A Comparison of Patient-Reported Outcomes Following Consent for Genetic Testing Using an Oncologist- or Genetic Counselor-Mediated Model of Care
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
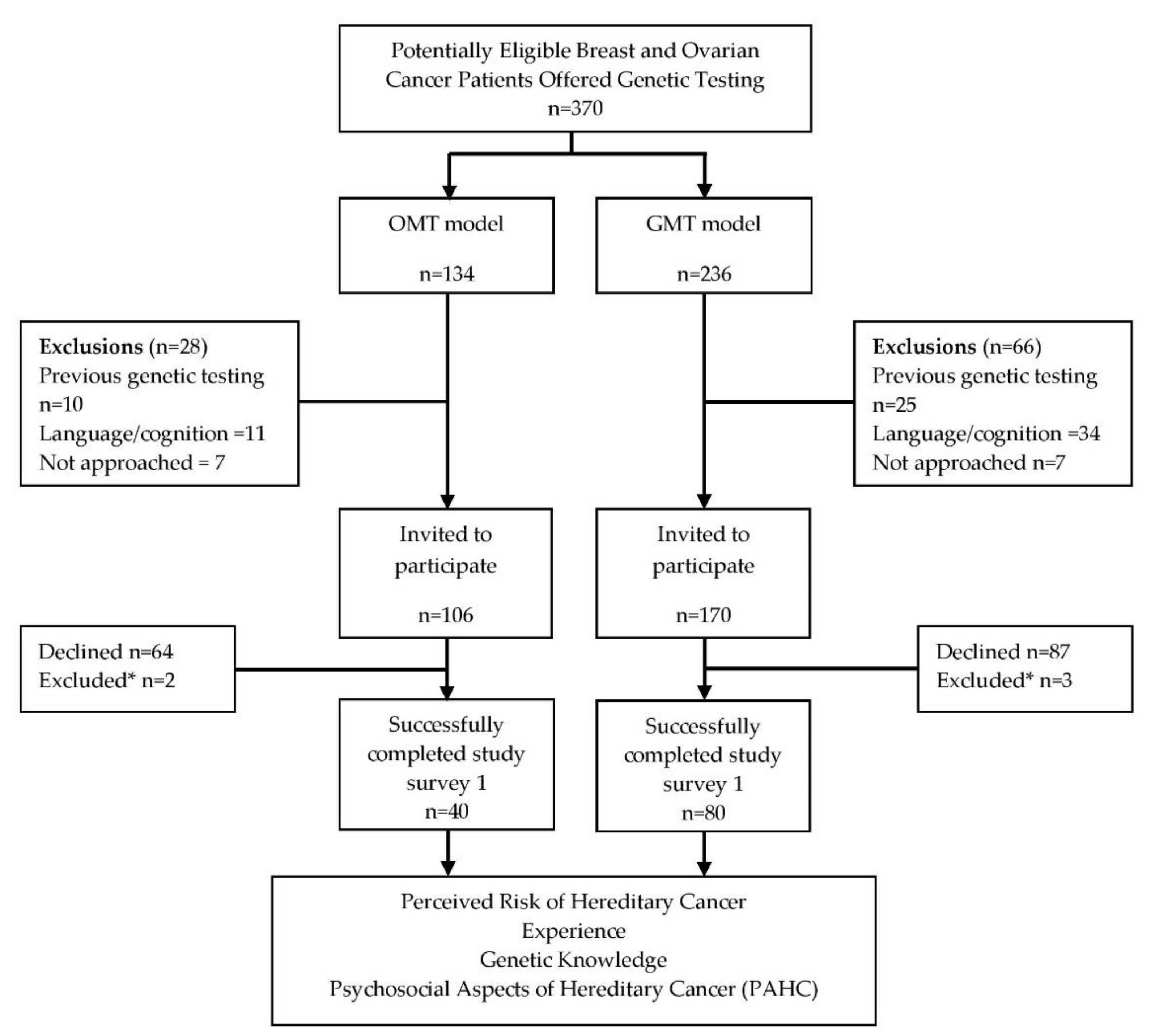
2.1. Study Population
2.2. Survey Responses Following Consent to Genetic Testing
2.2.1. Experience and Understanding of Genetic Testing
2.2.2. Genetic Testing Knowledge and Perceived Hereditary Cancer Risk
2.3. Psychosocial Aspects of Hereditary Cancer (PAHC)
2.4. Survey Responses Following Receipt of Genetic Testing Results
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Design
4.1.1. Study Participants and Recruitment
4.1.2. Genetic Counselor-Mediated Genetic Testing
4.1.3. Oncologist-Mediated Genetic Testing
4.2. Study Measurements
4.2.1. Experience and Understanding of Genetic Testing
4.2.2. Knowledge and Perceived Hereditary Cancer Risk
4.2.3. Modified Psychosocial Aspects of Hereditary Cancer (PAHC) Tool
4.2.4. Items Included in Survey Administered Following Results Disclosure
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tung, N.M.; Boughey, J.C.; Pierce, L.J.; Robson, M.E.; Bedrosian, I.; Dietz, J.R.; Dragun, A.; Gelpi, J.B.; Hofstatter, E.W.; Isaacs, C.J.; et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J. Clin. Oncol. 2020, 38, 2080–2106. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Norquist, B.; Lacchetti, C.; Armstrong, D.; Grisham, R.N.; Goodfellow, P.J.; Kohn, E.C.; Levine, D.A.; Liu, J.F.; Lu, K.H.; et al. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1222–1245. [Google Scholar] [CrossRef]
- Kolor, K.; Chen, Z.; Grosse, S.D.; Rodriguez, J.L.; Green, R.F.; Dotson, W.D.; Bowen, M.S.; Lynch, J.A.; Khoury, M.J. BRCA Genetic Testing and Receipt of Preventive Interventions among Women Aged 18–64 Years with Employer-Sponsored Health Insurance in Nonmetropolitan and Metropolitan Areas—United States, 2009–2014. MMWR Surveill. Summ. 2017, 66, 1–11. [Google Scholar] [CrossRef]
- Childers, C.P.; Childers, K.K.; Maggard-Gibbons, M.; Macinko, J. National Estimates of Genetic Testing in Women with a History of Breast or Ovarian Cancer. J. Clin. Oncol. 2017, 35, 3800–3806. [Google Scholar] [CrossRef]
- Hoskovec, J.M.; Bennett, R.L.; Carey, M.E.; DaVanzo, J.E.; Dougherty, M.; Hahn, S.E.; LeRoy, B.S.; O’Neal, S.; Richardson, J.G.; Wicklund, C.A. Projecting the Supply and Demand for Certified Genetic Counselors: A Workforce Study. J. Genet. Couns. 2018, 27, 16–20. [Google Scholar] [CrossRef]
- Cancer Care Ontario Recommendation Report for Ontario’s Clinical Genetic Services. Available online: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/ClinicalGeneticServicesRecommendationReport.pdf (accessed on 12 September 2020).
