Circulating miR-618 Has Prognostic Significance in Patients with Metastatic Colon Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Healthy Controls
2.2. RNA Extraction, cDNA Synthesis and qPCR
2.3. DNA Extraction and Single Nucleotide Polymorphism (SNP) Genotyping
2.4. Statistical Analysis
3. Results
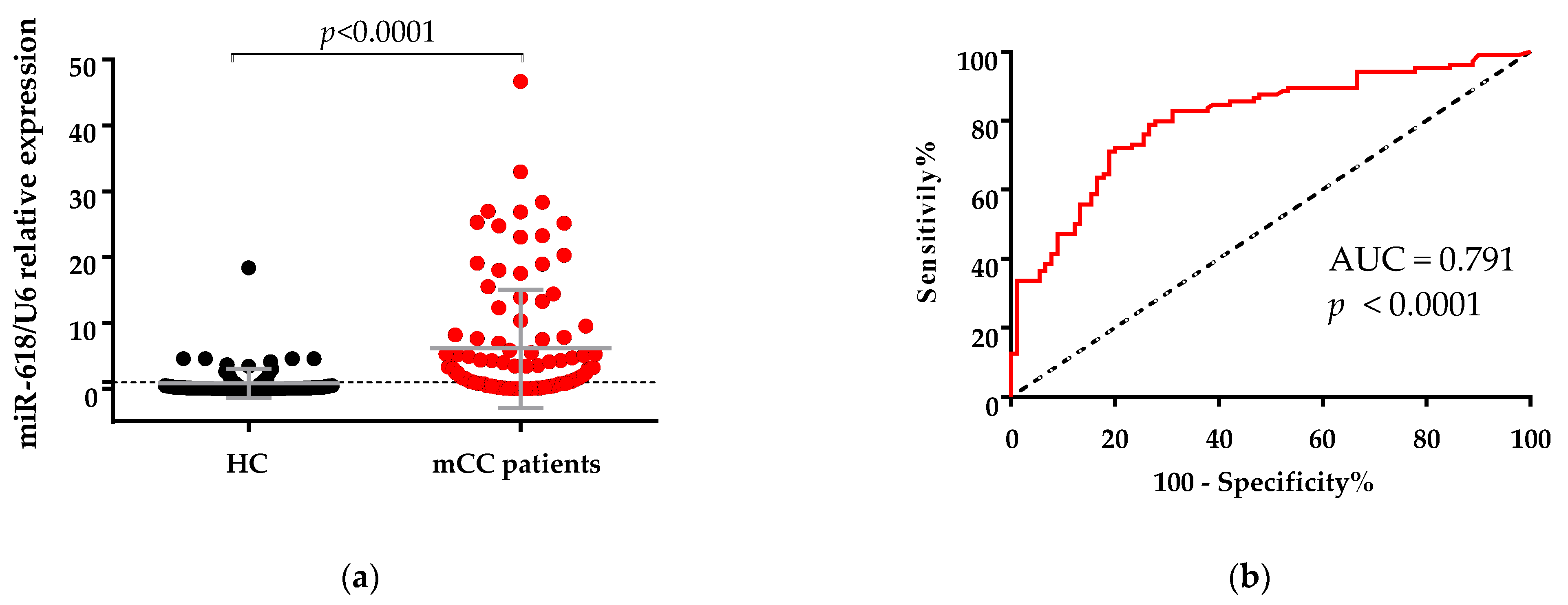
3.1. Expression of miR-618 in Serum Samples of Metastatic Colon Cancer (mCC) Patients Was Significantly Higher Than in Serum Samples of Healthy Controls
3.2. miR-618 Serum Levels Was Not Related Significantly with Clinicopathological Characteristics of the Patients
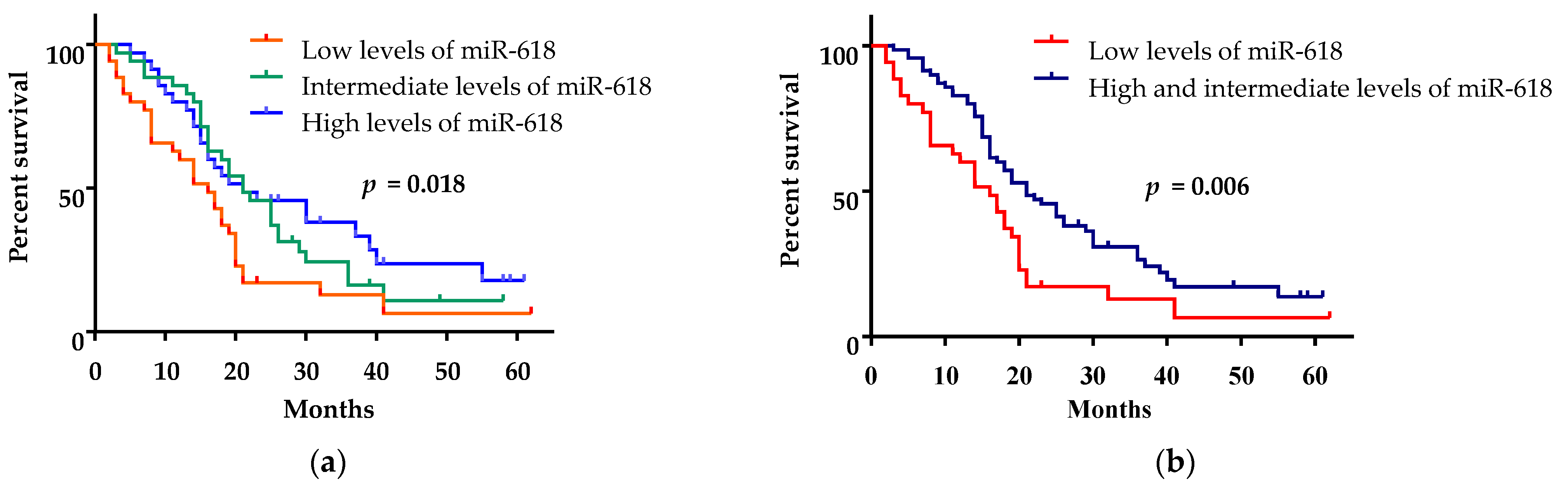
3.3. Low miR-618 Levels in Serum Were Associated with Short Survival
3.4. The Presence of rs2682818 Did Not Determine Serum Levels of miR-618
3.5. rs2682818 AC Genotype Was Associated with a Decreased Risk of Colon Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D.; ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. 3), iii1–iii9. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.M.H. Circulating microRNAs as biomarkers in cancer diagnosis. Life Sci. 2020, 248, 117473. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenet. 2018, 10, 59. [Google Scholar] [CrossRef]
- Ding, H.X.; Lv, Z.; Yuan, Y.; Xu, Q. MiRNA Polymorphisms and Cancer Prognosis: A Systematic Review and Meta-Analysis. Front. Oncol. 2018, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Song, F.J.; Chen, K.X. Single-nucleotide polymorphisms among microRNA: Big effects on cancer. Chin. J. Cancer. 2011, 30, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yao, Y.; Leng, K.; Ji, D.; Qu, L.; Liu, Y.; Cui, Y. Increased Expression of Circular RNA circ_0005230 Indicates Dismal Prognosis in Breast Cancer and Regulates Cell Proliferation and Invasion via miR-618/ CBX8 Signal Pathway. Cell Physiol. Biochem. 2018, 51, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Bahramian, S.T.; Shamsabadi, F.; Fazel, A.; Delshad, E.; Amini, A.; Memari, F.; Shafiee, M. Evaluation of Arylsulfatase D (ARSD) and long noncoding RNA ARSD-AS1 gene expression in breast cancer patients and their association with oncogenic transcription factors. J. BUON 2020, 25, 1805–1813. [Google Scholar] [PubMed]
