On the Prognosis of Multifocal Glioblastoma: An Evaluation Incorporating Volumetric MRI
Abstract
1. Introduction
2. Methods
2.1. Patient Selection and Treatment
2.2. Clinical, Pathological and Radiological Assessment
2.3. Statistical Analysis
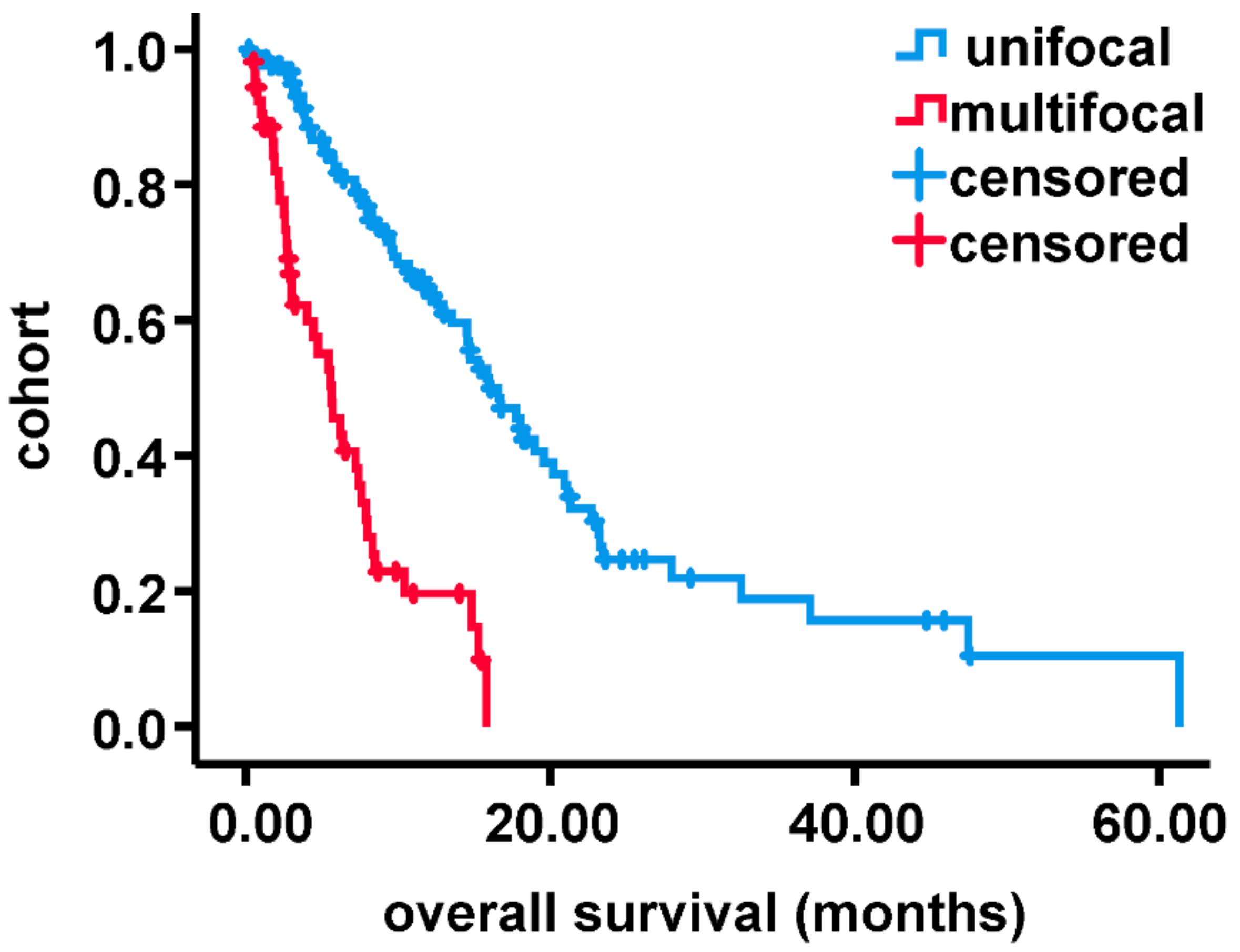
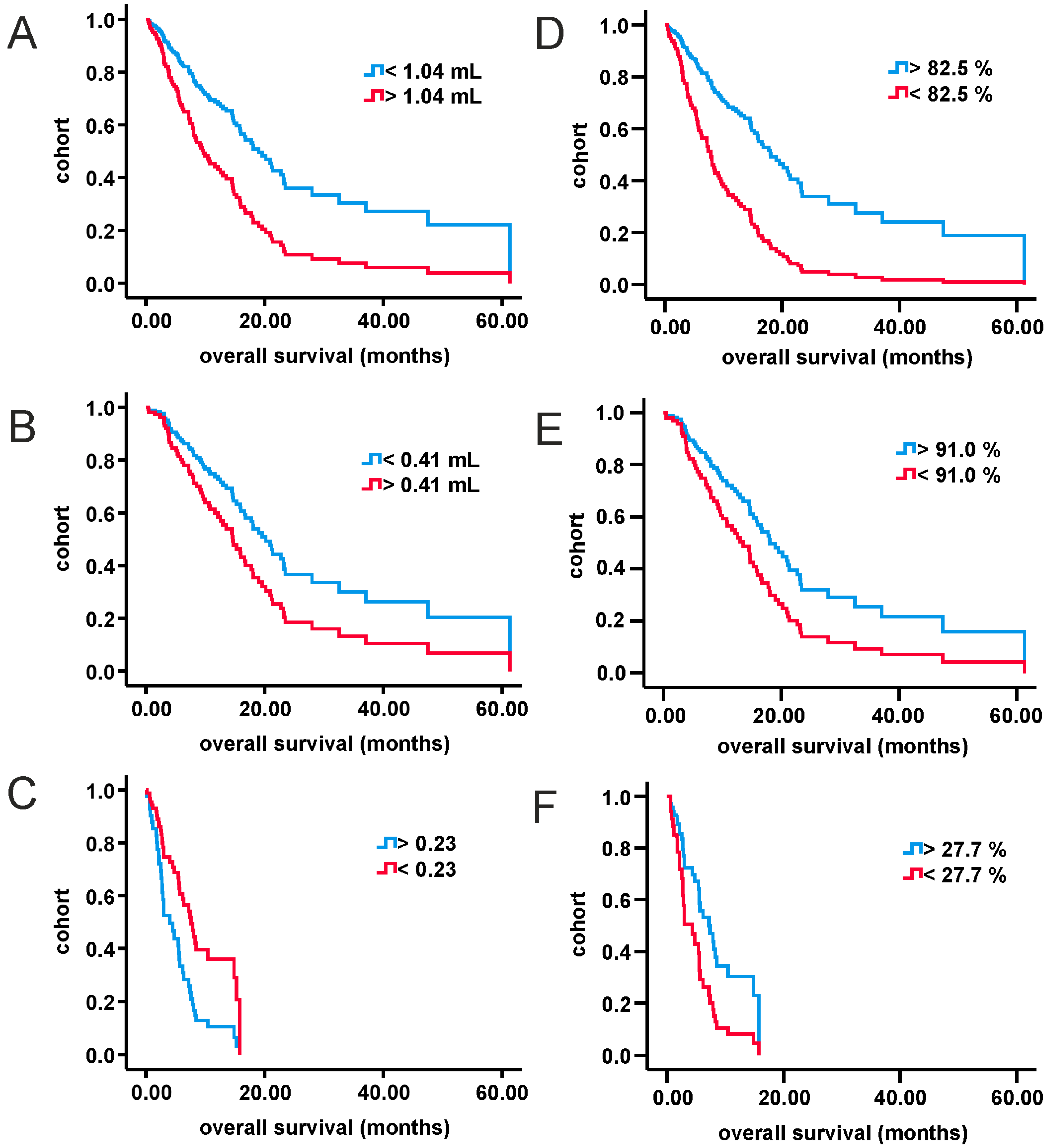
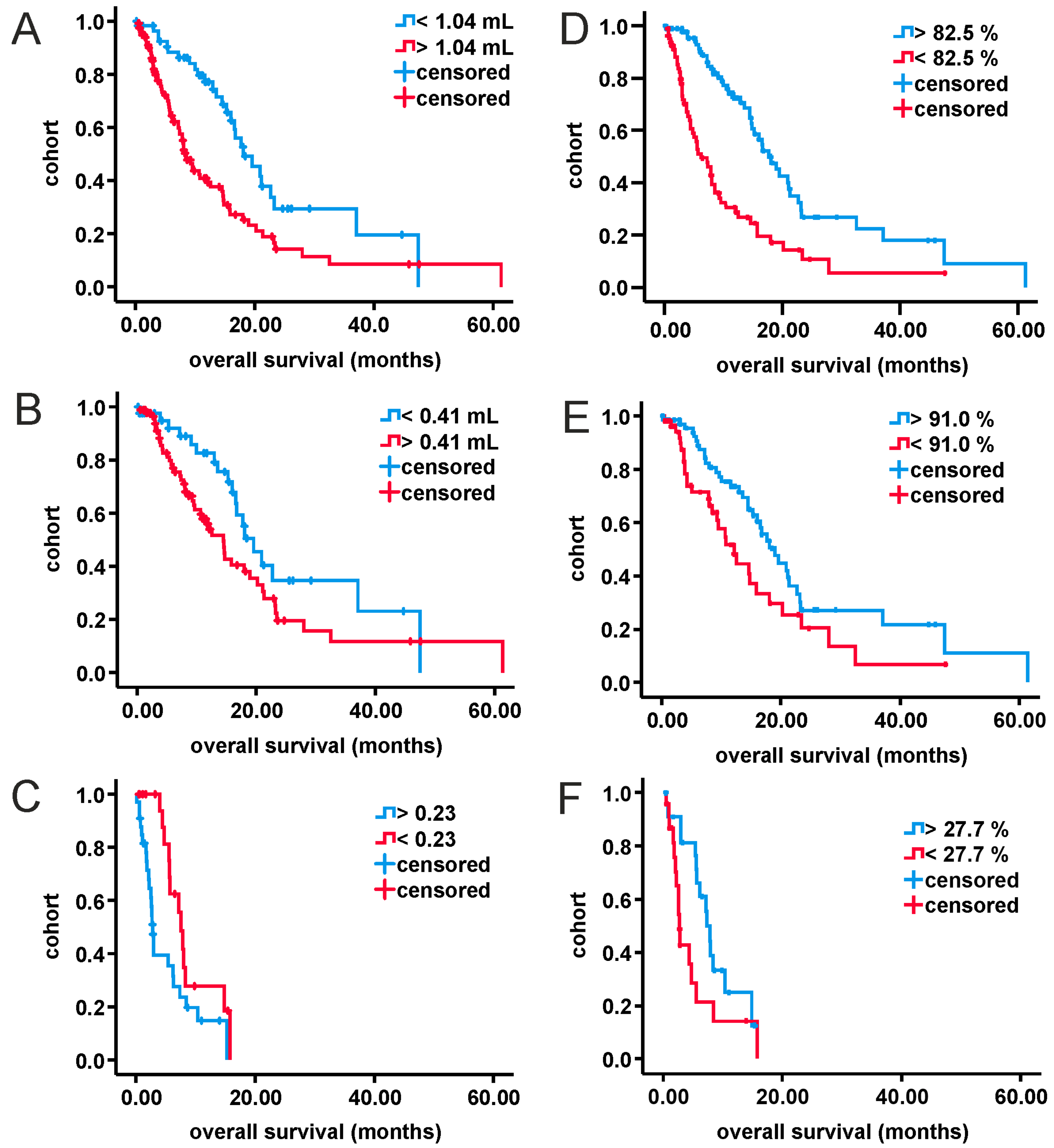
3. Results
3.1. Patient Cohort
3.2. Analysis of MRI-Volumetric Parameters
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-Oncology 2018, 20, iv1–iv86. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Hopkins, K.; Tonn, J.C.; Stupp, R.; Falini, A.; Cohen-Jonathan-Moyal, E.; Frappaz, D.; Henriksson, R.; Balana, C.; et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014, 15, e395–e403. [Google Scholar] [CrossRef]
- Wick, W.; Osswald, M.; Wick, A.; Winkler, F. Treatment of glioblastoma in adults. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418790452. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Noch, E.K.; Ramakrishna, R.; Magge, R. Challenges in the Treatment of Glioblastoma: Multisystem Mechanisms of Therapeutic Resistance. World Neurosurg. 2018, 116, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Fabian, D.; Guillermo Prieto Eibl, M.D.P.; Alnahhas, I.; Sebastian, N.; Giglio, P.; Puduvalli, V.; Gonzalez, J.; Palmer, J.D. Treatment of Glioblastoma (GBM) with the Addition of Tumor-Treating Fields (TTF): A Review. Cancers 2019, 11, 174. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMTGene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Miyake, K.; Tamiya, T. Glioblastoma Treatment in the Elderly. Neurol. Med. Chir. 2017, 57, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Dietterle, J.; Wende, T.; Wilhelmy, F.; Eisenlöffel, C.; Jähne, K.; Taubenheim, S.; Arlt, F.; Meixensberger, J. The prognostic value of peri-operative neurological performance in glioblastoma patients. Acta Neurochir. 2020, 162, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef]
