Patient and Patient Group Engagement in Cancer Clinical Trials: A Stakeholder Charter
Abstract
1. Introduction
2. Materials and Methods
2.1. Step 1: Drafting the Charter
2.2. Step 2: Socializing the Charter
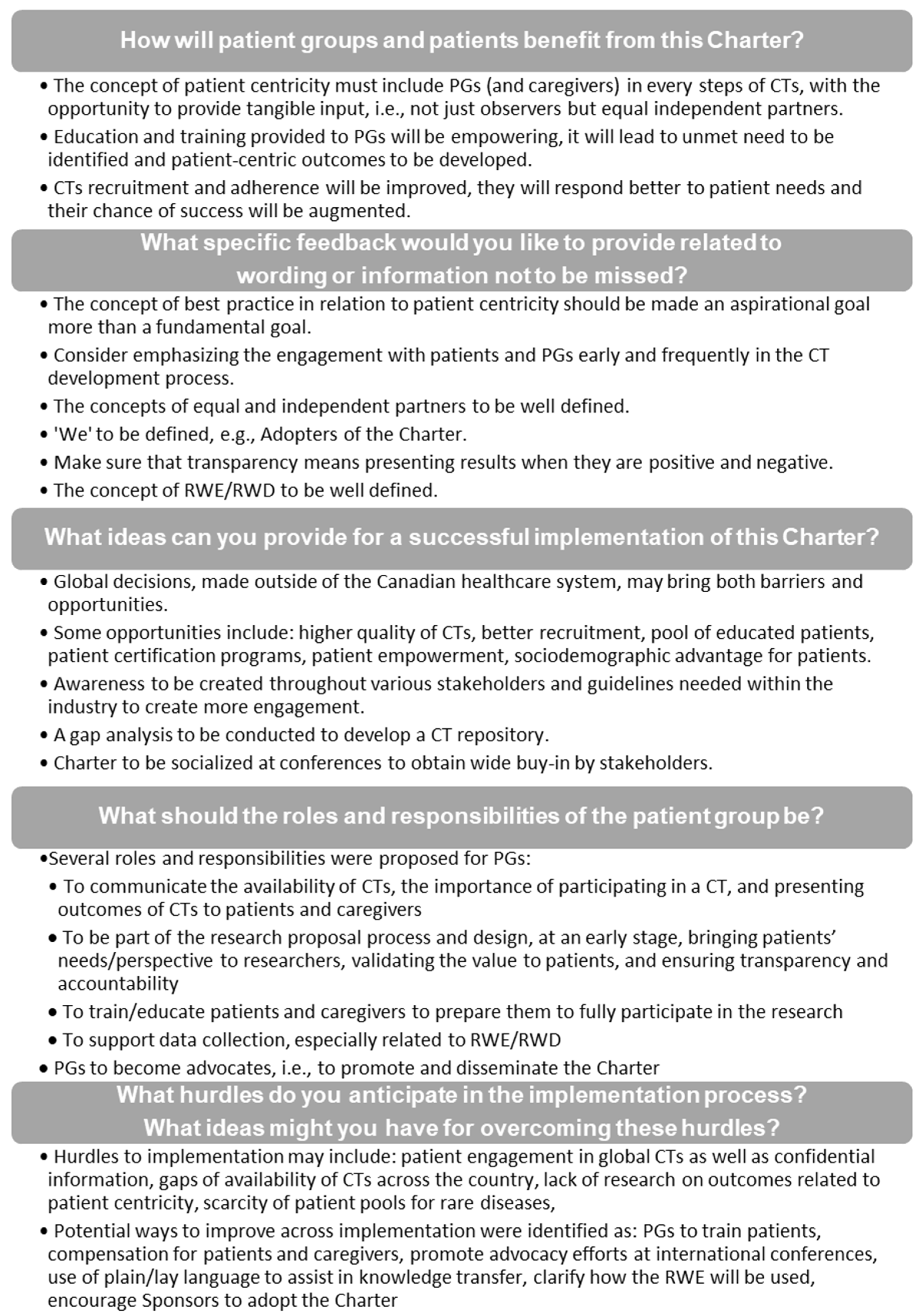
- How will PGs and patients benefit from this Charter?