- Abdalla, M.A.; Haj-Ahmad, Y. Promising Candidate Urinary MicroRNA Biomarkers for the Early Detection of Hepatocellular Carcinoma among High-Risk Hepatitis C Virus Egyptian Patients. J. Cancer 2012, 3, 19–31. [Google Scholar] [CrossRef]
- Hui, L.; Wu, H.; Yang, N.; Guo, X.; Jang, X. Identification of prognostic microRNA candidates for head and neck squamous cell carcinoma. Oncol. Rep. 2016, 35, 3321–3330. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, X.; Xu, X.; Lu, X. MiR-618 inhibits anaplastic thyroid cancer by repressing XIAP in one ATC cell line. Ann. Endocrinol. 2014, 75, 187–193. [Google Scholar] [CrossRef]
- Yi, L.; Yuan, Y. MicroRNA-618 modulates cell growth via targeting PI3K/Akt pathway in human thyroid carcinomas. Indian J. Cancer 2015, 52 (Suppl. 3), E186–E189. [Google Scholar] [CrossRef]
- Das, P.K.; Asha, S.Y.; Abe, I.; Islam, F.; Lam, A.K. Roles of Non-Coding RNAs on Anaplastic Thyroid Carcinomas. Cancers 2020, 12, 3159. [Google Scholar] [CrossRef] [PubMed]
- Ivanovic, R.F.; Viana, N.I.; Morais, D.R.; Moura, C.; Silva, I.A.; Leite, K.R.; Pontes-Junior, J.; Nahas, W.C.; Srougi, M.; Reis, S.T. miR-618: Possible control over TIMP-1 and its expression in localized prostate cancer. BMC Cancer 2018, 18, 992. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Tang, Y.; Lei, X.H.; Zhao, S.C.; Wu, Z.Q. miR-618 Inhibits Prostate Cancer Migration and Invasion by Targeting FOXP2. J. Cancer 2017, 8, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gong, L.; Chen, L.; Luo, J.; Song, G.; Xu, J.; Lv, Z.; Tao, H.; Xia, Y.; Ye, Z. miR-618 Suppresses Metastasis in Gastric Cancer by Downregulating the Expression of TGF-β2. Anat. Rec. 2019, 302, 931–940. [Google Scholar] [CrossRef]
- Xu, Y.; Pasche, B. TGF-beta signaling alterations and susceptibility to colorectal cancer. Hum. Mol. Genet. 2007, 16, R14–R20. [Google Scholar] [CrossRef] [PubMed]
- Tölle, A.; Jung, M.; Rabenhorst, S.; Kilic, E.; Jung, K.; Weikert, S. Identification of microRNAs in blood and urine as tumour markers for the detection of urinary bladder cancer. Oncol. Rep. 2013, 30, 1949–1956. [Google Scholar] [CrossRef]