- Ellingson, B.M.E.; Abrey, L.; Nelson, S.J.; Kaufmann, T.J.; Garcia, J.; Chinot, O.; Saran, F.; Nishikawa, R.; Henriksson, R.; Mason, W.P.; et al. Validation of postoperative residual contrast-enhancing tumor volume as an independent prognostic factor for overall survival in newly diagnosed glioblastoma. Neuro-Oncol. 2018, 20, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Henker, C.; Kriesen, T.; Glass, Ä.; Schneider, B.; Piek, J. Volumetric quantification of glioblastoma: Experiences with different measurement techniques and impact on survival. J. Neuro-Oncol. 2017, 135, 391–402. [Google Scholar] [CrossRef]
- Mackintosh, C.; Butterfield, R.; Zhang, N.; Lorence, J.; Zlomanczuk, P.; Bendok, B.R.; Zimmerman, R.S.; Swanson, K.; Porter, A.; Mrugala, M.M. Does location matter? Characterisation of the anatomic locations, molecular profiles, and clinical features of gliomas. Neurol. Neurochir. Polska 2020, 54, 456–465. [Google Scholar] [CrossRef]
- Di, L.; Heath, R.N.; Shah, A.H.; Sanjurjo, A.D.; Eichberg, D.G.; Luther, E.M.; De La Fuente, M.I.; Komotar, R.J.; Ivan, M.E. Resection versus biopsy in the treatment of multifocal glioblastoma: A weighted survival analysis. J. Neuro-Oncol. 2020, 148, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Haque, W.; Thong, Y.; Verma, V.; Rostomily, R.; Butler, E.B.; Teh, B.S. Patterns of management and outcomes of unifocal versus multifocal glioblastoma. J. Clin. Neurosci. 2020, 74, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Dono, A.; Wang, E.; Lopez-Rivera, V.; Ramesh, A.V.; Tandon, N.; Ballester, L.Y.; Esquenazi, Y. Molecular characteristics and clinical features of multifocal glioblastoma. J. Neuro-Oncol. 