Cancer Screening Interventions in Indigenous Populations: A Rapid Review
Abstract
1. Background
1.1. Disparities in Cancer Screening Among Indigenous Populations
1.2. Increasing Cancer Screening in Indigenous Populations
- (1)
- Attitudes and beliefs about cancer;
- (2)
- Health system challenges;
- (3)
- Lack of trusting relationships with health care providers and health organizations;
- (4)
- Lack of knowledge or awareness about cancer and cancer screening;
- (5)
- Barriers associated with demographics and health determinants;
- (6)
- Impacts of colonialism, discrimination, and/or racism.
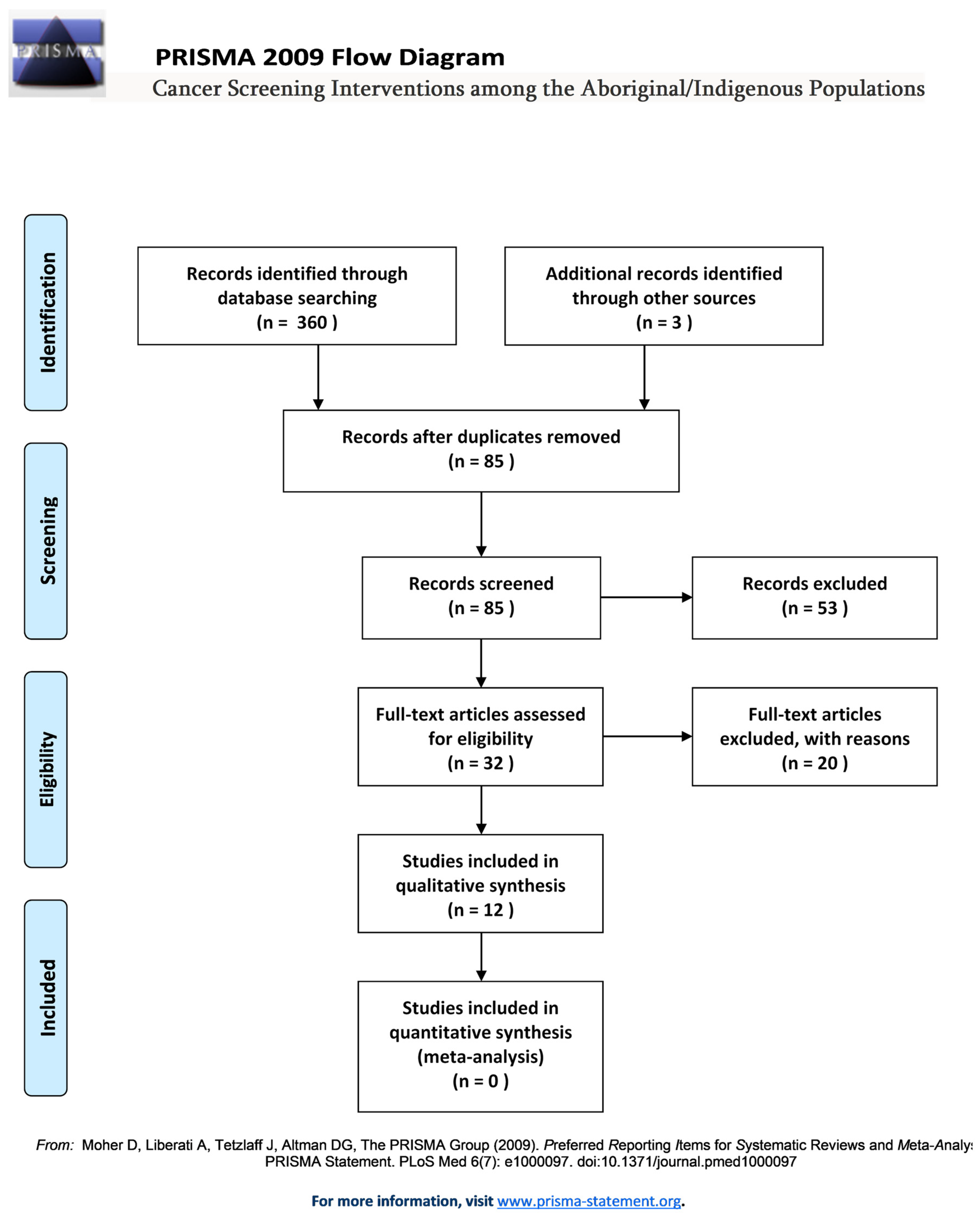
2. Methods
- (1)
- Increased breast, colorectal, or cervical cancer screening participation in Indigenous populations?