- Guo, W.; Yu, Q.; Zhang, M.; Li, F.; Liu, Y.; Jiang, W.; Jiang, H.; Li, H. Long intergenic non-protein coding RNA 511 promotes the progression of osteosarcoma cells through sponging microRNA 618 to upregulate the expression of maelstrom. Aging 2019, 11, 5351–5367. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Zhao, Q.; Wu, D.; Zhang, C.; Zhao, K.; Song, Y.; Gao, C. MicroRNA-618 Directly Targets Metadherin mRNA to Suppress the Malignant Phenotype of Osteosarcoma Cells by Reducing PTEN-AKT Pathway Output. OncoTargets Ther. 2019, 12, 9795–9807. [Google Scholar] [CrossRef]
- Wu, X.; Ajani, J.A.; Gu, J.; Chang, D.W.; Tan, W.; Hildebrandt, M.A.; Huang, M.; Wang, K.K.; Hawk, E. MicroRNA expression signatures during malignant progression from Barrett’s esophagus to esophageal adenocarcinoma. Cancer Prev. Res. 2013, 6, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Hoffman, A.E.; Liu, R.; Jacobs, D.I.; Zheng, T.; Zhu, Y. Targetome profiling and functional genetics implicate miR-618 in lymphomagenesis. Epigenetics 2014, 9, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Koveitypour, Z.; Panahi, F.; Vakilian, M.; Peymani, M.; Forootan, F.S.; Esfahani, M.H.N.; Ghaedi, K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019, 9, 97. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Yu, B.H.; Li, D.L.; Ke, H.L.; Guo, X.Z.; Xiao, X.Y. PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J. Gastroenterol. 2012, 18, 3745–3751. [Google Scholar] [CrossRef]
- Luo, J.; Chen, X.Q.; Li, P. The Role of TGF-β and Its Receptors in Gastrointestinal Cancers. Transl. Oncol. 2019, 12, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Bachman, K.E.; Park, B.H. Duel nature of TGF-beta signaling: Tumor suppressor vs. tumor promoter. Curr. Opin. Oncol. 2005, 17, 49–54. [Google Scholar] [CrossRef]
- Fukada, M.; Matsuhashi, N.; Takahashi, T.; Sugito, N.; Heishima, K.; Akao, Y.; Yoshida, K. Tumor Tissue MIR92a and Plasma MIRs21 and 29a as Predictive Biomarkers Associated with Clinicopathological Features and Surgical Resection in a Prospective Study on Colorectal Cancer Patients. J. Clin. Med. 2020, 9, 2509. [Google Scholar] [CrossRef]
- Cui, C.; Cui, Q. The relationship of human tissue microRNAs with those from body fluids. Sci. Rep. 2020, 10, 5644. [Google Scholar] [CrossRef]
- Takahashi, M.; Cuatrecasas, M.; Balaguer, F.; Hur, K.; Toiyama, Y.; Castells, A.; Boland, C.R.; Goel, A. The clinical significance of MiR-148a as a predictive biomarker in patients with advanced colorectal cancer. PLoS ONE 2012, 7, e46684. [Google Scholar] [CrossRef]