2020, 148, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Ahmadipour, Y.; Jabbarli, R.; Gembruch, O.; Pierscianek, D.; Oppong, M.D.; Dammann, P.; Wrede, K.; Özkan, N.; Müller, O.; Sure, U.; et al. Impact of Multifocality and Molecular Markers on Survival of Glioblastoma. World Neurosurg. 2019, 122, e461–e466. [Google Scholar] [CrossRef] [PubMed]
- Tunthanathip, T.; Sangkhathat, S.; Tanvejsilp, P.; Kanjanapradit, K. The clinical characteristics and prognostic factors of multiple lesions in glioblastomas. Clin. Neurol. Neurosurg. 2020, 195, 105891. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Beteta, J.; Molina-García, D.; Villena, M.; Rodríguez, M.; Velásquez, C.; Martino, J.; Meléndez-Asensio, B.; De Lope, Á.R.; Morcillo, R.; Sepúlveda, J.; et al. Morphologic Features on MR Imaging Classify Multifocal Glioblastomas in Different Prognostic Groups. Am. J. Neuroradiol. 2019, 40, 634–640. [Google Scholar] [CrossRef]
- Lasocki, A.; Gaillard, F.; Tacey, M.; Drummond, K.; Stuckey, S. Multifocal and multicentric glioblastoma: Improved characterisation with FLAIR imaging and prognostic implications. J. Clin. Neurosci. 2016, 31, 92–98. [Google Scholar] [CrossRef]
- Bleehen, N.M.; Stenning, S.P. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. Br. J. Cancer 1991, 64, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D.T.; Cagnazzo, F.; Benedetto, N.; Morganti, R.; Perrini, P. Multiple high-grade gliomas: Epidemiology, management, and outcome. A systematic review and meta-analysis. Neurosurg. Rev. 2019, 42, 263–275. [Google Scholar] [CrossRef]