- (2)
- Promise for increasing breast, colorectal, or cervical cancer screening in Indigenous populations based on process indicators of the outcome (e.g., knowledge, attitude, or intent to screen)?
2.1. Search Strategy
2.2. Selection Strategy
3. Interventions That Increased Screening Participation
3.1. Randomized Controlled Trials (RCTs)
3.1.1. Text-Message Reminders
3.1.2. Telephone Call Reminders
3.1.3. HPV Self-Sampling
3.1.4. Mailing of FIT Kits
3.2. Pilot Projects
3.2.1. Opportunistic Screening Pilot
3.2.2. Mobile Screening Pilot.
3.3. Translational Research
Plan-Do-Study-Act (PDSA) Cycles.
4. Interventions That Improved Knowledge, Attitude, or Intention to Screen
4.1. Community-Based Participatory Research
4.1.1. Peer-Led Intervention
4.1.2. Multicomponent Intervention
4.2. Preference and Acceptability of HPV Self-Sampling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability
Acknowledgments
Conflicts of Interest
References
- Alberta First Nations Information Goverance Centre and Alberta Health. Top Types of Cancer among First Nations in Alberta: Proportion of Total Cancer Cases by Cancer Type and First Nations Status; 2006–2015; Government of Alberta (AFNIGC): Edmonton, AB, Canada, 2017. [Google Scholar]
- Health Canada. First Nations Health Status Report—Alberta Region; Health Canada: Edmonton, AB, Canada, 2012.
- Ahmed, S.; Shahid, R.K.; Episkenew, J.A. Disparity in cancer prevention and screening in aboriginal populations: Recommendations for action. Curr. Oncol. 2015, 22, 417–426. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hutchinson, P.; Tobin, P.; Muirhead, A.; Robinson, N. Closing the gaps in cancer screening with First Nations, Inuit, and Métis populations: A narrative literature review. J. Indig. Wellbeing 2018, 3, 3–17. [Google Scholar]
- Mema, S.C.; Yang, H.; Elnitsky, S.; Jiang, Z.; Vaska, M.; Xu, L. Enhancing access to cervical and colorectal cancer screening for women in rural and remote northern Alberta: A pilot study. CMAJ Open 2017, 5, E740. [Google Scholar] [CrossRef] [PubMed]
- Aboriginal Affairs and Northern Development Canada. First Nations in Alberta; Aboriginal Affairs and Northern Development Canada: Edmonton, 2014. Available online: https://www.aadnc-aadnc.gc.ca/DAM/DAM-INTER-AB/STAGING/texte-text/fnamarch11_1315587933961_eng.pdf (accessed on 3 May 2021).
- Muller, C.J.; Robinson, R.F.; Smith, J.J.; Jernigan, M.A.; Hiratsuka, V.; Dillard, D.A.; Buchwald, D. Text message reminders increased colorectal cancer screening in a randomized trial with Alaska Native and American Indian people. Cancer 2017, 123, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Sandiford, P.; Buckley, A.; Holdsworth, D.; Tozer, G.; Scott, N. Reducing ethnic inequalities in bowel screening participation in New Zealand: A randomised controlled trial of telephone follow-up for non-respondents. J. Med. Screen. 2019, 26, 139–146. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, E.J.; Geller, S.; Sibanda, N.; Stevenson, K.; Denmead, L.; Adcock, A.; Cram, F.; Hibma, M.; Sykes, P.; Lawton, B. Reaching under-screened/never-screened indigenous peoples with human papilloma virus self-testing: A community-based cluster randomised controlled trial. Aust. N. Z. J. Obstet. Gynaecol. 2020, 61, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, D.; English, K.; Jacobs-Wingo, J.; Tjemsland, A.; Espey, D. Peer Reviewed: Effectiveness of Interventions to Increase Colorectal Cancer Screening Among American Indians and Alaska Natives. Prev. Chronic Dis. 2020, 17, E62. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.; Bale, S.; Sky, F.; Wesley, S.; Beach, L.; Hyett, S.; Heiskanen, T.; Gillis, K.-J.; Harris, C.P. The Wequedong Lodge Cancer Screening Program: Implementation of an opportunistic cancer screening pilot program for residents of rural and remote Indigenous communities in Northwestern Ontario, Canada. Rural Remote Health 2020, 20, 246. [Google Scholar] [CrossRef] [PubMed]
- Dorrington, M.S.; Herceg, A.; Douglas, K.; Tongs, J.; Bookallil, M. Increasing Pap smear rates at an urban Aboriginal Community Controlled Health Service through translational research and continuous quality improvement. Aust. J. Prim. Health 2015, 21, 417–422. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services Office of Minority Health. Profile: American Indian/Alaska Native; U.S. Department of Health and Human Services Office of Minority Health: Rockville, MD, USA, 2021. Available online: https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=62 (accessed on 3 May 2021).