- What specific feedback would you like to provide related to wording or information not to be missed?
- What ideas can you provide for successful implementation of this Charter?
- What should the roles and responsibilities of the PG be? and
- What hurdles do you anticipate in the implementation process? What ideas might you have for overcoming these hurdles?
2.3. Step 3: Finalizing the Charter
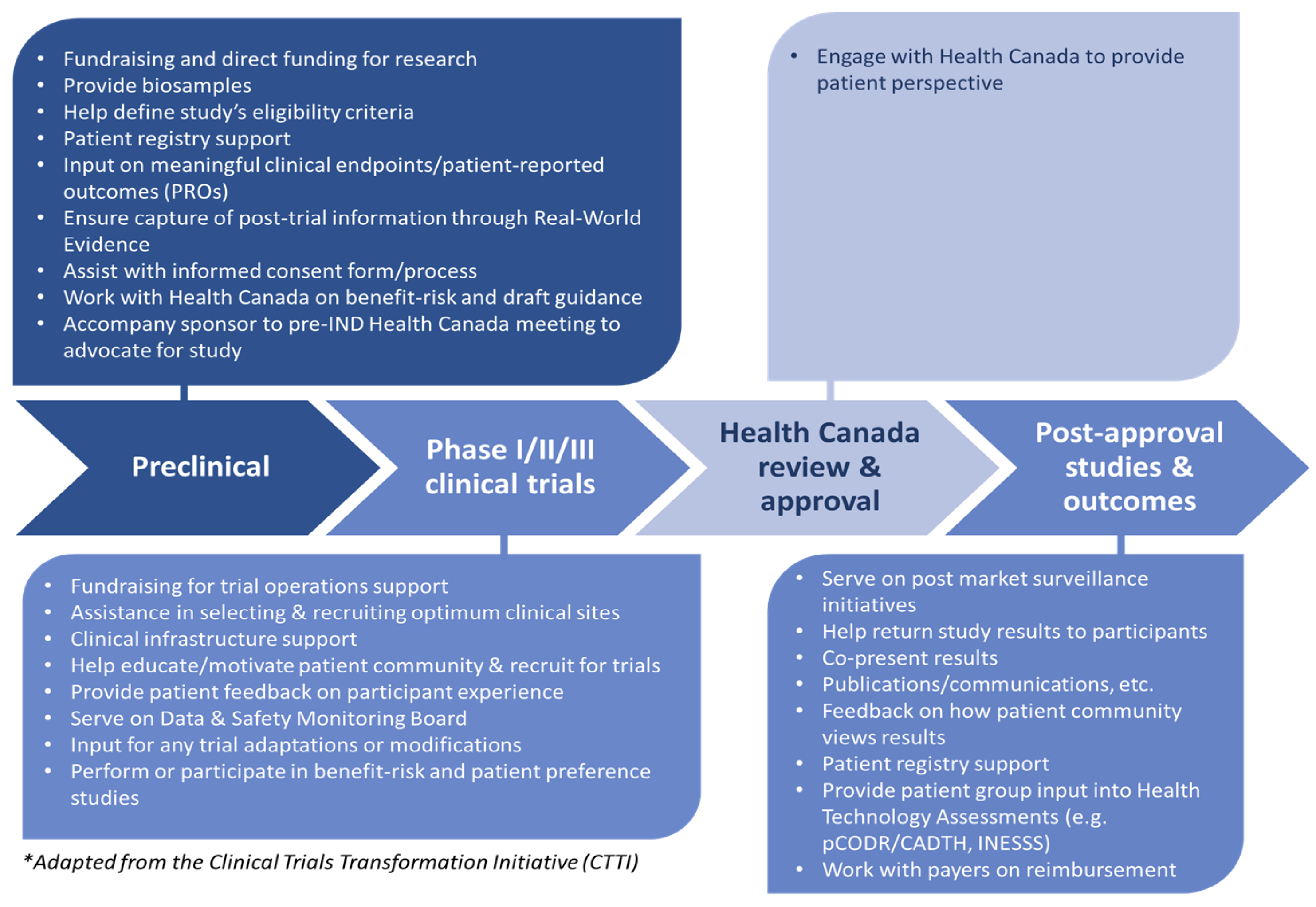
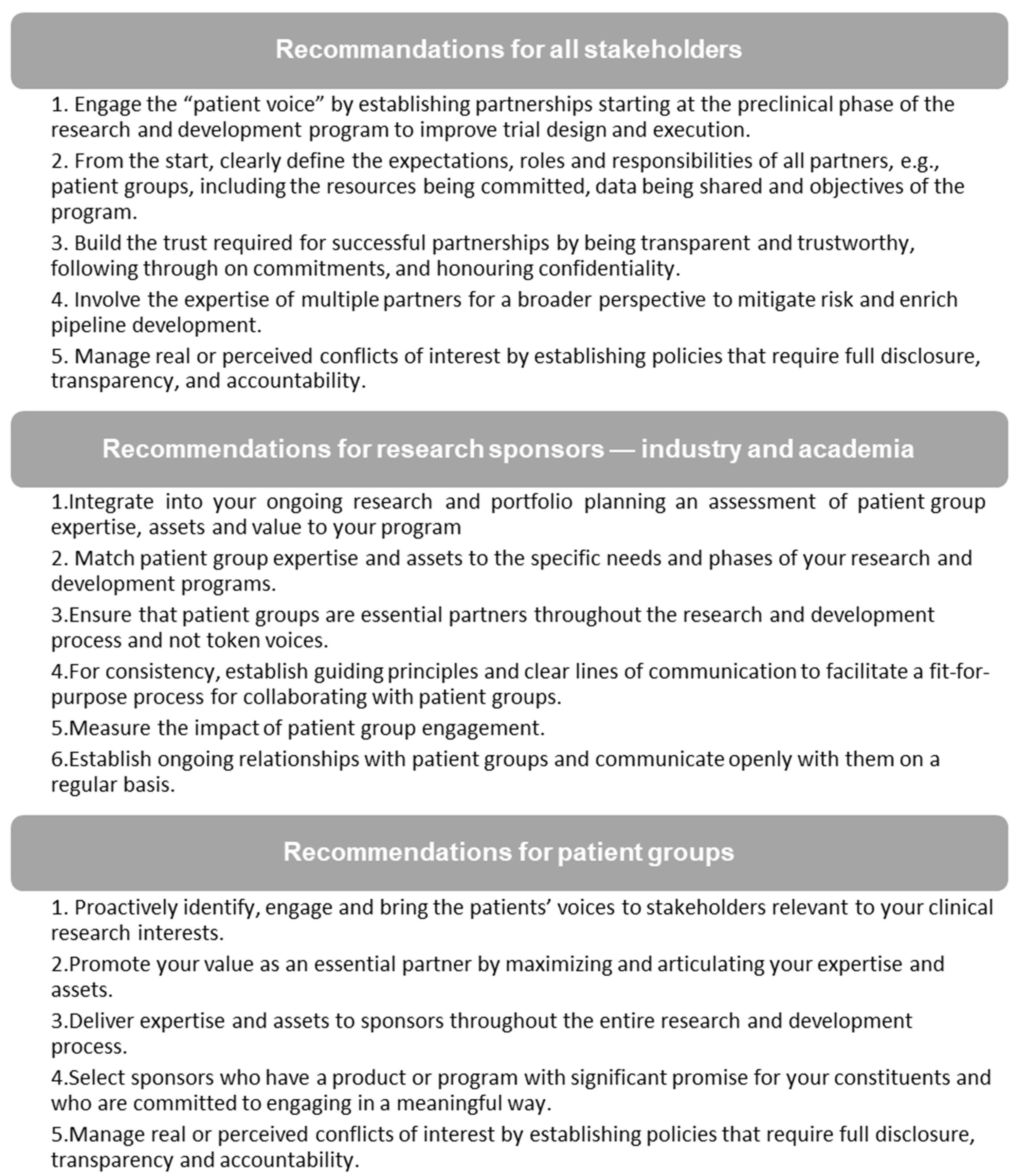
3. Results
3.1. Consolidated Feedback from Stakeholders
3.2. The Canadian Cancer Trials Stakeholder Charter
3.2.1. Tenet 1: Making Patient Centricity a Norm in Clinical Trials
- Ensure that studies are designed to realize outcomes that are relevant to patients and includes their preferences and trade-offs, achieve clinically meaningful results and enhance patient quality of life and health outcomes, while minimizing the burden of disease and treatment on patients;
- Increase access to CTs by reducing barriers and ensuring that eligibility criteria are fair and appropriate;
- Simplify the informed consent document to provide transparent, comprehensible CT information in a language that is relevant for patients;
- Engage patients in a two-way communication throughout the CT continuum (CTC), solicit and incorporate their feedback, and provide access to mechanisms, such as digital and mobile health technologies where possible;
- Connect patients to internal and external support programs and other resources through convenient and user-friendly channels; and
- Provide patients with uninterrupted access to CT therapies.