- Tsai, H.L.; Yang, I.P.; Huang, C.W.; Ma, C.J.; Kuo, C.H.; Lu, C.Y.; Juo, S.H.; Wang, J.Y. Clinical significance of microRNA-148a in patients with early relapse of stage II stage and III colorectal cancer after curative resection. Transl. Res. 2013, 162, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Gmerek, L.; Martyniak, K.; Horbacka, K.; Krokowicz, P.; Scierski, W.; Golusinski, P.; Golusinski, W.; Schneider, A.; Masternak, M.M. MicroRNA regulation in colorectal cancer tissue and serum. PLoS ONE 2019, 14, e0222013. [Google Scholar] [CrossRef]
- Shimizu, C.; Kim, J.; Stepanowsky, P.; Trinh, C.; Lau, H.D.; Akers, J.C.; Chen, C.; Kanegaye, J.T.; Tremoulet, A.; Ohno-Machado, L.; et al. Differential expression of miR-145 in children with Kawasaki disease. PLoS ONE 2013, 8, e58159. [Google Scholar] [CrossRef] [PubMed]
- Santos-Bezerra, D.P.; Santos, A.S.; Guimarães, G.C.; Admoni, S.N.; Perez, R.V.; Machado, C.G.; Pelaes, T.S.; Passarelli, M.; Machado, U.F.; Queiroz, M.S.; et al. Micro-RNAs 518d-3p and 618 Are Upregulated in Individuals with Type 1 Diabetes with Multiple Microvascular Complications. Front. Endocrinol. 2019, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Julian, M.W.; Bicer, S.; Ghai, V.; Kim, T.K.; Maier, L.A.; Gillespie, M.; Hamzeh, N.Y.; Wang, K. Circulating exosomal microRNA expression patterns distinguish cardiac sarcoidosis from myocardial ischemia. PLoS ONE 2021, 16, e0246083. [Google Scholar] [CrossRef]
- Feng, X.; Ji, D.; Liang, C.; Fan, S. Does miR-618 rs2682818 variant affect cancer susceptibility? Evidence from 10 case-control studies. Biosci. Rep. 2019, 39, BSR20190741. [Google Scholar] [CrossRef]
- Chen, Y.; Du, M.; Chen, W.; Zhu, L.; Wu, C.; Zhang, Z.; Wang, M.; Chu, H.; Gu, D.; Chen, J. Polymorphism rs2682818 in miR-618 is associated with colorectal cancer susceptibility in a Han Chinese population. Cancer Med. 2018, 7, 1194–1200. [Google Scholar] [CrossRef]
- Zhang, Q.; Miao, Y.; Fu, Q.; Hu, H.; Chen, H.; Zeng, A.; Jin, Y.; Jiang, Y.; Qian, L.; Wu, L.; et al. CircRNACCDC66 regulates cisplatin resistance in gastric cancer via the miR-618/BCL2 axis. Biochem. Biophys. Res. Commun. 2020, 526, 713–720. [Google Scholar] [CrossRef]