- Adcock, A.; Cram, F.; Lawton, B.; Geller, S.; Hibma, M.; Sykes, P.; MacDonald, E.J.; Dallas-Katoa, W.; Rendle, B.; Cornell, T. Acceptability of self-taken vaginal HPV sample for cervical screening among an under-screened Indigenous population. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Cassel, K.D.; Hughes, C.; Higuchi, P.; Lee, P.; Fagan, P.; Lono, J.; Ho, R.; Wong, N.; Brady, S.K.; Ahuna, W. No Ke Ola Pono o Nā Kāne: A Culturally Grounded Approach to Promote Health Improvement in Native Hawaiian Men. Am. J. Men Health 2020, 14, 1557988319893886. [Google Scholar] [CrossRef] [PubMed]
- Tolma, E.; Thomas, C.; Neely, N.; Chery, E.; Edwards, T.; Canfield, V. The development of a culturally sensitive brochure on breast health for American Indian women. Eur. J. Public Health 2018, 28, cky213.477. [Google Scholar] [CrossRef]
- Zehbe, I.; Jackson, R.; Wood, B.; Weaver, B.; Escott, N.; Severini, A.; Krajden, M.; Bishop, L.; Morrisseau, K.; Ogilvie, G. Community-randomised controlled trial embedded in the Anishinaabek Cervical Cancer Screening Study: Human papillomavirus self-sampling versus Papanicolaou cytology. BMJ Open 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Winer, R.L.; Gonzales, A.A.; Noonan, C.J.; Cherne, S.L.; Buchwald, D.S. Assessing acceptability of self-sampling kits, prevalence, and risk factors for human papillomavirus infection in American Indian Women. J. Community Health 2016, 41, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Zehbe, I.; Wakewich, P.; King, A.-D.; Morrisseau, K.; Tuck, C. Self-administered versus provider-directed sampling in the Anishinaabek cervical Cancer screening study (ACCSS): A qualitative investigation with Canadian first nations women. BMJ Open 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Brewer, N.; Foliaki, S.; Bromhead, C.; Viliamu-Amusia, I.; Pelefoti-Gibson, L.; Jones, T.; Pearce, N.; Potter, J.D.; Douwes, J. Acceptability of human papillomavirus self-sampling for cervical-cancer screening in under-screened Māori and Pasifika women: A pilot study. N. Z. Med. J. 2019, 132, 21–31. [Google Scholar] [PubMed]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Palmer, C.; Bik, E.M.; Cardenas, J.P.; Nuñez, H.; Kraal, L.; Bird, S.W.; Bowers, J.; Smith, A.; Walton, N.A. Self-sampling for human papillomavirus testing: Increased cervical cancer screening participation and incorporation in international screening programs. Front. Public Health 2018, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
| Search Terms | |
|---|---|
| Databases searched | Native Health Database, MEDLINE (Ovid), Cochrane Library, PsycINFO, PubMed, PubMed Central, CINAHL, MEDLINE (Ebsco), Psychology & Behavioral Sciences Collection, and HealthSTAR |
| Population-specific terms/phrases used | Aboriginal, Indigenous, Inuit, First Nations, Métis, native people, native Canadian, Māori, and Native American |
| Disease-specific terms/phrases used | Breast cancer screening, cervical cancer screening, colorectal cancer screening, early detection of cancer, mammogram, mammography, pap, pap smear, fecal immunochemical test, faecal immunochemical test, fecal occult blood test, faecal occult blood test, breast, cervix, colon, rectum, cancer, carcinoma, neoplasm, tumour, oncology, and mass screening |
| Articles | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population |
|
|
| Intervention |
|
|
| Outcome |
|
|
| Other |
|
|
| Citation | Cancer Screening Type | Setting | Sample | Study Design & Intervention | Outcome: Screening Participation |
|---|---|---|---|---|---|
| Muller et al., 2017 [7] | Colorectal cancer | Anchorage, Alaska | 2386 Alaskan Native and Native American men and women, aged 40 to 75 years | RCT: Addition of text message reminders to existing electronic reminders | Age groups:
|