- Hoberg-Vetti, H.; Bjorvatn, C.; Fiane, B.E.; Aas, T.; Woie, K.; Espelid, H.; Rusken, T.; Eikesdal, H.P.; Listol, W.; Haavind, M.T.; et al. BRCA1/2 testing in newly diagnosed breast and ovarian cancer patients without prior genetic counselling: The DNA-BONus study. Eur. J. Hum. Genet. 2016, 24, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Quinn, V.F.; Meiser, B.; Kirk, J.; Tucker, K.M.; Watts, K.J.; Rahman, B.; Peate, M.; Saunders, C.; Geelhoed, E.; Gleeson, M.; et al. Streamlined genetic education is effective in preparing women newly diagnosed with breast cancer for decision making about treatment-focused genetic testing: A randomized controlled noninferiority trial. Genet. Med. 2017, 19, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Sie, A.S.; Spruijt, L.; van Zelst-Stams, W.A.; Mensenkamp, A.R.; Ligtenberg, M.J.; Brunner, H.G.; Prins, J.B.; Hoogerbrugge, N. High Satisfaction and Low Distress in Breast Cancer Patients One Year after BRCA-Mutation Testing without Prior Face-to-Face Genetic Counseling. J. Genet. Couns. 2016, 25, 504–514. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Riddell, D.; Seal, S.; Talukdar, S.; Mahamdallie, S.; Ruark, E.; Cloke, V.; Slade, I.; Kemp, Z.; Gore, M.; et al. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci. Rep. 2016, 6, 29506. [Google Scholar] [CrossRef]
- Berliner, J.L.; Fay, A.M.; Cummings, S.A.; Burnett, B.; Tillmanns, T. NSGC practice guideline: Risk assessment and genetic counseling for hereditary breast and ovarian cancer. J. Genet. Couns. 2013, 22, 155–163. [Google Scholar] [CrossRef]
- Madlensky, L.; Trepanier, A.M.; Cragun, D.; Lerner, B.; Shannon, K.M.; Zierhut, H. A Rapid Systematic Review of Outcomes Studies in Genetic Counseling. J. Genet. Couns. 2017, 26, 361–378. [Google Scholar] [CrossRef]
- Plaskocinska, I.; Shipman, H.; Drummond, J.; Thompson, E.; Buchanan, V.; Newcombe, B.; Hodgkin, C.; Barter, E.; Ridley, P.; Ng, R.; et al. New paradigms for BRCA1/BRCA2 testing in women with ovarian cancer: Results of the Genetic Testing in Epithelial Ovarian Cancer (GTEOC) study. J. Med. Genet. 2016, 53, 655–661. [Google Scholar] [CrossRef]
- Colombo, N.; Huang, G.; Scambia, G.; Chalas, E.; Pignata, S.; Fiorica, J.; Van Le, L.; Ghamande, S.; González-Santiago, S.; Bover, I.; et al. Evaluation of a Streamlined Oncologist-Led BRCA Mutation Testing and Counseling Model for Patients with Ovarian Cancer. J. Clin. Oncol. 2018, 36, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Kemp, Z.; Turnbull, A.; Yost, S.; Seal, S.; Mahamdallie, S.; Poyastro-Pearson, E.; Warren-Perry, M.; Eccleston, A.; Tan, M.M.; Teo, S.H.; et al. Evaluation of Cancer-Based Criteria for Use in Mainstream BRCA1 and BRCA2 Genetic Testing in Patients With Breast Cancer. JAMA Netw. Open 2019, 2, e194428. [Google Scholar] [CrossRef] [PubMed]
- Rumford, M.; Lythgoe, M.; McNeish, I.; Gabra, H.; Tookman, L.; Rahman, N.; George, A.; Krell, J. Oncologist-led BRCA ‘mainstreaming’ in the ovarian cancer clinic: A study of 255 patients and its impact on their management. Sci. Rep. 2020, 10, 3390. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.; Min, H.J.; Hong, Q.; Compton, K.; Mung, S.W.; Lohn, Z.; Nuk, J.; McCullum, M.; Portigal-Todd, C.; Karsan, A.; et al. Oncology Clinic-Based Hereditary Cancer Genetic Testing in a Population-Based Health Care System. Cancers 2020, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, S.; Power, J.; Foulkes, W.D.; Weber, E.; Palma, L.; Schiavi, A.; Ambrosio, E.; Konci, R.; Gilbert, L.; Jardon, K.; et al. BRCA testing in women with high-grade serous ovarian cancer: Gynecologic oncologist-initiated testing compared with genetics referral. Int. J. Gynecol. Cancer 2020, 159, 221–228. [Google Scholar] [CrossRef]
