The Out-of-Pocket Cost Burden of Cancer Care—A Systematic Literature Review
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Search Strategies
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Data Synthesis
2.5. Quality Assessment
3. Results
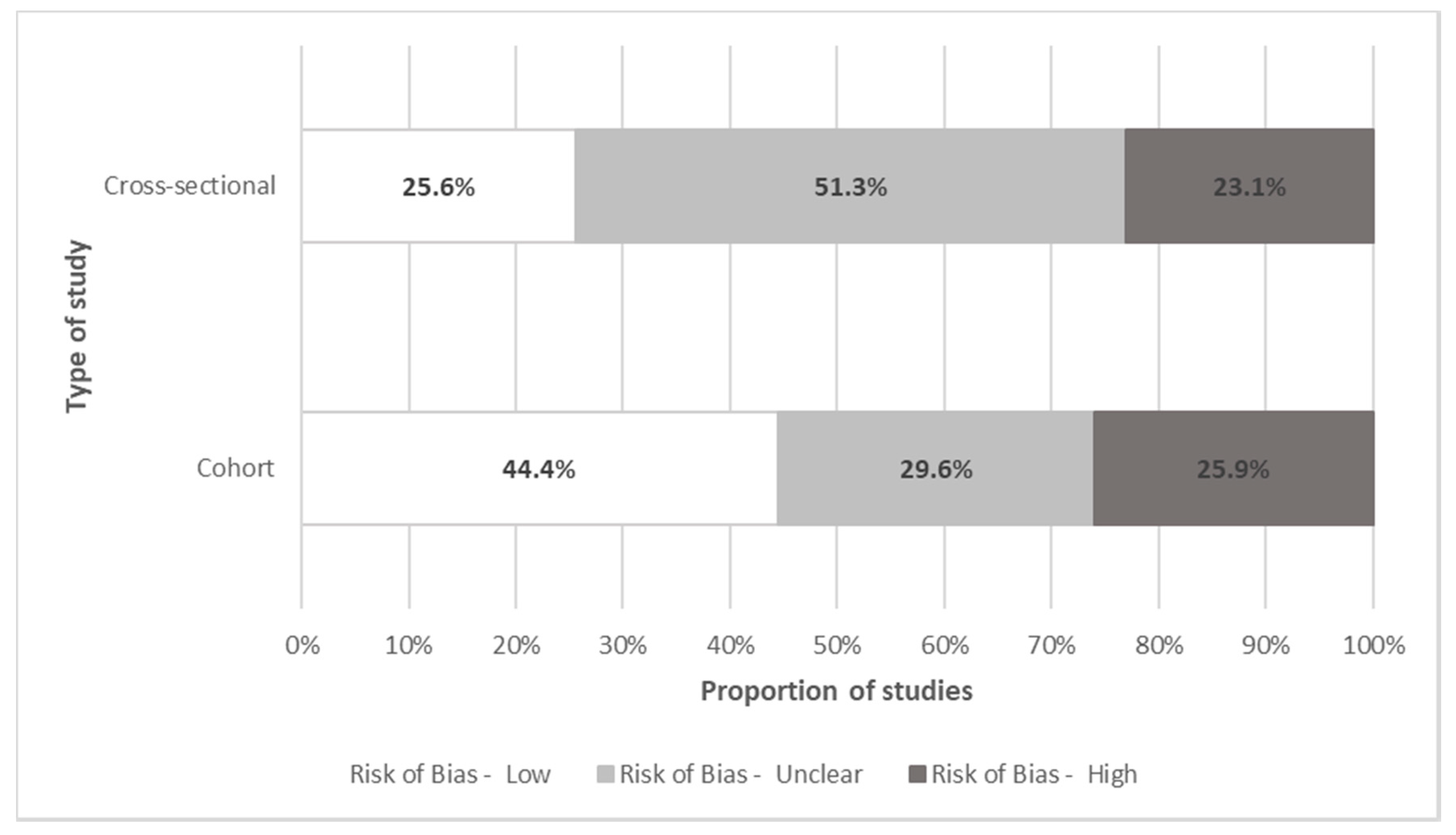
Quality Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef]
- Richard, P.; Walker, R.; Alexandre, P. The burden of out of pocket costs and medical debt faced by households with chronic health conditions in the United States. PLoS ONE 2018, 13, e0199598. [Google Scholar] [CrossRef]
- Landwehr, M.S.; Watson, S.E.; Macpherson, C.F.; Novak, K.A.; Johnson, R.H. The cost of cancer: A retrospective analysis of the financial impact of cancer on young adults. Cancer Med. 2016, 5, 863–870. [Google Scholar] [CrossRef]
- Cancer-Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 22 August 2019).