| Sandiford et al., 2019 [8] | Colorectal cancer | New Zealand | 7601 Māori, Pacific, and Asian men and women, aged 50 to 74 years | RCT: Addition of a telephone call to existing letter reminders | Ethnic groups:
|
| MacDonald et al., 2021 [9] | Cervical Cancer | Northland, New Zealand | 931 Māori women, aged 25–69 years | RCT: Addition of HPV self-test |
|
| Haverkamp et al., 2020 [10] | Colorectal cancer | Southwest United States | 1288 Alaskan Natives and American Indians | RCT: Addition of mailed FIT kits or mailed FIT kits plus follow-up outreach by phone/home visit |
|
| Chow et al., 2020 [11] | Breast, cervical, colorectal cancer | Wequedong Lodge in Ontario | First Nations men and women, aged 50–74 years (breast and colorectal) and 21–69 years (cervical) | Pilot study: Education and opportunistic cancer screening | Year:
|
| Mema et al., 2017 [5] | Cervical and colorectal cancer | Northern Alberta | First Nations, Métis and Hutterite women, aged 50 to 74 years | Pilot study: Integration of cervical and colorectal screening with the Screen Test mobile mammography program | Cancer type: Total screened: Usual Practice (Screen-Test mobile mammography)
|
| Dorrington et al., 2015 [12] | Cervical cancer | Australia | Aboriginal and Torres Strait Islander women, aged 18 to 70 years | PDSA Cycles: translational research and continuous quality improvement | Year: 2012: 40% * increase |
| Citation | Cancer Type | Setting | Sample | Study Design & Intervention | Outcome |
|---|---|---|---|---|---|
| Cassel et al., 2020 [15] | Colorectal | Hawaii, USA | 378 Native Hawaiian men, aged 18+, with focus on ages 50+ for use of FIT | Peer-led model: group discussions and educational sessions. | 92% improved their knowledge about colon health and 76% agreed to complete a FIT. |
| Tolma et al., 2018 [16] | Breast | Oklahoma City, USA | 21 American Indian/ Alaska Native women, aged 52–74 years | Formative evaluation: Multicomponent (clinic and community components) | 30% improved their intention to do a mammogram. 52% had a mammogram by six months post-intervention. |
| Adcock et al., 2019 [14] | Cervical | New Zealand | 503 Māori women, aged 25+ years and 17 healthcare providers | Mixed qualitative and quantitative: Focus groups/interviews, survey | 75% of Māori survey participants reported being likely/very likely to do an HPV self-test |
| Zehbe et al., 2016 [17] | Cervical | NW Ontario, Canada | 834 First Nations Women, aged 25–69 years | Community RCT: HPV self-sampling (Arm A) and Pap testing (Arm B) | Initial uptake in Arm A was 1.4-fold higher than arm B Range of uptake: Arm A: 0.0% to 62.1% Arm B: 0.0% to 47.1%. |
| Winer et al., 2016 [18] | Cervical | NE Arizona, USA | 329 Hopi women, aged 21–65 years | Cross-sectional: Recruitment within community to complete HPV self-sampling | 62% reported a preference for self-sampling |
| Citation | Intervention Development | Community Needs and Preferences | Community Engagement | Workforce Preparation | Communication Methods |
|---|---|---|---|---|---|
| Muller et al., 2017 [7] | Developed in coordination with SCF, a tribally owned and operated health care organization. | Previous survey findings showed the majority of customer-owners over 50 used text messaging. | Text message content was developed with input from SCF customer-owners and tribal leadership. | The intervention was integrated into an existing SCF program. | The intervention group received up to 3 text messages sent 1 month apart. |
| Sandiford et al., 2019 [8] | Follow-up to an existing Bowel Screening Pilot using mailed invitation and reminder letters. | Patient and cultural barriers | During telephone calls, community coordinators sought to remove any barriers to screening, such as how to perform the test. | The callers’ script was reviewed by health literacy experts. | All non-respondents were sent reminder letters. The intervention group also received 3+ phone calls over 4 weeks. Community coordinators spoke with participants in their native languages. |