- Parsa, A.T.; Wachhorst, S.; Lamborn, K.R.; Prados, M.D.; McDermott, M.W.; Berger, M.S.; Chang, S.M. Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. J. Neurosurg. 2005, 102, 622–628. [Google Scholar] [CrossRef]
- Patil, C.G.; Yi, A.; Elramsisy, A.; Hu, J.; Mukherjee, D.; Irvin, D.K.; Yu, J.S.; Bannykh, S.I.; Black, K.L.; Nuño, M. Prognosis of patients with multifocal glioblastoma: A case-control study. J. Neurosurg. 2012, 117, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Salvati, M.; Caroli, E.; Orlando, E.R.; Frati, A.; Artizzu, S.; Ferrante, L. Multicentric glioma: Our experience in 25 patients and critical review of the literature. Neurosurg. Rev. 2003, 26, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Di Russo, P.; Perrini, P.; Pasqualetti, F.; Meola, A.; Vannozzi, R. Management and outcome of high-grade multicentric gliomas: A contemporary single-institution series and review of the literature. Acta Neurochir. 2013, 155, 2245–2251. [Google Scholar] [CrossRef]
- Hassaneen, W.; Levine, N.B.; Suki, D.; Salaskar, A.L.; Lima, A.D.M.; McCutcheon, I.E.; Prabhu, S.S.; Lang, F.F.; Demonte, F.; Rao, G.; et al. Multiple craniotomies in the management of multifocal and multicentric glioblastoma. J. Neurosurg. 2011, 114, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, M.A.; Sawaya, R.; Shi, W.; Thall, P.F.; Leeds, N.E. Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J. Neuro-Oncol. 1996, 27, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; Demonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]
- Pierallini, A.; Bonamini, M.; Pantano, P.; Palmeggiani, F.; Raguso, M.; Osti, M.F.; Anaveri, G.; Bozzao, L. Radiological assessment of necrosis in glioblastoma: Variability and prognostic value. Neuroradiology 1998, 40, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Nowosielski, M.; Gorlia, T.; Bromberg, J.E.; Sahm, F.; Harting, I.; Kickingereder, P.; Brandes, A.A.; Taphoorn, M.J.; Taal, W.; Domont, J.; et al. Imaging necrosis during treatment is associated with worse survival in EORTC 26101 study. Neurology 2019, 92, e2754–e2763. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Xu, K.; Wang, Z.; Fan, X.; Zhang, C.; Li, S.; Qiu, X.; Jiang, T. Relationship between necrotic patterns in glioblastoma and patient survival: Fractal dimension and lacunarity analyses using magnetic resonance imaging. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Henker, C.; Hiepel, M.C.; Kriesen, T.; Scherer, M.; Glass, Ä.; Herold-Mende, C.; Bendszus, M.; Langner, S.; Weber, M.-A.; Schneider, B.; et al. Volumetric assessment of glioblastoma and its predictive value for survival. Acta Neurochir. 2019, 161, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Henker, C.; Kriesen, T.; Scherer, M.; Glass, Ä.; Von Deimling, A.; Bendszus, M.; Weber, M.-A.; Herold-Mende, C.; Unterberg, A.; Piek, J. Association Between Tumor Compartment Volumes, the Incidence of Pretreatment Seizures, and Statin-Mediated Protective Effects in Glioblastoma. Neurosurgery 2019, 85, E722–E729. [Google Scholar] [CrossRef]
- Raza, S.M.; Lang, F.F.; Aggarwal, B.B.; Fuller, G.N.; Wildrick, D.M.; Sawaya, R. Necrosis and Glioblastoma: A Friend or a Foe? A Review and a Hypothesis. Neurosurgery 2002, 51, 2–13. [Google Scholar] [CrossRef]
- Noch, E.; Khalili, K. Molecular mechanisms of necrosis in glioblastoma: The role of glutamate excitotoxicity. Cancer Biol. Ther. 2009, 8, 1791–1797. [Google Scholar] [CrossRef]
- Brat, D.J.; Van Meir, E.G. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab. Investig. 2004, 84, 397–405. [Google Scholar] [CrossRef]
- Soni, S.; Padwad, Y.S. HIF-1 in cancer therapy: Two decade long story of a transcription factor. Acta Oncol. 2017, 56, 503–515. [Google Scholar] [CrossRef]