3.2.2. Tenet 2: Supporting Education, Training and Development of Patient Group Members for Effective Participation in the Design and Implementation of Clinical Trial Protocols on Behalf of Patients
- Support training of PGs and evaluation initiatives while working collaboratively with them, encouraging their input and active participation in the development, implementation and reporting of a CT;
- Support PGs’ ability to record patient values and preferences both during and post CT;
- Support training of other research Stakeholders including the Sponsor representatives, to ensure best practices are met in their engagement with PGs; and
- Evaluate and share the impact of our engagement with PGs.
3.2.3. Tenet 3: Collaborating with Patient Groups as Equal and Independent Partners to Optimize the Success of Clinical Trials
- Build strong partnerships with PGs and all Stakeholders by agreeing on joint expectations, responsibilities, and the commitment to promote co-operation;
- Include patient insight in the development of the consent process and patient-facing materials;
- Facilitate the connection between trial participants and PGs;
- Promote awareness and education of CTs among PGs, while integrating their involvement in the design and implementation of CTs;
- Work with PGs to integrate patient needs from the conception of CTs, to expedite and facilitate access to CT information, patient-facing materials and CT consent; and
- Act with integrity and respect the independence of PGs.
3.2.4. Tenet 4: Adhering to Transparency and Accountability throughout the Clinical Trial Continuum
- Bolster trust through open dialogue and interaction with PGs and seeking their input throughout to ensure CT lifecycle and following the CT, as required;
- Work collaboratively with PGs to better understand and address patient unmet needs, preferences and trade-offs, the burden of current treatments and disease;
- Share information with PGs and patients in a neutral, uninfluencing and objective manner, where data is presented clearly and accurately as well as in a balanced and fair context, to allow PGs to form their own independent opinion and interpretation;
- Hold ourselves to highest levels of accountability by ensuring that the independence of all stakeholders involved is maintained and by implementing clear conflict of interest and disclosure guidelines;
- Develop trust and confidence in the methods used;
- Transparently share the aggregate results of CTs with patients and PGs, regardless of the trial outcome, in a timely, efficient and comprehensible manner; and
- Transparently share the individual results with the patient and/or patient guardian in the case of a pediatric study, regardless of the outcome, in a timely, efficient and comprehensible manner.