| MacDonald et al., 2021 [9] | Follow-up to a survey showing high acceptability for HPV self-testing among Māori women. | Patient and cultural barriers | The study was under-taken in partnership with primary care and the Northland District Health. | Clinic staff were given an educational update on HPV, informed consent, and the HPV self-test. | Text, email, letter, and phone calls from clinics and outreach by kaiāwhina. |
| Haverkamp et al., 2020 [10] | Developed in partnership with 3 tribally operated health facilities that participated in study. | Patient and structural barriers † | American Indian CHRs contacted intervention nonrespondents to discuss the importance of CRC screening and how to use the FIT kit. | Clinic admin and staff were informed about the study and CHRs were educated about screening recommendations and intervention protocol. | FIT kits were mailed to intervention groups and CHRs provided outreach (i.e., phone calls and home visits). |
| Chow et al., 2020 [11] | Developed in partnership with the Wequedong Lodge, TBRHSC, the Nishnawbe Aski Nation Chiefs Assembly, and CCO’s. Indigenous Cancer Care Unit. | Geographic, transportation, and cultural barriers | Cancer screening education and opportunistic screening was provided for those staying at the lodge (mostly from rural FN populations). | Community chiefs and physicians were notified about the program and given information about program logistics and patient follow-up. | A FN liaison spoke with clients in their native language. A FN-specific education toolkit was used during appointments. |
| Mema et al., 2017 [5] | Provision of ‘one stop shop’ cancer screening services in many communities, including FN. | Geographical barriers—communities were chosen based on their need for cancer screening services using a readiness assessment tool. | Leverage existing relationships with mobile mamography service. | Local clinical staff provided Pap and FIT tests. | Recall letters were sent to all clients who had participated in Screen Test in the past and were due for breast cancer screening. |
| Dorrington et al., 2015 [12] | Interventions were designed based on PDSA cycles and tested for cultural acceptability with the ACCHS Women’s Group. | Patient barriers | Client surveys and focus groups with stakeholders | The Social Health Team was educated on women’s preventative health and cervical cancer screening to faciliate discussions with ACCHS clients. HCPs were educated on how to use a data collection tool for Pap smears. | Promotional material was used to raise awareness of cervical screening. A reminder letter was updated to include culturally appropriate cervical cancer screening information. |
| Cassel et al., 2020 [15] | A peer-led intervention facilitated by kāne and Native Hawaiian physicians. | CRC health dispartities among Native Hawaiian men | Discussions about CRC were held at community-based venues and participants were given a FIT kit. | Education materials and curricula were developed by Native Hawaiian physicans and modified based on community feedback. | 21 community sessions on CRC screening. |
| Tolma et al., (2018) [16] | Formative evaluation to determine the feasibility and early impact of a CBPR intervention. | Geographical disparties | Clinic and community-based components on multiple system levels. | Evaluation planning based on years of formative research in the community. | Communication with HCP, discussion groups, and a congratulatory gift. |
| Adcock et al., 2019 [11] | This study explored the potential acceptability of an intervention. | Desire for bodily autonomy (privacy, control over ones body) | Focus groups, interviews, and surveys with never/underscreened Māori women. | Not addressed | CBRs recruited Māori women for interviews and focus groups. Participants surveyed up to 10 Māori female peers. |