- Pisu, M.; Azuero, A.; McNees, P.; Burkhardt, J.; Benz, R.; Meneses, K. The out of pocket cost of breast cancer survivors: A review. J. Cancer Surviv. 2010, 4, 202–209. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.; Weir, S.; Rangrej, J.; Krahn, M.D.; Mittmann, N.; Hoch, J.S.; Chan, K.K.W.; Peacock, S. The economic burden of cancer care in Canada: A population-based cost study. CMAJ Open 2018, 6, E1–E10. [Google Scholar] [CrossRef]
- Luengo-Fernandez, R.; Leal, J.; Gray, A.; Sullivan, R. Economic burden of cancer across the European Union: A population-based cost analysis. Lancet Oncol. 2013, 14, 1165–1174. [Google Scholar] [CrossRef]
- Bekelman, J.E.; Sylwestrzak, G.; Barron, J.; Liu, J.; Epstein, A.J.; Freedman, G.; Malin, J.; Emanuel, E.J. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008–2013. JAMA 2014, 312, 2542–2550. [Google Scholar] [CrossRef]
- Longo, C.J.; Fitch, M.; Deber, R.B.; Williams, A.P. Financial and family burden associated with cancer treatment in Ontario, Canada. Support. Care Cancer 2006, 14, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Peppercorn, J.; Sikora, K.; Zalcberg, J.; Meropol, N.J.; Amir, E.; Khayat, D.; Boyle, P.; Autier, P.; Tannock, I.F.; et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011, 12, 933–980. [Google Scholar] [CrossRef]
- Prager, G.W.; Braga, S.; Bystricky, B.; Qvortrup, C.; Criscitiello, C.; Esin, E.; Sonke, G.S.; Martinez, G.A.; Frenel, J.S.; Karamouzis, M.; et al. Global cancer control: Responding to the growing burden, rising costs and inequalities in access. ESMO Open 2018, 3, e000285. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar] [PubMed]
- Higgins, J.P.T.; Green, S. Obtaining standard deviations from standard errors and confidence intervals for group means. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: Chichester, UK, 2011. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- World Bank. Global Economic Monitor; DataBank: Geneva, Switzerland, 2019. [Google Scholar]
- World Bank. World Bank Country and Lending Groups-Country Classification; DataBank: Geneva, Switzerland, 2020. [Google Scholar]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; The Hospital of Ottawa: Ottawa, ON, Canada, 2019. [Google Scholar]
- Ceilleachair, A.O.; Hanly, P.; Skally, M.; O’Leary, E.; O’Neill, C.; Fitzpatrick, P.; Kapur, K.; Staines, A.; Sharp, L. Counting the cost of cancer: Out-of-pocket payments made by colorectal cancer survivors. Support. Care Cancer 2017, 25, 2733–2741. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.; Tsimicalis, A.; Lederman, S.; Bagai, P.; Martiniuk, A.; Srinivas, S.; Arora, R.S. A pilot study to determine out-of-pocket expenditures by families of children being treated for cancer at public hospitals in New Delhi, India. Psychooncology 2019, 4, 4. [Google Scholar] [CrossRef]
- Alexander, L.C.; Erqi, L.P.; Yushen, Q.; Albert, C.K.; Daniel, T.C. Impact of Intensity-Modulated Radiotherapy on Health Care Costs of Patients With Anal Squamous Cell Carcinoma. J. Oncol. Pract. 2017, 13, 992–1001. [Google Scholar] [CrossRef]
- Andreas, S.; Chouaid, C.; Danson, S.; Siakpere, O.; Benjamin, L.; Ehness, R.; Dramard-Goasdoue, M.H.; Barth, J.; Hoffmann, H.; Potter, V.; et al. Economic burden of resected (stage IB-IIIA) non-small cell lung cancer in France, Germany and the United Kingdom: A retrospective observational study (LuCaBIS). Lung Cancer 2018, 124, 298–309. [Google Scholar] [CrossRef]
- Azzani, M.; Roslani, A.; Su, T.; Roslani, A.C.; Su, T.T. Financial burden of colorectal cancer treatment among patients and their families in a middle-income country. Support. Care Cancer 2016, 24, 4423–4432. [Google Scholar] [CrossRef]
- Baili, P.; Di Salvo, F.; de Lorenzo, F.; Maietta, F.; Pinto, C.; Rizzotto, V.; Vicentini, M.; Rossi, P.G.; Tumino, R.; Rollo, P.C.; et al. Out-of-pocket costs for cancer survivors between 5 and 10 years from diagnosis: An Italian population-based study. Support. Care Cancer 2016, 24, 2225–2233. [Google Scholar] [CrossRef]
- Bao, Y.; Maciejewski, R.C.; Garrido, M.M.; Shah, M.A.; Maciejewski, P.K.; Prigerson, H.G. Chemotherapy Use, End-of-Life Care, and Costs of Care Among Patients Diagnosed With Stage IV Pancreatic Cancer. J. Pain Symptom. Manag. 2018, 55, 1113–1121. [Google Scholar] [CrossRef]