3.2.5. Tenet 5: Maximizing the Potential to Collect and Utilize RWE/RWD Captured in All Clinical Trials
- Consider RWE/RWD in the collection of data in order to render the results more generalizable to achieve greater external validity, better support access, appropriateness of use and affordability of the therapeutic interventions being tested in CTs;
- Communicate (in lay language for good comprehension by PGs and patients), the research goals, methods, procedures, RWE/RWD collected as well as the findings resulting from the use and analysis of this data;
- Ensure that the RWE/RWD data is complete, reliable, and processed in a consistent manner. Best practices in data collection and analysis should be applied from the initiation of the trial study design and maintained throughout the CTC; and
- Share RWE/RWD data post CT in a timely manner to ensure the greatest impact of patients/caregivers to help with decision making.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Slattery, P.; Saeri, A.; Bragge, P. Research co-design in health: A rapid overview of reviews. Health Res. Policy Syst. 2020, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.; Bjørnnes, A.; Toupin-April, K.; Najam, A.; Wells, D.; Sivakumar, A.; Richards, D.; Ceroni, T.; Park, M.; Ellis, A.; et al. Patient Engagement Partnerships in Clinical Trials: Development of Patient Partner and Investigator Decision Aids. Patient 2020, 13, 745–756. [Google Scholar] [CrossRef]
- Frisch, N.; Atherton, P.; Doyle-Waters, M.; MacLeod, M.; Mallidou, A.; Sheane, V.; Ward, J.; Woodley, J. Patient-oriented research competencies in health (PORCH) for researchers, patients, healthcare providers, and decision-makers: Results of a scoping review. Res. Involv. Engagem. 2020, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Fergusson, D.; Hawrysh, T.; Presseau, J.; Kekre, N.; Schwartz, S.; Castillo, G.; Asad, S.; Fox, G.; Atkins, H.; et al. Partnering with patients to get better outcomes with chimeric antigen receptor T-cell therapy: Towards engagement of patients in early phase trials. Res. Involv. Engagem. 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Heckert, A.; Forsythe, L.; Carman, K.; Frank, L.; Hemphill, R.; Elstad, E.; Esmail, L.; Lesch, J. Researchers, patients, and other stakeholders’ perspectives on challenges to and strategies for engagement. Res. Involv. Engagem. 2020, 6, 60. [Google Scholar] [CrossRef]
- Skovlund, P.; Nielsen, B.; Thaysen, H.; Schmidt, H.; Finset, A.; Hansen, K.; Lomborg, K. The impact of patient involvement in research: A case study of the planning, conduct and dissemination of a clinical, controlled trial. Res. Involv. Engagem. 2020, 6, 43. [Google Scholar] [CrossRef]
- Arnstein, L.; Wadsworth, A.; Yamamoto, B.; Stephens, R.; Sehmi, K.; Jones, R.; Sargent, A.; Gegeny, T.; Woolley, K. Patient involvement in preparing health research peer-reviewed publications or results summaries: A systematic review and evidence-based recommendations. Res. Involv. Engagem. 2020, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Vat, L.; Warren, M.; Goold, S.; Davidge, E.; Porter, N.; Schuitmaker-Warnaar, T.; Broerse, J.; Etchegary, H. Giving patients a voice: A participatory evaluation of patient engagement in Newfoundland and Labrador Health Research. Res. Involv. Engagem. 2020, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Hovén, E.; Eriksson, L.; Månsson D’Souza, Å.; Sörensen, J.; Hill, D.; Viklund, C.; Wettergren, L.; Lampic, C. What makes it work? Exploring experiences of patient research partners and researchers involved in a long-term co-creative research collaboration. Res. Involv. Engagem. 2020, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Poger, J.; Mayer, V.; Duru, O.; Nauman, B.; Holderness, H.; Warren, N.; Vasquez, C.; Bibi, S.; Rasmussen-Torvik, L.; Hosseinian, Z.; et al. Network Engagement in Action: Stakeholder Engagement Activities to Enhance Patient-centeredness of Research. Med. Care 2020, 58, S66–S74. [Google Scholar] [CrossRef] [PubMed]