| Zehbe et al., 2016 [17] | Designed with 11 FN partner communities using a PAR framework. | Geographic and cultural barriers | Interviews and focus groups with HCPs and women living on reserves about CC screening barriers. | CBRAs invited women to participate after an educational event and other recruitment strategies. | CBRAs facilitated screening implementation and data collection. Participants were asked how they wanted to be contact if they had a positive HPV test result. |
| Winer et al., 2016 [18] | Designed with input from Hopi tribal partners, local project staff, and community advisors. | Patient barriers | In-person community recruitment events | Not addressed | Recruitment flyers and informational brochures were given at community events, door-to-door health education campaigns, and tribal radio announcements. HPV test results were communicated by letter or telephone, based on preference. |
| Studies | Citations | Study Design | Cancer Screening Types | Sample | Outcomes |
|---|---|---|---|---|---|
| Seven studies reported an increase in cancer screening participation | Muller, 2017 [7] | RCT | CRC | Alaskan Native; Native American | Age group: 40–49: 24% increase 50–75: 42% increase All ages: 30% * increase |
| Sandiford, 2019 [8] | Māori, Pacific | Ethnic group: Māori: 5.2% * increase Pacific: 3.6% * increase Asian: 0.7% increase | |||
| MacDonald et al., 2021 [9] | CC | Māori | Standard care: 21.8% screened HPV Self-sampling: 59.0% screened (2.8 * times higher) | ||
| Haverkamp et al., 2020 [10] | CRC | Alaskan Native/American Indian | Standard care: 6.4% screened Mailed FIT kit: 16.9% * screened Mailed FIT kit + outreach: 18.8% * screened | ||
| Chow, 2020 [11] | Pilot | CRC, CC, BC | First Nations | Year: 2014–2015: 62% increase 2015–2016: 68% increase | |
| Mema, 2017 [5] | CC, BC | First Nations, Métis, Hutterite | Total screened: Usual Practice (Screen-Test mobile mammography)
| ||
| Dorrington, 2015 [12] | PDSA cycles | CC | Torres Strait Islander | Year: 2012: 40% * increase | |
| Five studies improved knowledge, attitude, or intent to screen | Cassel, 2020 [15] | Peer-led | CC | Native Hawaiian | 92% improved their knowledge 76% agreed to complete a FIT |
| Tolma, 2018 [16] | Multi-level | BC | Native American | 30% improved their intent to screen 52% had a mammogram by 6 months post-intervention. | |
| Zehbe, 2016 [17] | RCT | CC (HPV self-sampling) | First Nations | Initial uptake in HPV self-sampling was 1.4-fold higher than clinician-sampling | |
| Adcock, 2019 [14] | Mixed | Māori | 75% reported being likely/very likely to do an HPV self-test | ||
| Winer, 2016 [18] | Cross-sectional | Hopi | 62% reported a preference for HPV self-sampling |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryant, J.; Patterson, K.; Vaska, M.; Chiang, B.; Letendre, A.; Bill, L.; Yang, H.; Kopciuk, K. Cancer Screening Interventions in Indigenous Populations: A Rapid Review. Curr. Oncol. 2021, 28, 1728-1743. https://doi.org/10.3390/curroncol28030161
Bryant J, Patterson K, Vaska M, Chiang B, Letendre A, Bill L, Yang H, Kopciuk K. Cancer Screening Interventions in Indigenous Populations: A Rapid Review. Current Oncology. 2021; 28(3):1728-1743. https://doi.org/10.3390/curroncol28030161
Chicago/Turabian StyleBryant, Janell, Kara Patterson, Marcus Vaska, Bonnie Chiang, Angeline Letendre, Lea Bill, Huiming Yang, and Karen Kopciuk. 2021. "Cancer Screening Interventions in Indigenous Populations: A Rapid Review" Current Oncology 28, no. 3: 1728-1743. https://doi.org/10.3390/curroncol28030161
APA StyleBryant, J., Patterson, K., Vaska, M., Chiang, B., Letendre, A., Bill, L., Yang, H., & Kopciuk, K. (2021). Cancer Screening Interventions in Indigenous Populations: A Rapid Review. Current Oncology, 28(3), 1728-1743. https://doi.org/10.3390/curroncol28030161