- Bargallo-Rocha, J.E.; Soto-Perez-de-Celis, E.; Pico-Guzman, F.J.; Quintero-Rodriguez, C.E.; Almog, D.; Santiago-Concha, G.; Flores-Balcazar, C.H.; Corona, J.; Vazquez-Romo, R.; Villarreal-Garza, C.; et al. The impact of the use of intraoperative radiotherapy on costs, travel time and distance for women with breast cancer in the Mexico City Metropolitan Area. J. Surg. Oncol. 2017, 116, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Basavaiah, G.; Rent, P.D.; Rent, E.G.; Sullivan, R.; Towne, M.; Bak, M.; Sirohi, B.; Goel, M.; Shrikhande, S.V. Financial Impact of Complex Cancer Surgery in India: A Study of Pancreatic Cancer. J. Glob. Oncol. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Bates, N.; Callander, E.; Lindsay, D.; Watt, K. CancerCostMod: A model of the healthcare expenditure, patient resource use, and patient co-payment costs for Australian cancer patients. Health Econ. Rev. 2018, 8, 28. [Google Scholar] [CrossRef]
- Bercow, A.S.; Chen, L.; Chatterjee, S.; Tergas, A.I.; Hou, J.Y.; Burke, W.M.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Cost of Care for the Initial Management of Ovarian Cancer. Obstet. Gynecol. 2017, 130, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Bernard, D.S.; Farr, S.L.; Fang, Z.; Bernard, D.S.M.; Farr, S.L.; Fang, Z. National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. J. Clin. Oncol. 2011, 29, 2821–2826. [Google Scholar] [CrossRef]
- Bloom, B.S.; Knorr, R.S.; Evans, A.E. The epidemiology of disease expenses. The costs of caring for children with cancer. JAMA 1985, 253, 2393–2397. [Google Scholar] [CrossRef]
- Boyages, J.; Xu, Y.; Kalfa, S.; Koelmeyer, L.; Parkinson, B.; Mackie, H.; Viveros, H.; Gollan, P.; Taksa, L. Financial cost of lymphedema borne by women with breast cancer. Psychooncology 2017, 26, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.L.; Kularatna, S.; Ward, E.C.; Hill, A.J.; Byrnes, J.; Kenny, L.M. Cost analysis of a speech pathology synchronous telepractice service for patients with head and neck cancer. Head. Neck 2017, 39, 2470–2480. [Google Scholar] [CrossRef]
- Buttner, M.; Konig, H.H.; Lobner, M.; Briest, S.; Konnopka, A.; Dietz, A.; Riedel-Heller, S.; Singer, S. Out-of-pocket-payments and the financial burden of 502 cancer patients of working age in Germany: Results from a longitudinal study. Support. Care Cancer 2018, 12, 12. [Google Scholar] [CrossRef]
- Calhoun, E.A.; Chang, C.H.; Welshman, E.E.; Fishman, D.A.; Lurain, J.R.; Bennett, C.L. Evaluating the total costs of chemotherapy-induced toxicity: Results from a pilot study with ovarian cancer patients. Oncologist 2001, 6, 441–445. [Google Scholar] [CrossRef]
- Callander, E.; Bates, N.; Lindsay, D.; Larkins, S.; Topp, S.M.; Cunningham, J.; Sabesan, S.; Garvey, G. Long-term out of pocket expenditure of people with cancer: Comparing health service cost and use for indigenous and non-indigenous people with cancer in Australia. Int. J. Equity Health 2019, 18, 32. [Google Scholar] [CrossRef]
- Chan, S.; Bo, Z.; Lei, L.; Shih, Y.-C.T. Financial Burden for Patients With Chronic Myeloid Leukemia Enrolled in Medicare Part D Taking Targeted Oral Anticancer Medications. J. Oncol. Pract. 2017, 13, e152–e162. [Google Scholar] [CrossRef]
- Chang, S.; Long, S.R.; Kutikova, L.; Bowman, L.; Finley, D.; Crown, W.H.; Bennett, C.L. Estimating the cost of cancer: Results on the basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999 to 2000. J. Clin. Oncol. 2004, 22, 3524–3530. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Prinja, S.; Ghoshal, S.; Verma, R.; Oinam, A.S. Cost of treatment for head and neck cancer in India. PLoS ONE 2018, 13, e0191132. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.L.; Bentley, J.P.; Pollom, E.L. The impact of state parity laws on copayments for and adherence to oral endocrine therapy for breast cancer. Cancer 2019, 125, 374–381. [Google Scholar] [CrossRef]
- Chino, F.; Peppercorn, J.M.; Rushing, C.; Nicolla, J.; Kamal, A.H.; Altomare, I.; Samsa, G.; Zafar, S.Y. Going for Broke: A Longitudinal Study of Patient-Reported Financial Sacrifice in Cancer Care. J. Oncol. Pract. 2018, 14, e533–e546. [Google Scholar] [CrossRef]
- Cohn, R.J.; Goodenough, B.; Foreman, T.; Suneson, J. Hidden financial costs in treatment for childhood cancer: An Australian study of lifestyle implications for families absorbing out-of-pocket expenses. J. Pediatr. Hematol. Oncol. 2003, 25, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Colby, M.S.; Esposito, D.; Goldfarb, S.; Ball, D.E.; Herrera, V.; Conwell, L.J.; Garavaglia, S.B.; Meadows, E.S.; Marciniak, M.D. Medication discontinuation and reinitiation among Medicare part D beneficiaries taking costly medications. Am. J. Pharm. Benefits 2011, 3, e102–e110. [Google Scholar]