- Chen, W.-S.; Hong, L.; Wang, F.; Li, J.-J. Investigation of dacomitinib on reducing cell necrosis and enhancing cell apoptosis in C6 glioma rat model by MRI. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- De Souza, P.C.; Balasubramanian, K.; Njoku, C.; Smith, N.; Gillespie, D.L.; Schwager, A.; Abdullah, O.; Ritchey, J.W.; Fung, K.-M.; Saunders, D.; et al. OKN-007 decreases tumor necrosis and tumor cell proliferation and increases apoptosis in a preclinical F98 rat glioma model. J. Magn. Reson. Imaging 2015, 42, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
| All | Unifocal | Multifocal | p-Value | ||
|---|---|---|---|---|---|
| Number of patients | 183 | 129 | 54 | - | |
| Age (years) | 67.4 ± 10.6 | 66.9 ± 10.9 | 68.5 ± 9.9 | 0.24 | |
| Gender ratio (male to female) | 1:0.48 | 1:0.5 | 1:0.45 | 0.68 | |
| IV (mL) | 33.1 ± 27 | 32.4 ± 25.6 | 34.8 ± 28.8 | 0.44 | |
| CEV- (mL) | 10.3 ± 13.3 | 11.3 ± 14.0 | 7.9 ± 11.2 | 0.34 | |
| CEV-/IV | 0.25 ± 0.2 | 0.27 ± 0.18 | 0.19 ± 0.16 | 0.05 | |
| RV (mL) | 9.6 ± 16.3 | 5.7 ± 8.9 | 20.5 ± 24.8 | <0.0001 | |
| EOR (%) | 67.6 ± 37.4 | 79.3 ± 30.2 | 36.9 ± 36.4 | <0.0001 | |
| MRC-NPS | 2.6 ± 0.9 | 2.6 ± 0.9 | 2.6 ± 0.9 | 0.29 | |
| MGMT (%) | 22.3 ± 20.6 | 22.2 ± 20.9 | 22.5 ± 20.1 | 0.47 | |
| Adjuvant therapy | w/o | 35 | 16 | 19 | 0.02 |
| Rx | 34 | 26 | 8 | ||
| RCx | 114 | 87 | 27 |
| All | Unifocal | Multifocal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | CI | p-Value | HR | CI | p-Value | HR | CI | p-Value | |
| Age | 1.012 | 0.99–1.03 | 0.282 | 0.998 | 0.99–1.01 | 0.704 | - | - | - |
| MGMT | 0.989 | 0.98–1.0 | 0.086 | 1.026 | 1.0–1.06 | 0.07 | - | - | - |
| MRC-NPS | 0.924 | 0.7–1.2 | 0.58 | 1.055 | 0.74–1.49 | 0.759 | - | - | - |