- Powell, C.B.; Laurent, C.; Ciaravino, G.; Garcia, C.; Han, L.; Hoodfar, E.; Karlea, A.; Kobelka, C.; Lee, J.; Littell, R.D.; et al. Streamlining genetic testing for women with ovarian cancer in a Northern California health care system. Gynecol. Oncol. 2020, 159, 221–228. [Google Scholar] [CrossRef]
- McCuaig, J.M.; Greenwood, C.M.; Shuman, C.; Chitayat, D.; Murphy, K.J.; Rosen, B.; Armel, S.R. Breast and ovarian cancer: The forgotten paternal contribution. J. Genet. Couns. 2011, 20, 442–449. [Google Scholar] [CrossRef]
- Eijzenga, W.; Bleiker, E.M.; Hahn, D.E.; Van der Kolk, L.E.; Sidharta, G.N.; Aaronson, N.K. Prevalence and detection of psychosocial problems in cancer genetic counseling. Fam. Cancer 2015, 14, 629–636. [Google Scholar] [CrossRef]
- Meiser, B.; Quinn, V.F.; Gleeson, M.; Kirk, J.; Tucker, K.M.; Rahman, B.; Saunders, C.; Watts, K.J.; Peate, M.; Geelhoed, E.; et al. When knowledge of a heritable gene mutation comes out of the blue: Treatment-focused genetic testing in women newly diagnosed with breast cancer. Eur. J. Hum. Genet. 2016, 24, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Eijzenga, W.; Bleiker, E.M.; Hahn, D.E.; Kluijt, I.; Sidharta, G.N.; Gundy, C.; Aaronson, N.K. Psychosocial aspects of hereditary cancer (PAHC) questionnaire: Development and testing of a screening questionnaire for use in clinical cancer genetics. Psycho Oncol. 2014, 23, 862–869. [Google Scholar] [CrossRef]
- Cella, D.; Hughes, C.; Peterman, A.; Chang, C.H.; Peshkin, B.N.; Schwartz, M.D.; Wenzel, L.; Lemke, A.; Marcus, A.C.; Lerman, C. A brief assessment of concerns associated with genetic testing for cancer: The Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002, 21, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Bjornslett, M.; Dahl, A.A.; Sorebo, O.; Dorum, A. Psychological distress related to BRCA testing in ovarian cancer patients. Fam. Cancer 2015, 14, 495–504. [Google Scholar] [CrossRef] [PubMed]

| Covariate | Full Sample (n = 120) | GMT (n = 80) | OMT (n = 40) | p-Value |
|---|---|---|---|---|
| Marital Status a (%) | 0.35 | |||
| In a Relationship | 10 (8.4) | 8 (10.1) | 2 (5.0) | |
| Married/Common-Law | 73 (61.3) | 45 (57.0) | 28 (70.0) | |
| Single/Widowed | 36 (30.3) | 26 (32.9) | 10 (25.0) | |
| Education Level b (%) | 0.67 | |||
| Elementary/Middle S | 3 (2.5) | 1 (1.3) | 2 (5.0) | |
| High School | 18 (15.2) | 12 (15.4) | 6 (15.0) | |
| Certificate Program | 11 (9.3) | 6 (7.7) | 5 (12.5) | |
| College/University | 58 (49.3) | 40 (51.3) | 18 (45.0) | |
| Post-Graduate | 28 (23.7) | 19 (24.4) | 9 (22.5) | |
| Diagnosis (%) | 1 | |||