- Collins, D.C.; Coghlan, M.; Hennessy, B.T.; Grogan, L.; Morris, P.G.; Breathnach, O.S. The impact of outpatient systemic anti-cancer treatment on patient costs and work practices. Ir. J. Med. Sci. 2017, 186, 81–87. [Google Scholar] [CrossRef]
- Darkow, T.; Maclean, J.R.; Joyce, G.F.; Goldman, D.; Lakdawalla, D.N. Coverage and Use of Cancer Therapies in the Treatment of Chronic Myeloid Leukemia. Am. J. Manag. Care 2012, 18, S272–S278. [Google Scholar]
- Davidoff, A.J.; Erten, M.; Shaffer, T.; Shoemaker, J.S.; Zuckerman, I.H.; Pandya, N.; Tai, M.H.; Ke, X.; Stuart, B. Out-of-pocket health care expenditure burden for Medicare beneficiaries with cancer. Cancer 2013, 119, 1257–1265. [Google Scholar] [CrossRef]
- de Oliveira, C.; Bremner, K.E.; Ni, A.; Alibhai, S.M.; Laporte, A.; Krahn, M.D. Patient time and out-of-pocket costs for long-term prostate cancer survivors in Ontario, Canada. J. Cancer Surviv. 2014, 8, 9–20. [Google Scholar] [CrossRef]
- de Souza, J.A.; Kung, S.; O’Connor, J.; Yap, B.J. Determinants of Patient-Centered Financial Stress in Patients With Locally Advanced Head and Neck Cancer. J. Oncol. Pract. 2017, 13, e310–e318. [Google Scholar] [CrossRef]
- Dean, L.T.; Moss, S.L.; Ransome, Y.; Frasso-Jaramillo, L.; Zhang, Y.; Visvanathan, K.; Nicholas, L.H.; Schmitz, K.H. “It still affects our economic situation”: Long-term economic burden of breast cancer and lymphedema. Support. Care Cancer 2019, 27, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Doshi, J.A.; Pengxiang, L.; Hairong, H.; Pettit, A.R.; Kumar, R.; Weiss, B.M.; Huntington, S.F. High Cost Sharing and Specialty Drug Initiation Under Medicare Part D: A Case Study in Patients With Newly Diagnosed Chronic Myeloid Leukemia. Am. J. Manag. Care 2016, 22, S78–S86. [Google Scholar] [PubMed]
- Dumont, S.; Jacobs, P.; Turcotte, V.; Turcotte, S.; Johnston, G. Palliative care costs in Canada: A descriptive comparison of studies of urban and rural patients near end of life. Palliat. Med. 2015, 29, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Dusetzina, S.B.; Huskamp, H.A.; Winn, A.N.; Basch, E.; Keating, N.L. Out-of-Pocket and Health Care Spending Changes for Patients Using Orally Administered Anticancer Therapy After Adoption of State Parity Laws. JAMA Oncol. 2018, 4, e173598. [Google Scholar] [CrossRef] [PubMed]
- Dusetzina, S.B.; Keating, N.L. Mind the Gap: Why Closing the Doughnut Hole Is Insufficient for Increasing Medicare Beneficiary Access to Oral Chemotherapy. J. Clin. Oncol. 2016, 34, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Dusetzina, S.B.; Winn, A.N.; Abel, G.A.; Huskamp, H.A.; Keating, N.L. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J. Clin. Oncol. 2014, 32, 306–311. [Google Scholar] [CrossRef]
- Ezeife, D.A.; Morganstein, B.J.; Lau, S.; Law, J.H.; Le, L.W.; Bredle, J.; Cella, D.; Doherty, M.K.; Bradbury, P.; Liu, G.; et al. Financial Burden Among Patients With Lung Cancer in a Publically Funded Health Care System. Clin. Lung Cancer 2018, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Farias, A.J.; Hansen, R.N.; Zeliadt, S.B.; Ornelas, I.J.; Li, C.I.; Thompson, B. The Association Between Out-of-Pocket Costs and Adherence to Adjuvant Endocrine Therapy Among Newly Diagnosed Breast Cancer Patients. Am. J. Clin. Oncol. 2018, 41, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Tangka, F.K.; Trogdon, J.G.; Sabatino, S.A.; Richardson, L.C. The personal financial burden of cancer for the working-aged population. Am. J. Manag. Care 2009, 15, 801–806. [Google Scholar]
- Geynisman, D.M.; Meeker, C.R.; Doyle, J.L.; Handorf, E.A.; Bilusic, M.; Plimack, E.R.; Wong, Y.N. Provider and patient burdens of obtaining oral anticancer medications. Am. J. Manag. Care 2018, 24, e128–e133. [Google Scholar] [PubMed]
- Ghatak, N.; Trehan, A.; Bansal, D. Financial burden of therapy in families with a child with acute lymphoblastic leukemia: Report from north India. Support. Care Cancer 2016, 24, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H.; Niu, J.; Chavez-MacGregor, M.; Zhao, H.; Zorzi, D.; Shih, Y.C.T.; Smith, B.D.; Shen, C. Estimating regimen-specific costs of chemotherapy for breast cancer: Observational cohort study. Cancer 2016, 122, 3447–3455. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.A.; Coleman, E.A.; Sullivan, E.; Easley, R.; McNatt, P.K.; Chowdhury, N.; Stewart, C.B. Personal Financial Effects of Multiple Myeloma and Its Treatment. Cancer Nurs. 2013, 36, 301–308. [Google Scholar] [CrossRef]