- Hsiao, K.Y.; Lin, Y.C.; Gupta, S.K.; Chang, N.; Yen, L.; Sun, H.S.; Tsai, S.J. Noncoding Effects of Circular RNA CCDC66 Promote Colon Cancer Growth and Metastasis. Cancer Res. 2017, 77, 2339–2350. [Google Scholar] [CrossRef] [PubMed]

| Clinicopathological Characteristics | High/Intermediate Expression of miR-618 Number (%) | Low Expression of miR-618 Number (%) | p-Value |
|---|---|---|---|
| Age | 0.863 | ||
| <65 | 42 (66.7) | 21 (33.3) | |
| ≥65 | 28 (68.3) | 13 (31.7) | |
| Sex | 0.149 | ||
| female | 19 (57.6) | 14 (42.4) | |
| male | 51 (71.8) | 20 (28.2) | |
| Liver Metastasis | 0.102 | ||
| yes | 52 (63.4) | 30 (36.6) | |
| no | 18 (81.8) | 4 (18.2) | |
| Peritoneum Metastasis | 0.753 | ||
| yes | 12 (70.6) | 5 (29.4) | |
| no | 58 (66.7) | 29 (33.8) | |
| Lung Metastasis | 0.422 | ||
| yes | 24 (72.7) | 9 (27.3) | |
| no | 46 (64.8) | 25 (35.2) | |
| RAS | 0.721 | ||
| WT | 34 (65.4) | 18 (34.6) | |
| M+ | 33 (68.8) | 15 (31.3) | |
| Primary Tumor Location | 0.469 | ||
| left colon | 54 (69.2) | 24 (30.8) | |
| right colon | 16 (61.5) | 10 (38.5) | |
| Grade | 0.242 | ||
| low | 60 (69.8) | 26 (30.2) | |
| high | 10 (55.6) | 8 (44.4) | |
| PS (ECOG) | 0.382 | ||
| 0 | 31 (72.1) | 12 (27.9) | |
| 1 | 39 (63.9) | 22 (36.1) | |
| CEA | 0.437 | ||
| ≤2 ULN | 26 (72.2) | 10 (27.8) | |
| >2 ULN | 44 (64.7) | 24 (35.3) |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| Age | ||||||
| <65 vs. ≥65 | 1.25 | 0.80–1.95 | 0.322 | 1.22 | 0.72–2.08 | 0.461 |
| Sex | ||||||
| male vs. female | 1.19 | 0.75–1.89 | 0.467 | 0.68 | 0.38–1.20 | 0.182 |
| Liver metastasis | ||||||
| no vs. yes | 0.76 | 0.44–1.31 | 0.318 | 0.77 | 0.41–1.47 | 0.773 |
| Peritoneum metastasis | ||||||
| no vs. yes | 0.86 | 0.49–1.49 | 0.58 | 0.69 | 0.36–1.34 | 0.27 |
| Lung metastasis | ||||||
| no vs. yes | 1.03 | 0.66–1.62 | 0.884 | 0.93 | 0.53–1.61 | 0.783 |
| RAS status | ||||||
| M+ vs. WT | 1.06 | 0.68–1.66 | 0.782 | 0.99 | 0.61–1.60 | 0.969 |
| Primary tumor location | ||||||
| left colon vs. right colon | 0.93 | 0.56–1.53 | 0.768 | 0.95 | 0.53–1.71 | 0.874 |
| Grade | ||||||
| low vs. high | 0.58 | 0.34–0.99 | 0.048 | 0.6 | 0.33–1.11 | 0.104 |
| PS (ECOG) | ||||||
| 0 vs. 1 | 0.64 | 0.41–1.007 | 0.053 | 0.65 | 0.38–1.10 | 0.107 |