| Breast | 33 (27.7) | 22 (27.8) | 11 (27.5) | |
| Ovarian c | 86 (72.3) | 57 (72.2) | 29 (72.5) | |
| Age at diagnosis | 0.18 | |||
| Mean (sd) | 57.4 (11.1) | 58.4 (11.0) | 55.4 (11.3) | |
| Median (Min,Max) | 57 (24,79) | 57 (32,79) | 54.5 (24,78) | |
| Family history of BR/OV cancer a (%) | 0.80 | |||
| No | 45 (37.8) | 31 (39.2) | 14 (35.0) | |
| Yes | 74 (62.2) | 48 (61.8) | 26 (65.0) | |
| Ethnicity | 0.69 | |||
| African | 1 (0.8) | 1 (1.3) | 0 (0) | |
| Ashkenazi Jewish | 10 (8.3) | 9 (11.3) | 1 (2.5) | |
| Asian | 15 (12.5) | 11 (13.8) | 4 (10.0) | |
| Caucasian | 74 (61.7) | 45 (56.3) | 29 (72.5) | |
| East Indian | 6 (5.0) | 4 (5.0) | 2 (5.0) | |
| Hispanic | 2 (1.7) | 1 (1.3) | 1 (2.5) | |
| Middle Eastern | 3 (2.5) | 2 (2.5) | 1 (2.5) | |
| Mixed | 2 (1.7) | 2 (2.5) | 0 (0) | |
| West Indies | 3 (2.5) | 2 (2.5) | 1 (2.5) | |
| Missing/Unknown | 4 (3.3) | 3 (3.8) | 1 (2.5) |
| Outcome of Interest | Full Sample | GMT | OMT | p-Value |
|---|---|---|---|---|
| Experience & Understanding | <0.001 | |||
| Median (Min,Max) | 10 (1,10) | 10 (3,10) | 8.5 (1,10) | |
| Mean (sd) | 8.8 (2.1) | 9.4 (1.3) | 7.7 (2.7) | |
| Knowledge Score | 0.025 | |||
| Median (Min,Max) | 8 (1,11) | 9 (4,11) | 8 (1,10) | |
| Mean (sd) | 7.8 (2.1) | 8.2 (1.8) | 7.1 (2.3) | |
| Perceived Risk a | 0.29 | |||
| Median (Min,Max) | 30 (0,100) | 40 (0,100) | 22.5 (0,100) | |
| Mean (sd) | 34.6 (29.4) | 36.1 (28.7) | 31.5 (30.9) | |
| PAHC-Screen Positive by domain (%) | ||||
| Hereditary Predisposition | 55 (45.8) | 44 (55.0) | 11 (27.5) | 0.005 |
| Practical Issues b | 28 (23.3) | 22 (27.5) | 6 (15.0) | 0.15 |
| Family & Social Issues | 9 (7.5) | 8 (10.0) | 1 (2.5) | 0.14 |
| Living with Cancer | 91 (75.8) | 57 (71.3) | 34 (85.0) | 0.10 |
| General Emotions | 57 (47.5) | 31 (38.8) | 26 (65.0) | 0.007 |
| Child-related issues c | 53 (65.4) | 30 (61.2) | 23 (71.9) | 0.33 |
| Any | 98 (86.7) | 62 (77.5) | 36 (90.0) | 0.10 |
| Thinking about How you Received Information about Genetic Testing, Please Answer the Following a | |||
|---|---|---|---|
| Staement Provided | % Agreed | p-Value | |
| GMT | OMT | ||
| a. The information that I was given about genetic testing was clear and helpful. | 96.2 | 75.0 | 0.01 |
| b. The information was given to me in a way that I could understand. | 97.4 | 77.5 | 0.01 |
| c. The information helped me understand why I was being offered genetic testing. | 96.3 | 85.0 | 0.06 |
| d. I knew that I could decide NOT to have genetic testing. | 93.8 | 90.0 | 0.48 |
| e. The information helped me understand how the result of genetic testing might impact me. | 91.3 | 69.2 | 0.003 |
| f. The information helped me understand how the result of genetic testing might impact my family. | 93.8 | 71.8 | 0.003 |
| g. I had enough information to decide whether or not I wanted to have genetic testing. | 96.3 | 92.5 | 0.39 |
| h. I understand the different types of test results I can receive from my genetic test (positive, negative, inconclusive). | 93.8 | 70.0 | 0.001 |