- Gordon, L.; Scuffham, P.; Hayes, S.; Newman, B. Exploring the economic impact of breast cancers during the 18 months following diagnosis. Psychooncology 2007, 16, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.G.; Elliott, T.M.; Olsen, C.M.; Pandeya, N.; Whiteman, D.C. Multiplicity of skin cancers in Queensland and their cost burden to government and patients. Aust. N. Z. J. Public Health 2018, 42, 86–91. [Google Scholar] [CrossRef]
- Gordon, L.G.; Elliott, T.M.; Olsen, C.M.; Pandeya, N.; Whiteman, D.C. Patient out-of-pocket medical expenses over 2 years among Queenslanders with and without a major cancer. Aust. J. Prim. Health 2018, 24, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.G.; Ferguson, M.; Chambers, S.K.; Dunn, J. Fuel, beds, meals and meds: Out-of-pocket expenses for patients with cancer in rural Queensland. Cancer Forum. 2009, 33, 202–208. [Google Scholar]
- Gordon, L.G.; Walker, S.M.; Mervin, M.C.; Lowe, A.; Smith, D.P.; Gardiner, R.A.; Chambers, S.K. Financial toxicity: A potential side effect of prostate cancer treatment among Australian men. Eur. J. Cancer Care 2017, 26. [Google Scholar] [CrossRef]
- Grange, F.; Mohr, P.; Harries, M.; Ehness, R.; Benjamin, L.; Siakpere, O.; Barth, J.; Stapelkamp, C.; Pfersch, S.; McLeod, L.D.; et al. Economic burden of advanced melanoma in France, Germany and the UK: A retrospective observational study (Melanoma Burden-of-Illness Study). Melanoma Res. 2017, 27, 607–618. [Google Scholar] [CrossRef]
- Group, A.S.; Jan, S.; Kimman, M.; Peters, S.A.; Woodward, M. Financial catastrophe, treatment discontinuation and death associated with surgically operable cancer in South-East Asia: Results from the ACTION Study. Surgery 2015, 157, 971–982. [Google Scholar] [CrossRef]
- Group, T.A.S. Policy and priorities for national cancer control planning in low- and middle-income countries: Lessons from the Association of Southeast Asian Nations (ASEAN) Costs in Oncology prospective cohort study. Eur. J. Cancer 2017, 74, 26–37. [Google Scholar] [CrossRef]
- Gupta, S.; Abouzaid, S.; Liebert, R.; Parikh, K.; Ung, B.; Rosenberg, A.S. Assessing the Effect of Adherence on Patient-reported Outcomes and Out of Pocket Costs Among Patients With Multiple Myeloma. Clin. Lymphoma Myeloma Leuk 2018, 18, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P., Jr.; Yabroff, K.R.; Ekwueme, D.U.; Virgo, K.S.; Han, X.; Banegas, M.P.; Soni, A.; Zheng, Z.; Chawla, N.; Geiger, A.M. Healthcare Expenditure Burden Among Non-elderly Cancer Survivors, 2008–2012. Am. J. Prev. Med. 2015, 49, S489–S497. [Google Scholar] [CrossRef]
- Hanly, P.; Ceilleachair, A.O.; Skally, M.; O’Leary, E.; Kapur, K.; Fitzpatrick, P.; Staines, A.; Sharp, L. How much does it cost to care for survivors of colorectal cancer? Caregiver’s time, travel and out-of-pocket costs. Support. Care Cancer 2013, 21, 2583–2592. [Google Scholar] [CrossRef]
- Hess, L.M.; Cui, Z.L.; Wu, Y.; Fang, Y.; Gaynor, P.J.; Oton, A.B. Current and projected patient and insurer costs for the care of patients with non-small cell lung cancer in the United States through 2040. J. Med. Econ. 2017, 20, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Housser, E.; Mathews, M.; Le Messurier, J.; Young, S.; Hawboldt, J.; West, R. Responses by breast and prostate cancer patients to out-of-pocket costs in Newfoundland and Labrador. Curr. Oncol. 2013, 20, 158–165. [Google Scholar] [CrossRef]
- Houts, P.S.; Lipton, A.; Harvey, H.A.; Martin, B.; Simmonds, M.A.; Dixon, R.H.; Longo, S.; Andrews, T.; Gordon, R.A.; Meloy, J. Nonmedical costs to patients and their families associated with outpatient chemotherapy. Cancer 1984, 53, 2388–2392. [Google Scholar] [CrossRef]
- Huang, H.Y.; Shi, J.F.; Guo, L.W.; Bai, Y.N.; Liao, X.Z.; Liu, G.X.; Mao, A.Y.; Ren, J.S.; Sun, X.J.; Zhu, X.Y.; et al. Expenditure and financial burden for the diagnosis and treatment of colorectal cancer in China: A hospital-based, multicenter, cross-sectional survey. Cancer Commun. 2017, 36, 41. [Google Scholar] [CrossRef]
- Isshiki, T. Outpatient treatment costs and their potential impact on cancer care. Cancer Med. 2014, 3, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.U.; Jacobs, D.; Stell, B.V.; Grimm, S.A.; Rademaker, A.; Rice, L.; Schwartz, M.A.; Chandler, J.; Muro, K.; Marymont, M.H.; et al. Financial burden experienced by patients undergoing treatment for malignant gliomas: Updated data. J. Clin. Oncol. Conf. ASCO Annu. Meet. 2011, 29, e19571. [Google Scholar] [CrossRef] [PubMed]