- Barger, S.; Sullivan, S.; Bell-Brown, A.; Bott, B.; Ciccarella, A.; Golenski, J.; Gorman, M.; Johnson, J.; Kreizenbeck, K.; Kurttila, F.; et al. Effective stakeholder engagement: Design and implementation of a clinical trial (SWOG S1415CD) to improve cancer care. BMC Med. Res. Methodol. 2019, 19, 119. [Google Scholar] [CrossRef]
- Michaels, D.; Lamberti, M.; Peña, Y.; Kunz, B.; Getz, K. Assessing Biopharmaceutical Company Experience with Patient-centric Initiatives. Clin. Ther. 2019, 41, 1427–1438. [Google Scholar] [CrossRef]
- Stephens, K.; Osterhage, K.; Fiore-Gartland, B.; Lovins, T.; Keppel, G.; Kim, K. Examining the Needs of Patient Stakeholders as Research Partners in Health Data Networks for Translational Research. AMIA Jt. Summits Transl. Sci. Proc. 2019, 2019, 363–369. [Google Scholar]
- Smith, A.; Chisolm, S.; Deal, A.; Spangler, A.; Quale, D.; Bangs, R.; Jones, J.; Gore, J. Patient-centered prioritization of bladder cancer research. Cancer 2018, 124, 3136–3144. [Google Scholar] [CrossRef]
- Fergusson, D.; Monfaredi, Z.; Pussegoda, K.; Garritty, C.; Lyddiatt, A.; Shea, B.; Duffett, L.; Ghannad, M.; Montroy, J.; Murad, M.; et al. The prevalence of patient engagement in published trials: A systematic review. Res. Involv. Engagem. 2018, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, B.; Harrison, M.; Genao, I.; Greene, A.; Pappas, M.; Glover, J.; Rosenthal, M. Patient, Family, and Community Advisory Councils in Health Care and Research: A Systematic Review. J. Gen. Intern. Med. 2018, 34, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Boaz, A.; Hanney, S.; Borst, R.; O’Shea, A.; Kok, M. How to engage stakeholders in research: Design principles to support improvement. Health Res. Policy Syst. 2018, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Patrick-Lake, B. Patient engagement in clinical trials: The Clinical Trials Transformation Initiative’s leadership from theory to practical implementation. Clin. Trials 2018, 15, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Archambault, P.; McGavin, C.; Dainty, K.; McLeod, S.; Vaillancourt, C.; Lee, J.; Perry, J.; Gauvin, F.; Boivin, A. Recommendations for patient engagement in patient-oriented emergency medicine research. Can. J. Emerg. Med. 2018, 20, 435–442. [Google Scholar] [CrossRef]
- Haynes, S.; Rudov, L.; Nauman, E.; Hendryx, L.; Angove, R.; Carton, T. Engaging Stakeholders to Develop a Patient-centered Research Agenda: Lessons Learned From the Research Action for Health Network (REACHnet). Med. Care 2018, 56, S27. [Google Scholar] [CrossRef]
- Warren, N.; Gaudino, J.J.; Likumahuwa-Ackman, S.; Dickerson, K.; Robbins, L.; Norman, K.; Lind, J.; D’Amato, S.; Foley, P.; Gold, R.; et al. Building Meaningful Patient Engagement in Research: Case Study From ADVANCE Clinical Data Research Network. Med. Care 2018, 56, S58–S63. [Google Scholar] [CrossRef]
- Concannon, T.; Grant, S.; Welch, V.; Petkovic, J.; Selby, J.; Crowe, S.; Synnot, A.; Greer-Smith, R.; Mayo-Wilson, E.; Tambor, E.; et al. Practical Guidance for Involving Stakeholders in Health Research. J. Gen. Intern. Med. 2019, 34, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Khodyakov, D.; Marie, K.; Taras, H.; Meeker, D.; Campos, H.; Ohno-Machado, L. Novel Stakeholder Engagement Approach for Patient-centered Outcomes Research. Med. Care 2018, 56, S41–S47. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, D.; Sake, J.; Halling, K.; Morgan, J.; Georgieva, A.; Bertelsen, N. Patient Centricity and Pharmaceutical Companies: Is It Feasible? Ther. Innov. Regul. Sci. 2017, 51, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, G.; Furlong, P.; Seres, M.; Binder, H.; Chung, H.; Garzya, V.; Jones, R. Defining patient centricity with patients for patients and caregivers: A collaborative endeavour. BMJ Innov. 2017, 3, 76–83. [Google Scholar] [CrossRef]
- Lamberti, M.; Awatin, J. Mapping the Landscape of Patient-centric Activities within Clinical Research. Clin. Ther. 2017, 39, 2196–2202. [Google Scholar] [CrossRef]
- Stegemann, S.; Ternik, R.; Onder, G.; Khan, M.; van Riet-Nales, D. Defining Patient Centric Pharmaceutical Drug Product Design. AAPS J. 2016, 18, 1047–1055. [Google Scholar] [CrossRef]