| i. I knew that I could contact a genetic counsellor if I had questions before deciding to have genetic testing. | 91.3 | 66.7 | 0.001 |
| j. Overall, I felt the process of having genetic testing worked well. | 91.1 | 74.4 | 0.02 |
| The Following Questions will Ask You about Hereditary Cancer. Please Answer the Following a | |||
|---|---|---|---|
| Statement Provided | % Correct | p-Value | |
| GMT | OMT | ||
| a. All people who have a mutation in a cancer gene will get cancer. (N) | 75.0 | 72.5 | 0.83 |
| b. A person who has a mutation in a cancer gene has an increased chance to get more than one cancer in their lifetime. (Y) | 73.8 | 55.0 | 0.06 |
| c. There are only two possible results of a genetic test (i.e., positive or negative). (N) | 63.3 | 40.0 | 0.02 |
| d. Genetic testing can determine if a cancer is hereditary. (Y) | 78.2 | 78.9 | 1.00 |
| e. The son of a woman with a mutation in an ovarian cancer gene has a 50% risk of having the mutation. (Y) | 53.2 | 20.0 | 0.001 |
| f. A genetic test can find 100% of all possible gene mutations. (N) | 68.4 | 59.0 | 0.41 |
| g. If someone has a mutation in a cancer gene, genetic testing becomes available to their family members. (Y) | 72.5 | 71.8 | 1.00 |
| h. Some people may feel anxious or guilty during or after genetic counselling and testing. (Y) | 83.8 | 77.5 | 0.46 |
| i. It is my responsibility to share my test results with my healthcare providers and family members. (Y) | 78.8 | 85.0 | 0.47 |
| j. For people who have a mutation in a cancer gene, there are medical options to reduce cancer risks. (Y) | 81.3 | 72.5 | 0.35 |
| k. Women who have a mutation in a cancer gene, only need to share the results with their female family members. (N) | 92.4 | 87.5 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCuaig, J.M.; Thain, E.; Malcolmson, J.; Keshavarzi, S.; Armel, S.R.; Kim, R.H. A Comparison of Patient-Reported Outcomes Following Consent for Genetic Testing Using an Oncologist- or Genetic Counselor-Mediated Model of Care. Curr. Oncol. 2021, 28, 1459-1471. https://doi.org/10.3390/curroncol28020138
McCuaig JM, Thain E, Malcolmson J, Keshavarzi S, Armel SR, Kim RH. A Comparison of Patient-Reported Outcomes Following Consent for Genetic Testing Using an Oncologist- or Genetic Counselor-Mediated Model of Care. Current Oncology. 2021; 28(2):1459-1471. https://doi.org/10.3390/curroncol28020138
Chicago/Turabian StyleMcCuaig, Jeanna M., Emily Thain, Janet Malcolmson, Sareh Keshavarzi, Susan Randall Armel, and Raymond H. Kim. 2021. "A Comparison of Patient-Reported Outcomes Following Consent for Genetic Testing Using an Oncologist- or Genetic Counselor-Mediated Model of Care" Current Oncology 28, no. 2: 1459-1471. https://doi.org/10.3390/curroncol28020138
APA StyleMcCuaig, J. M., Thain, E., Malcolmson, J., Keshavarzi, S., Armel, S. R., & Kim, R. H. (2021). A Comparison of Patient-Reported Outcomes Following Consent for Genetic Testing Using an Oncologist- or Genetic Counselor-Mediated Model of Care. Current Oncology, 28(2), 1459-1471. https://doi.org/10.3390/curroncol28020138