- Jagsi, R.; Pottow, J.A.; Griffith, K.A.; Bradley, C.; Hamilton, A.S.; Graff, J.; Katz, S.J.; Hawley, S.T. Long-term financial burden of breast cancer: Experiences of a diverse cohort of survivors identified through population-based registries. J. Clin. Oncol. 2014, 32, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Jagsi, R.; Ward, K.C.; Abrahamse, P.; Wallner, L.P.; Kurian, A.W.; Hamilton, A.S.; Katz, S.J.; Hawley, S.T. Unmet need for clinician engagement about financial toxicity after diagnosis of breast cancer. J. Clin. Oncol. Conf. 2018, 36. [Google Scholar] [CrossRef]
- Jayadevappa, R.; Schwartz, J.S.; Chhatre, S.; Gallo, J.J.; Wein, A.J.; Malkowicz, S.B. The burden of out-of-pocket and indirect costs of prostate cancer. Prostate 2010, 70, 1255–1264. [Google Scholar] [CrossRef]
- Jeah, J.; Wendy Yi, X.; Chelim, C. In-Gap Discounts in Medicare Part D and Specialty Drug Use. Am. J. Manag. Care 2017, 23, 553–559. [Google Scholar]
- John, G.; Hershman, D.; Falci, L.; Shi, Z.; Tsai, W.-Y.; Greenlee, H.; John, G.M.; Hershman, D.L. Complementary and alternative medicine use among US cancer survivors. J. Cancer Surviv. 2016, 10, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Kaisaeng, N.; Harpe, S.E.; Carroll, N.V. Out-of-pocket costs and oral cancer medication discontinuation in the elderly. J. Manag. Care Spec. Pharm. 2014, 20, 669–675. [Google Scholar] [CrossRef]
- Kircher, S.M.; Johansen, M.; Davis, M.M. Impact of Medicare Part D on out-of-pocket drug costs and utilization for patients with cancer. J. Clin. Oncol. 2013, 31, 275. [Google Scholar] [CrossRef]
- Kodama, Y.; Morozumi, R.; Matsumura, T.; Kishi, Y.; Murashige, N.; Tanaka, Y.; Takita, M.; Hatanaka, N.; Kusumi, E.; Kami, M.; et al. Increased financial burden among patients with chronic myelogenous leukaemia receiving imatinib in Japan: A retrospective survey. BMC Cancer 2012, 12, 152. [Google Scholar] [CrossRef]
- Koskinen, J.P.; Farkkila, N.; Sintonen, H.; Saarto, T.; Taari, K.; Roine, R.P. The association of financial difficulties and out-of-pocket payments with health-related quality of life among breast, prostate and colorectal cancer patients. Acta Oncol 2019. [Google Scholar] [CrossRef] [PubMed]
- Langa, K.M.; Fendrick, A.M.; Chernew, M.E.; Kabeto, M.U.; Paisley, K.L.; Hayman, J.A. Out-of-pocket health-care expenditures among older Americans with cancer. Value Health 2004, 7, 186–194. [Google Scholar] [CrossRef]
- Lansky, S.B.; Cairns, N.U.; Clark, G.M.; Lowman, J.; Miller, L.; Trueworthy, R. Childhood cancer: Nonmedical costs of the illness. Cancer 1979, 43, 403–408. [Google Scholar] [CrossRef]
- Lauzier, S.; Levesque, P.; Mondor, M.; Drolet, M.; Coyle, D.; Brisson, J.; Masse, B.; Provencher, L.; Robidoux, A.; Maunsell, E. Out-of-pocket costs in the year after early breast cancer among Canadian women and spouses. J. Natl. Cancer Inst. 2013, 105, 280–292. [Google Scholar] [CrossRef]
- Leopold, C.; Wagner, A.K.; Zhang, F.; Lu, C.Y.; Earle, C.C.; Nekhlyudov, L.; Ross-Degnan, D.; Wharam, J.F. Total and out-of-pocket expenditures among women with metastatic breast cancer in low-deductible versus high-deductible health plans. Breast Cancer Res. Treat. 2018, 171, 449–459. [Google Scholar] [CrossRef]
- Liao, X.Z.; Shi, J.F.; Liu, J.S.; Huang, H.Y.; Guo, L.W.; Zhu, X.Y.; Xiao, H.F.; Wang, L.; Bai, Y.N.; Liu, G.X.; et al. Medical and non-medical expenditure for breast cancer diagnosis and treatment in China: A multicenter cross-sectional study. Asia Pac J. Clin. Oncol. 2018, 14, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.J.; Bereza, B.G. A comparative analysis of monthly out-of-pocket costs for patients with breast cancer as compared with other common cancers in Ontario, Canada. Curr. Oncol. 2011, 18, e1–e8. [Google Scholar] [CrossRef]
- Mahal, A.; Karan, A.; Fan, V.Y.; Engelgau, M. The economic burden of cancers on Indian households. PLoS ONE 2013, 8, e71853. [Google Scholar] [CrossRef]
- Markman, M.; Luce, R. Impact of the cost of cancer treatment: An internet-based survey. J. Oncol. Pract. 2010, 6, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Marti, J.; Hall, P.S.; Hamilton, P.; Hulme, C.T.; Jones, H.; Velikova, G.; Ashley, L.; Wright, P. The economic burden of cancer in the UK: A study of survivors treated with curative intent. Psychooncology 2016, 25, 77–83. [Google Scholar] [CrossRef]