| CEA | ||||||
| ≤2 ULN vs. >2 ULN | 0.53 | 0.33–0.84 | 0.007 | 0.58 | 0.34–0.98 | 0.041 |
| miR-618 | ||||||
| high/intermediate vs. low expression | 0.56 | 0.36–0.89 | 0.013 | 0.51 | 0.30–0.86 | 0.012 |
| rs2682818 | mCC Patients, Number | Healthy Controls, Number | Odd | 95% CI | p-Value |
|---|---|---|---|---|---|
| (Proportion) | (Proportion) | Ratio | |||
| Alleles | 0.67 | 0.33–1.33 | 0.25 | ||
| C | 192 (0.92) | 160 (0.89) | |||
| A | 16 (0.08) | 20 (0.11) | |||
| Genotypes | 0.39 | 0.17–0.88 | 0.024 | ||
| Codominant model | |||||
| CC | 91 (0.87) | 70 (0.78) | |||
| AC | 10 (0.10) | 20 (0.22) | |||
| AA | 3 (0.03) | 0 (0.00) | |||
| Dominant model | 0.5 | 0.23–1.07 | 0.076 | ||
| CC vs. | 91 (0.87) | 70 (0.78) | |||
| AC and AA | 13 (0.13) | 20 (0.22) | |||
| Overdominant model | 0.37 | 0.16–0.85 | 0.018 | ||
| CC and AA vs. | 94 (0.90) | 70 (0.78) | |||
| AC | 10 (0.10) | 20 (0.22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radanova, M.; Mihaylova, G.; Mihaylova, Z.; Ivanova, D.; Tasinov, O.; Nazifova-Tasinova, N.; Pavlov, P.; Mirchev, M.; Conev, N.; Donev, I. Circulating miR-618 Has Prognostic Significance in Patients with Metastatic Colon Cancer. Curr. Oncol. 2021, 28, 1204-1215. https://doi.org/10.3390/curroncol28020116
Radanova M, Mihaylova G, Mihaylova Z, Ivanova D, Tasinov O, Nazifova-Tasinova N, Pavlov P, Mirchev M, Conev N, Donev I. Circulating miR-618 Has Prognostic Significance in Patients with Metastatic Colon Cancer. Current Oncology. 2021; 28(2):1204-1215. https://doi.org/10.3390/curroncol28020116
Chicago/Turabian StyleRadanova, Maria, Galya Mihaylova, Zhasmina Mihaylova, Desislava Ivanova, Oskan Tasinov, Neshe Nazifova-Tasinova, Pavel Pavlov, Milko Mirchev, Nikolay Conev, and Ivan Donev. 2021. "Circulating miR-618 Has Prognostic Significance in Patients with Metastatic Colon Cancer" Current Oncology 28, no. 2: 1204-1215. https://doi.org/10.3390/curroncol28020116
APA StyleRadanova, M., Mihaylova, G., Mihaylova, Z., Ivanova, D., Tasinov, O., Nazifova-Tasinova, N., Pavlov, P., Mirchev, M., Conev, N., & Donev, I. (2021). Circulating miR-618 Has Prognostic Significance in Patients with Metastatic Colon Cancer. Current Oncology, 28(2), 1204-1215. https://doi.org/10.3390/curroncol28020116






