 |
| No. of metastatic sites | ||||
| 1 | 6 | 22 | 17 | 47 |
| 2 | 12 | 44 | 15 | 42 |
| ≥3 | 9 | 34 | 4 | 11 |
| Site of metastatic disease | ||||
| Liver | 20 | 74 | 28 | 78 |
| Lung | 8 | 30 | 9 | 25 |
| Lymph nodes | 18 | 49 | 16 | 45 |
| Peritoneum | 6 | 22 | 8 | 22 |
| Other | 5 | 19 | 0 | 0 |
| Biliary stenting | ||||
| Yes | 5 | 19 | 4 | 11 |
| No | 22 | 81 | 32 | 89 |
| Ca 19.9 baseline | ||||
| Median | 68.3 | 336.5 | ||
| Range | (0.95–41,251) | (0.80–136,505) | ||
| Nab-p (mg/m2) | Level | No. Pts | Nab-FOLFIRI (Arm A) | No. pts | Nab-FOLFOX (Arm B) |
|---|---|---|---|---|---|
| 90 | I | 3 | 3 | ||
| 100 | II | 3 | 3 | ||
| 110 | III | 6 | Liver toxicity G3 (1pt) | 3 | |
| 120 | IV | 6 | MTD | 3 | |
| 130 | V | 3 + 3 ↑↓ | Neutropenia G4, leucopenia G3 (1 pt) anemia G3, neutropenia G4, thrombocytopenia G3, and mucositis G2 (1 pt) | 3 | |
| 140 | VI | 3 | Neutropenia G4, leucopenia G3, and thrombocytopenia G3 (1 pt) Fever G1, asthenia G3, and hospitalization (1 pt) DLT | 6 | Mucositis G3, diarrhea G3, and anorexia G3 (1 pt) |
| 150 | VII | NA | 6 | ||
| 160 | VIII | NA | 6 | Nausea G3 (1 pt) MTD | |
| 170 | IX | NA * | 3 | Sepsis (1 pt) Febrile neutropenia G4 and leucopenia G3 (1 pt) DLT |
| Patient’s Characteristics | Arm A (Nab-FOLFIRI) | Arm B (Nab-FOLFOX) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Male | 26 | 62 | 27 | 64 |
| Female | 16 | 38 | 15 | 36 |
| Age | ||||
| Median | 60 (range 29–75) | 64 (range 47–74) | ||
| ECOG | ||||
| PS 0 | 25 | 60 | 29 | 69 |
| PS 1 | 17 | 40 | 13 | 31 |
| Site of primary | ||||
| Head | 18 | 43 | 19 | 45 |
| Body/tail | 24 | 57 | 23 | 55 |
| Previous surgery | ||||
| Yes | 12 | 29 | 4 | 10 |
| Previous adjuvant treatment | ||||
| Yes | 8 | 19 | 1 | 2 |
| Biliary stenting | 7 | 17 | 6 | 14 |
| M + liver | 33 | 79 | 30 | 71 |
| Ca 19.9 (U/mL) | ||||
| median | 566 (range 3–634,657) | 1101 (range 0–167,350) | ||
| BEST OVERALL RESPONSE | ARM A (Nab-FOLFIRI) | ARM B (Nab-FOLFOX) | ||
|---|---|---|---|---|
| N | % | N | % | |
| CR | 0 | 0 | 0 | 0 |
| PR | 13 | 31 | 13 | 31 |
| SD | 16 | 38 | 17 | 40 |
| PD | 11 | 26 | 7 | 17 |
| Not assessed | 2 | 5 | 5 | 12 |
| CLINICAL BENEFIT RATE | ||||
| CR + PR + SD | 29 | 69 | 30 | 71 |
| Other | 13 | 31 | 12 | 29 |
| Phase II: Most Relevant Grade 3 or 4 Adverse Events and Historical Controls. | ||||||
|---|---|---|---|---|---|---|
| Event | Nab-FOLFIRI n | Nab-FOLFIRI % | Nab-FOLFOX n | Nab-FOLFOX % | FOLFIRINOX Conroy, 2011 n (%) | Nab-FOLFOX Safran, 2015 n (%) |
| Hematologic | ||||||
| Neutropenia | 8 | 19.1 | 12 | 28.6 | 75/164 (45.7) | 6/35 (17.1) |
| Febrile neutropenia | 5 | 11.9 | 4 | 9.5 | 9/166 (5.4) | NR |
| Thrombocytopenia | 1 | 2.4 | 0 | 0 | 15/166 (9.1) | 0/35 (0.0) |
| Anemia | 3 | 7.1 | 4 | 9.5 | 13/166 (7.8) | 2/35 (5.7) |
| Non hematologic | ||||||
| Fatigue | 2 | 4.8 | 6 | 14.3 | 39/165 (23.6) | 9/35(25.7) |
| Vomiting | 0 | 0.0 | 0 | 0.0 | 24/166 (14.5) | 0/35 (0.0) |
| Diarrhea | 1 | 2.4 | 0 | 0 | 21/165 (12.7) | 2/35 (5.7) |
| Sensory neuropathy | 0 | 0 | 3 | 7.1 | 15/166 (9.0) | 2/35 (5.7) |
| Liver toxicity | 2 | 4.8 | 1 | 2.4 | 12/165 (7.3) | 1/35 (2.9) |
| Thromboembolism | 0 | 0.0 | 0 | 0.0 | 11/166 (6.6) | 0/35 (0.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giommoni, E.; Maiello, E.; Vaccaro, V.; Rondini, E.; Vivaldi, C.; Tortora, G.; Toppo, L.; Giordano, G.; Latiano, T.P.; Lamperini, C.; et al. Activity and Safety of NAB-FOLFIRI and NAB-FOLFOX as First-Line Treatment for metastatic Pancreatic Cancer (NabucCO Study). Curr. Oncol. 2021, 28, 1761-1772. https://doi.org/10.3390/curroncol28030164
Giommoni E, Maiello E, Vaccaro V, Rondini E, Vivaldi C, Tortora G, Toppo L, Giordano G, Latiano TP, Lamperini C, et al. Activity and Safety of NAB-FOLFIRI and NAB-FOLFOX as First-Line Treatment for metastatic Pancreatic Cancer (NabucCO Study). Current Oncology. 2021; 28(3):1761-1772. https://doi.org/10.3390/curroncol28030164
Chicago/Turabian StyleGiommoni, Elisa, Evaristo Maiello, Vanja Vaccaro, Ermanno Rondini, Caterina Vivaldi, Giampaolo Tortora, Laura Toppo, Guido Giordano, Tiziana Pia Latiano, Cinzia Lamperini, and et al. 2021. "Activity and Safety of NAB-FOLFIRI and NAB-FOLFOX as First-Line Treatment for metastatic Pancreatic Cancer (NabucCO Study)" Current Oncology 28, no. 3: 1761-1772. https://doi.org/10.3390/curroncol28030164
APA StyleGiommoni, E., Maiello, E., Vaccaro, V., Rondini, E., Vivaldi, C., Tortora, G., Toppo, L., Giordano, G., Latiano, T. P., Lamperini, C., Pillozzi, S., Boni, L., Antonuzzo, L., & Di Costanzo, F. (2021). Activity and Safety of NAB-FOLFIRI and NAB-FOLFOX as First-Line Treatment for metastatic Pancreatic Cancer (NabucCO Study). Current Oncology, 28(3), 1761-1772. https://doi.org/10.3390/curroncol28030164