- Massa, S.T.; Osazuwa-Peters, N.; Adjei Boakye, E.; Walker, R.J.; Ward, G.M. Comparison of the Financial Burden of Survivors of Head and Neck Cancer With Other Cancer Survivors. JAMA Otolaryngol. Head Neck Surg. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.K.; Nicholas, L.H. Out-of-Pocket Spending and Financial Burden among Medicare Beneficiaries with Cancer. JAMA Oncol. 2017, 3, 757–765. [Google Scholar] [CrossRef]
- Newton, J.C.; Johnson, C.E.; Hohnen, H.; Bulsara, M.; Ives, A.; McKiernan, S.; Platt, V.; McConigley, R.; Slavova-Azmanova, N.S.; Saunders, C. Out-of-pocket expenses experienced by rural Western Australians diagnosed with cancer. Support. Care Cancer 2018, 26, 3543–3552. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, A.J.; Dusetzina, S.B.; Eaton, C.B.; Davidoff, A.J.; Trivedi, A.N. Subsidies for Oral Chemotherapy and Use of Immunomodulatory Drugs Among Medicare Beneficiaries With Myeloma. J. Clin. Oncol. 2017, 35, 3306–3314. [Google Scholar] [CrossRef]
- O’Neill, K.M.; Mandigo, M.; Pyda, J.; Nazaire, Y.; Greenberg, S.L.; Gillies, R.; Damuse, R. Out-of-pocket expenses incurred by patients obtaining free breast cancer care in Haiti. Lancet 2015, 385 (Suppl. 2), S48. [Google Scholar] [CrossRef]
- Pisu, M.; Azuero, A.; Benz, R.; McNees, P.; Meneses, K. Out-of-pocket costs and burden among rural breast cancer survivors. Cancer Med. 2017, 6, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Pisu, M.; Azuero, A.; Meneses, K.; Burkhardt, J.; McNees, P. Out of pocket cost comparison between Caucasian and minority breast cancer survivors in the Breast Cancer Education Intervention (BCEI). Breast Cancer Res. Treat 2011, 127, 521–529. [Google Scholar] [CrossRef][Green Version]
- Raborn, M.L.; Pelletier, E.M.; Smith, D.B.; Reyes, C.M. Patient Out-of-Pocket Payments for Oral Oncolytics: Results From a 2009 US Claims Data Analysis. J. Oncol. Pract. 2012, 8, 9s–15s. [Google Scholar] [CrossRef]
- Roberts, M.C.; Dusetzina, S.B. Use and Costs for Tumor Gene Expression Profiling Panels in the Management of Breast Cancer From 2006 to 2012: Implications for Genomic Test Adoption Among Private Payers. J. Oncol. Pract. 2015, 11, 273–277. [Google Scholar] [CrossRef]
- Sculpher, M.; Palmer, M.K.; Heyes, A. Costs incurred by patients undergoing advanced colorectal cancer therapy. A comparison of raltitrexed and fluorouracil plus folinic acid. Pharmacoeconomics 2000, 17, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.C.; Smieliauskas, F.; Geynisman, D.M.; Kelly, R.J.; Smith, T.J. Trends in the Cost and Use of Targeted Cancer Therapies for the Privately Insured Nonelderly: 2001 to 2011. J. Clin. Oncol. 2015, 33, 2190–2196. [Google Scholar] [CrossRef]
- Shiroiwa, T.; Fukuda, T.; Tsutani, K. Out-of-pocket payment and cost-effectiveness of XELOX and XELOX plus bevacizumab therapy: From the perspective of metastatic colorectal cancer patients in Japan. Int. J. Clin. Oncol. 2010, 15, 256–262. [Google Scholar] [CrossRef]
- Sneha, L.M.; Sai, J.; Ashwini, S.; Ramaswamy, S.; Rajan, M.; Scott, J.X. Financial Burden Faced by Families due to Out-of-pocket Expenses during the Treatment of their Cancer Children: An Indian Perspective. Indian J. Med. Paediatr. Oncol. 2017, 38, 4–9. [Google Scholar] [CrossRef]
- Stommel, M.; Given, C.W.; Given, B.A. The cost of cancer home care to families. Cancer 1993, 71, 1867–1874. [Google Scholar] [CrossRef]
- Suidan, R.S.; He, W.; Sun, C.C.; Zhao, H.; Rauh-Hain, J.A.; Fleming, N.D.; Lu, K.H.; Giordano, S.H.; Meyer, L.A. Total and out-of-pocket costs of different primary management strategies in ovarian cancer. Am. J. Obstet. Gynecol. 2019, 6, 6. [Google Scholar] [CrossRef]
- Tangka, F.K.; Trogdon, J.G.; Richardson, L.C.; Howard, D.; Sabatino, S.A.; Finkelstein, E.A. Cancer treatment cost in the United States: Has the burden shifted over time? Cancer 2010, 116, 3477–3484. [Google Scholar] [CrossRef]
- Thompson, B.S.; Pandeya, N.; Olsen, C.M.; Dusingize, J.C.; Green, A.C.; Neale, R.E.; Whiteman, D.C. Keratinocyte cancer excisions in Australia: Who performs them and associated costs. Australas. J. Dermatol. 2019, 22, 22. [Google Scholar] [CrossRef]
- Tomic, K.; Long, S.; Li, X.; Fu, A.C.; Yu, T.C.; Barron, R. A retrospective study of patients’ out-of-pocket costs for granulocyte colony-stimulating factors. J. Oncol. Pharm. Pract. 2013, 19, 328–337. [Google Scholar] [CrossRef]
- Tsimicalis, A.; Stevens, B.; Ungar, W.J.; Greenberg, M.; McKeever, P.; Agha, M.; Guerriere, D.; Barr, R.; Naqvi, A.; Moineddin, R. Determining the costs of families’ support networks following a child’s cancer diagnosis. Cancer Nurs. 2013, 36, E8–E19. [Google Scholar] [CrossRef] [PubMed]