- Petersen, C.; Austin, R.; Backonja, U.; Campos, H.; Chung, A.; Hekler, E.; Hsueh, P.; Kim, K.; Pho, A.; Salmi, L.; et al. Citizen science to further precision medicine: From vision to implementation. JAMIA Open 2020, 3, 2–8. [Google Scholar] [CrossRef]
- Forsythe, L.; Heckert, A.; Margolis, M.; Schrandt, S.; Frank, L. Methods and impact of engagement in research, from theory to practice and back again: Early findings from the Patient-Centered Outcomes Research Institute. Qual. Life Res. 2018, 27, 17–31. [Google Scholar] [CrossRef]
- Manafo, E.; Petermann, L.; Mason-Lai, P.; Vandall-Walker, V. Patient engagement in Canada: A scoping review of the ‘how’ and ‘what’ of patient engagement in health research. Health Res. Policy Syst. 2018, 16, 5. [Google Scholar] [CrossRef]
- Hansen, M.; Nørgaard, L.; Hallgreen, C. How and Why to Involve Patients in Drug Development: Perspectives From the Pharmaceutical Industry, Regulatory Authorities, and Patient Organizations. Ther. Innov. Regul. Sci. 2019, 7, 577–585. [Google Scholar] [CrossRef]
- Vat, L.; Finlay, T.; Jan Schuitmaker-Warnaar, T.; Fahy, N.; Robinson, P.; Boudes, M.; Diaz, A.; Ferrer, E.; Hivert, V.; Purman, G.; et al. Evaluating the “return on patient engagement initiatives” in medicines research and development: A literature review. Health Expect. 2019, 23, 5–18. [Google Scholar] [CrossRef]
- Domecq, J.; Prutsky, G.; Elraiyah, T.; Wang, Z.; Nabhan, M.; Shippee, N.; Brito, J.; Boehmer, K.; Hasan, R.; Firwana, B.; et al. Patient engagement in research: A systematic review. BMC Health Serv. Res. 2014, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Concannon, T.; Fuster, M.; Saunders, T.; Patel, K.; Wong, J.; Leslie, L.; Lau, J. A systematic review of stakeholder engagement in comparative effectiveness and patient-centered outcomes research. J. Gen. Intern. Med. 2014, 29, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Tenaerts, P.; Madre, L.; Landray, M. A decade of the Clinical Trials Transformation Initiative: What have we accomplished? What have we learned? Clin. Trials 2018, 15, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Initiative, C.T.T. CTTI Tool—Stakeholder Identification and Analysis Tool. Available online: https://www.ctti-clinicaltrials.org/files/recruitment-tool-2-stakeholder.pdf (accessed on 11 December 2020).
- Clinical Trials Transformation Initiative. CTTI Recommendations: Patient Groups and Clinical Trials. Available online: https://www.ctti-clinicaltrials.org/sites/www.ctti-clinicaltrials.org/files/7-revised_pgct-recommendations-2019_final.pdf (accessed on 22 November 2020).
- Kendall, C.; Fitzgerald, M.; Kang, R.; Wong, S.; Katz, A.; Fortin, M.; Dionne, E.; Kuluski, K.; O’Brien, M.; Ploeg, J.; et al. “Still learning and evolving in our approaches”: Patient and stakeholder engagement among Canadian community-based primary health care researchers. Res. Involv. Engagem. 2018, 4, 47. [Google Scholar] [CrossRef]
- Canadian Institutes of Health Research. Strategy for Patient-Oriented Research—Patient Engagement Framework. Available online: https://cihr-irsc.gc.ca/e/documents/spor_framework-en.pdf (accessed on 22 November 2020).
- Frank, L.; Forsythe, L.; Ellis, L.; Schrandt, S.; Sheridan, S.; Gerson, J.; Konopka, K.; Daugherty, S. Conceptual and practical foundations of patient engagement in research at the patient-centered outcomes research institute. Qual. Life Res. 2015, 24, 1033–1041. [Google Scholar] [CrossRef]
- Brett, J.; Staniszewska, S.; Mockford, C.; Herron-Marx, S.; Hughes, J.; Tysall, C.; Suleman, R. Mapping the impact of patient and public involvement on health and social care research: A systematic review. Health Expect. 2014, 17, 637–650. [Google Scholar] [CrossRef]
- Selva, A.; Sanabria, A.; Pequeño, S.; Zhang, Y.; Solà, I.; Pardo-Hernandez, H.; Selva, C.; Schünemann, H.; Alonso-Coello, P. Incorporating patients’ views in guideline development: A systematic review of guidance documents. J. Clin. Epidemiol. 2017, 88, 102–112. [Google Scholar] [CrossRef]
- Colorectal Cancer Canada. Learn about us. Available online: https://www.colorectalcancercanada.com/learn-about-us/ (accessed on 22 November 2020).