| Adjuvant therapy | 0.352 | 0.24–0.51 | <0.0001 | 0.391 | 0.23–0.67 | 0.001 | 0.429 | 0.27–0.7 | 0.002 |
| CEV- | - | - | - | - | - | - | 1.011 | 0.95–1.07 | 0.709 |
| CEV-/IV | 0.998 | 0.99–1.01 | 0.703 | - | - | - | 0.965 | 0.93–1.01 | 0.104 |
| RV | 1.011 | 1.0–1.03 | 0.175 | 1.037 | 1.0–1.08 | 0.085 | - | - | - |
| EOR | 0.992 | 0.985–0.998 | 0.017 | 0.977 | 0.962–0.99 | 0.003 | 0.998 | 0.99–1.01 | 0.699 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasper, J.; Hilbert, N.; Wende, T.; Fehrenbach, M.K.; Wilhelmy, F.; Jähne, K.; Frydrychowicz, C.; Hamerla, G.; Meixensberger, J.; Arlt, F. On the Prognosis of Multifocal Glioblastoma: An Evaluation Incorporating Volumetric MRI. Curr. Oncol. 2021, 28, 1437-1446. https://doi.org/10.3390/curroncol28020136
Kasper J, Hilbert N, Wende T, Fehrenbach MK, Wilhelmy F, Jähne K, Frydrychowicz C, Hamerla G, Meixensberger J, Arlt F. On the Prognosis of Multifocal Glioblastoma: An Evaluation Incorporating Volumetric MRI. Current Oncology. 2021; 28(2):1437-1446. https://doi.org/10.3390/curroncol28020136
Chicago/Turabian StyleKasper, Johannes, Nicole Hilbert, Tim Wende, Michael Karl Fehrenbach, Florian Wilhelmy, Katja Jähne, Clara Frydrychowicz, Gordian Hamerla, Jürgen Meixensberger, and Felix Arlt. 2021. "On the Prognosis of Multifocal Glioblastoma: An Evaluation Incorporating Volumetric MRI" Current Oncology 28, no. 2: 1437-1446. https://doi.org/10.3390/curroncol28020136
APA StyleKasper, J., Hilbert, N., Wende, T., Fehrenbach, M. K., Wilhelmy, F., Jähne, K., Frydrychowicz, C., Hamerla, G., Meixensberger, J., & Arlt, F. (2021). On the Prognosis of Multifocal Glioblastoma: An Evaluation Incorporating Volumetric MRI. Current Oncology, 28(2), 1437-1446. https://doi.org/10.3390/curroncol28020136