- Batist, G.; Michaud, S.; Richards, D.; Servidio-Italiano, F.; Stein, B. Developing a model of a patient-group pathway to accessing cancer clinical trials in Canada. Curr. Oncol. 2018, 25, e597–e609. [Google Scholar] [CrossRef]
- Schwaber, K.; Sutherland, J. The Scrum Guide™—The Definitive Guide to Scrum: The Rules of the Game; Creative Commons: Mountain View, CA, USA, 2017; p. 19. [Google Scholar]
- Wrike. What Is a Sprint in Agile? Available online: https://www.wrike.com/project-management-guide/faq/what-is-a-sprint-in-agile/ (accessed on 1 December 2020).
- Canada, C.C. Canadian Cancer Clinical Trials Stakeholder Charter. Available online: https://www.colorectalcancercanada.com/app/uploads/2020/09/Canadian-Cancer-Clinical-Trials-Stakeholder-Charter-Document-2020.pdf (accessed on 22 November 2020).
- Novartis. The Novartis Commitment to Patients and Caregivers. Available online: https://www.novartis.com/our-focus/patients-caregivers/novartis-commitment-patients-and-caregivers (accessed on 4 November 2020).
- Ghinea, N. Citizen Science and the Politicization of Epistemology. Am. J. Bioeth. 2019, 19, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Selig, W.; Harker, M.; Roberts, J.; Hesterlee, S.; Leventhal, D.; Klein, R.; Patrick-Lake, B.; Abernethy, A. Patient Engagement Practices in Clinical Research among Patient Groups, Industry, and Academia in the United States: A Survey. PLoS ONE 2015, 10, e0140232. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials Transformation Initiative. Use of Real-World Data to Plan Eligibility Criteria and Enhance Recruitment. Available online: https://www.ctti-clinicaltrials.org/sites/www.ctti-clinicaltrials.org/files/rwd-recommendations_final.pdf (accessed on 22 November 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michaud, S.; Needham, J.; Sundquist, S.; Johnson, D.; Hanna, S.; Hosseinzadeh, S.; Bartekian, V.; Steele, P.; Benchimol, S.; Ross, N.; et al. Patient and Patient Group Engagement in Cancer Clinical Trials: A Stakeholder Charter. Curr. Oncol. 2021, 28, 1447-1458. https://doi.org/10.3390/curroncol28020137
Michaud S, Needham J, Sundquist S, Johnson D, Hanna S, Hosseinzadeh S, Bartekian V, Steele P, Benchimol S, Ross N, et al. Patient and Patient Group Engagement in Cancer Clinical Trials: A Stakeholder Charter. Current Oncology. 2021; 28(2):1447-1458. https://doi.org/10.3390/curroncol28020137
Chicago/Turabian StyleMichaud, Stéphanie, Judy Needham, Stephen Sundquist, Dominique Johnson, Sabrina Hanna, Sharareh Hosseinzadeh, Vatche Bartekian, Patricia Steele, Sarita Benchimol, Nathalie Ross, and et al. 2021. "Patient and Patient Group Engagement in Cancer Clinical Trials: A Stakeholder Charter" Current Oncology 28, no. 2: 1447-1458. https://doi.org/10.3390/curroncol28020137
APA StyleMichaud, S., Needham, J., Sundquist, S., Johnson, D., Hanna, S., Hosseinzadeh, S., Bartekian, V., Steele, P., Benchimol, S., Ross, N., & Stein, B. D. (2021). Patient and Patient Group Engagement in Cancer Clinical Trials: A Stakeholder Charter. Current Oncology, 28(2), 1447-1458. https://doi.org/10.3390/curroncol28020137