- Tsimicalis, A.; Stevens, B.; Ungar, W.J.; McKeever, P.; Greenberg, M.; Agha, M.; Guerriere, D.; Barr, R.; Naqvi, A.; Moineddin, R. A prospective study to determine the costs incurred by families of children newly diagnosed with cancer in Ontario. Psychooncology 2012, 21, 1113–1123. [Google Scholar] [CrossRef]
- Van Houtven, C.H.; Ramsey, S.D.; Hornbrook, M.C.; Atienza, A.A.; van Ryn, M. Economic burden for informal caregivers of lung and colorectal cancer patients. Oncologist 2010, 15, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Wong, M.; Hsu, L.Y.; Chan, A. Costs associated with febrile neutropenia in solid tumor and lymphoma patients-an observational study in Singapore. BMC Health Serv. Res. 2014, 14, 434. [Google Scholar] [CrossRef] [PubMed]
- Wenhui, M.; Shenglan, T.; Ying, Z.; Zening, X.; Wen, C. Financial burden of healthcare for cancer patients with social medical insurance: A multi-centered study in urban China. Int. J. Equity Health 2017, 16, 1–12. [Google Scholar] [CrossRef]
- Wood, R.; Taylor-Stokes, G. Cost burden associated with advanced non-small cell lung cancer in Europe and influence of disease stage. BMC Cancer 2019, 19, 214. [Google Scholar] [CrossRef]
- Ya-Chen Tina, S.; Ying, X.; Lei, L.; Smieliauskas, F.; Shih, Y.-C.T.; Xu, Y.; Liu, L. Rising Prices of Targeted Oral Anticancer Medications and Associated Financial Burden on Medicare Beneficiaries. J. Clin. Oncol. 2017, 35, 2482–2489. [Google Scholar] [CrossRef]
- Yu, M.; Guerriere, D.N.; Coyte, P.C. Societal costs of home and hospital end-of-life care for palliative care patients in Ontario, Canada. Health Soc. Care Community 2015, 23, 605–618. [Google Scholar] [CrossRef]
- Faiman, B. Oral cancer therapy: Policy implications for the uninsured and underinsured populations. J. Adv. Pract. Oncol. 2013, 4, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, V.; Ramsey, S. Addressing the Financial Burden of Cancer Treatment: From Copay to Can’t Pay. JAMA Oncol. 2015, 1, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Essue, B.M.; Birch, S. Blunt Policy Instruments Deliver Blunt Policy Outcomes: Why Cost Sharing is Not Effective at Controlling Utilization and Improving Health System Efficiency. Med. Care 2016, 54, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Sirintrapun, S.J.; Lopez, A.M. Telemedicine in Cancer Care. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 540–545. [Google Scholar] [CrossRef]
- Bentur, N.; Resnizky, S.; Balicer, R.; Eilat-Tsanani, T. Quality of end-of-life care for cancer patients: Does home hospice care matter? Am. J. Manag. Care 2014, 20, 988–992. [Google Scholar]
- Tucker-Seeley, R.D.; Yabroff, K.R. Minimizing the “financial toxicity” associated with cancer care: Advancing the research agenda. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Altice, C.K.; Banegas, M.P.; Tucker-Seeley, R.D.; Yabroff, K.R. Financial Hardships Experienced by Cancer Survivors: A Systematic Review. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Gordon, L.G.; Merollini, K.M.D.; Lowe, A.; Chan, R.J. A Systematic Review of Financial Toxicity Among Cancer Survivors: We Can’t Pay the Co-Pay. Patient 2017, 10, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Diller, L. Late Effects of Childhood Cancer; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Jan, S.; Essue, B.M.; Leeder, S.R. Falling through the cracks: The hidden economic burden of chronic illness and disability on Australian households. Med. J. Aust. 2012, 196, 29–31. [Google Scholar] [CrossRef]
- Sanyal, C.; Aprikian, A.G.; Chevalier, S.; Cury, F.L.; Dragomir, A. Direct cost for initial management of prostate cancer: A systematic review. Curr. Oncol. 2013, 20, e522–e531. [Google Scholar] [CrossRef][Green Version]
- Meeker, C.R.; Geynisman, D.M.; Egleston, B.L.; Hall, M.J.; Mechanic, K.Y.; Bilusic, M.; Plimack, E.R.; Martin, L.P.; von Mehren, M.; Lewis, B.; et al. Relationships Among Financial Distress, Emotional Distress, and Overall Distress in Insured Patients With Cancer. J. Oncol. Pract. 2016, 12, e755–e764. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Lund, J.; Kepka, D.; Mariotto, A. Economic burden of cancer in the United States: Estimates, projections, and future research. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2006–2014. [Google Scholar] [CrossRef]
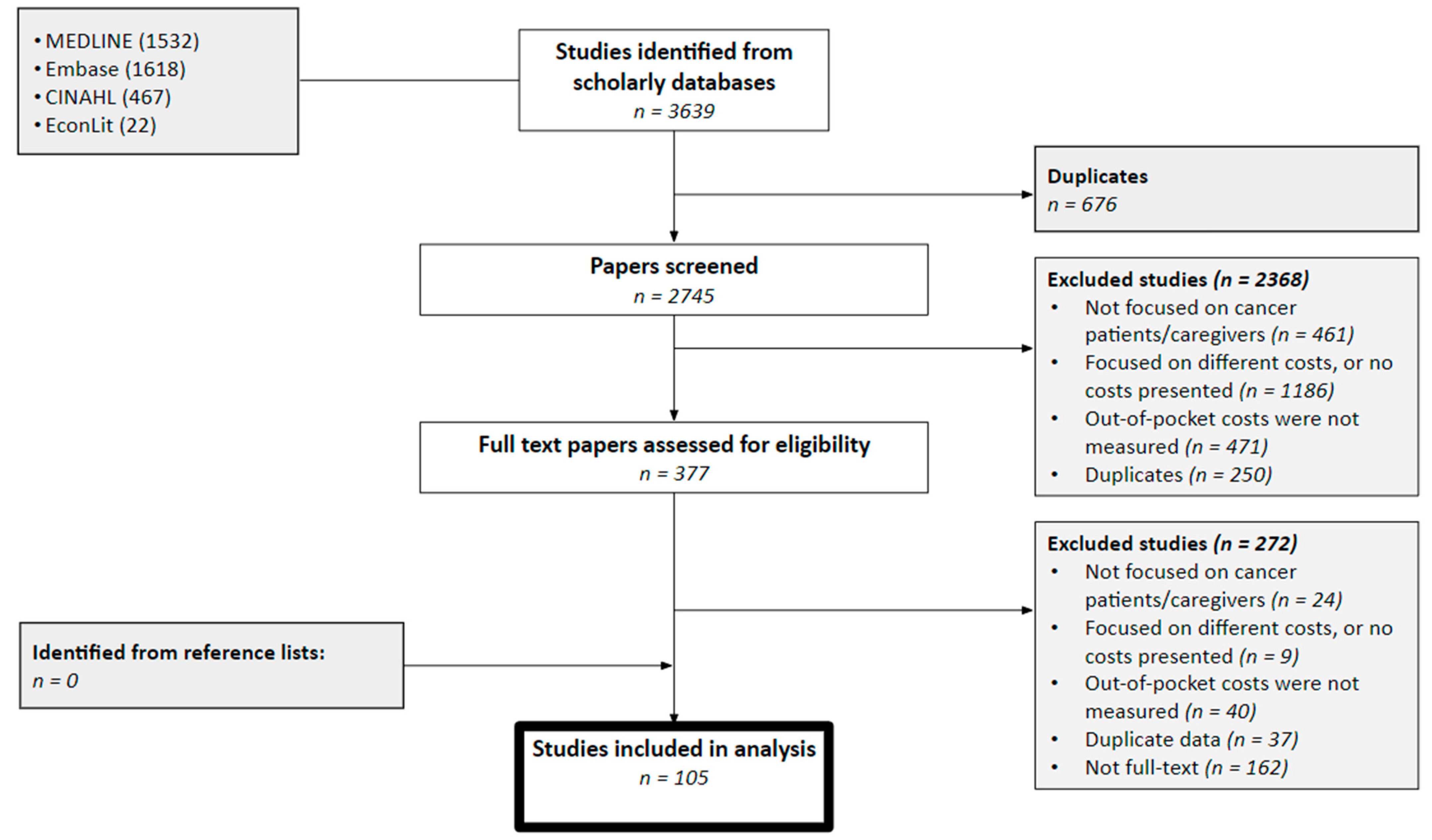
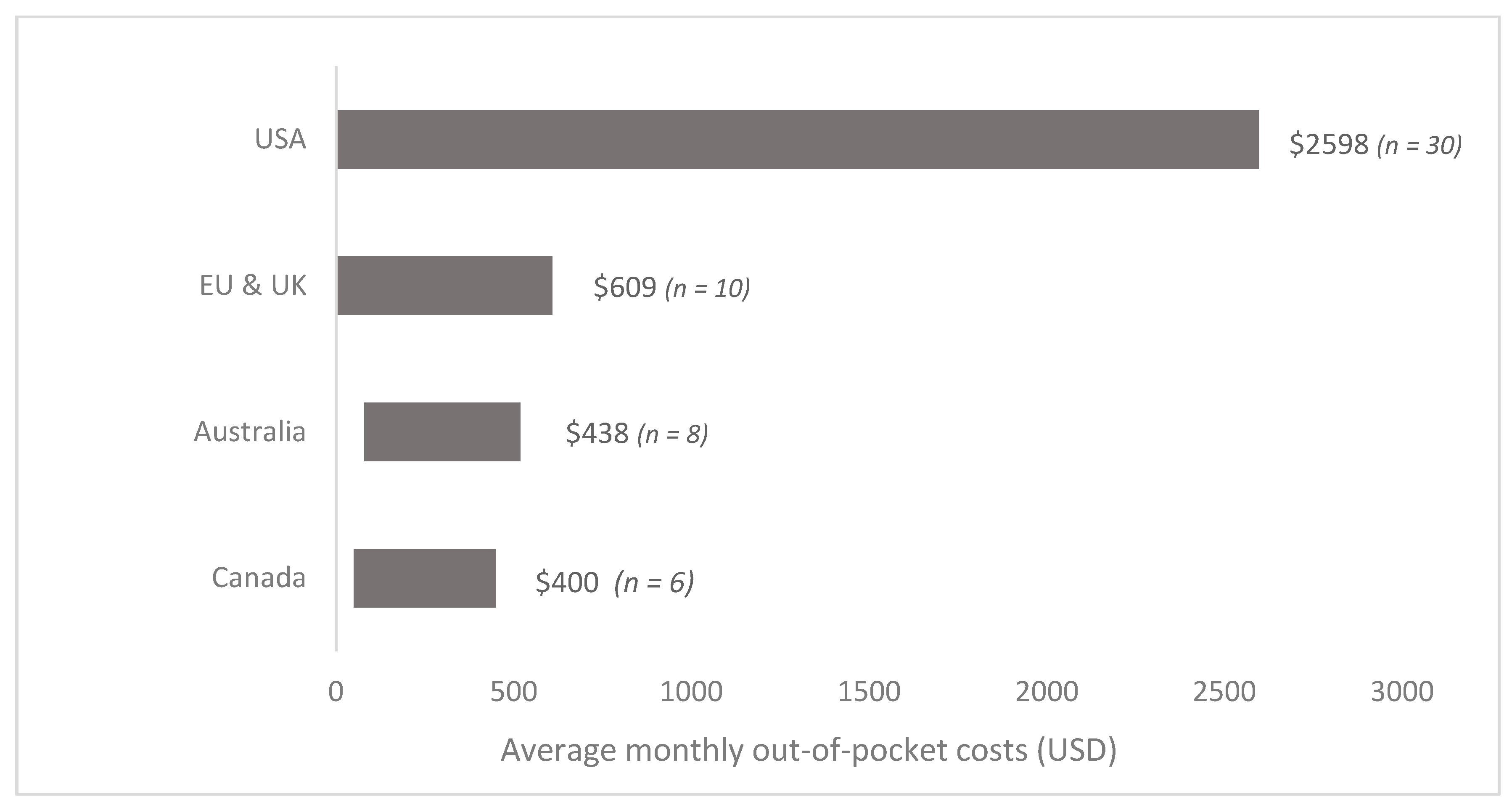
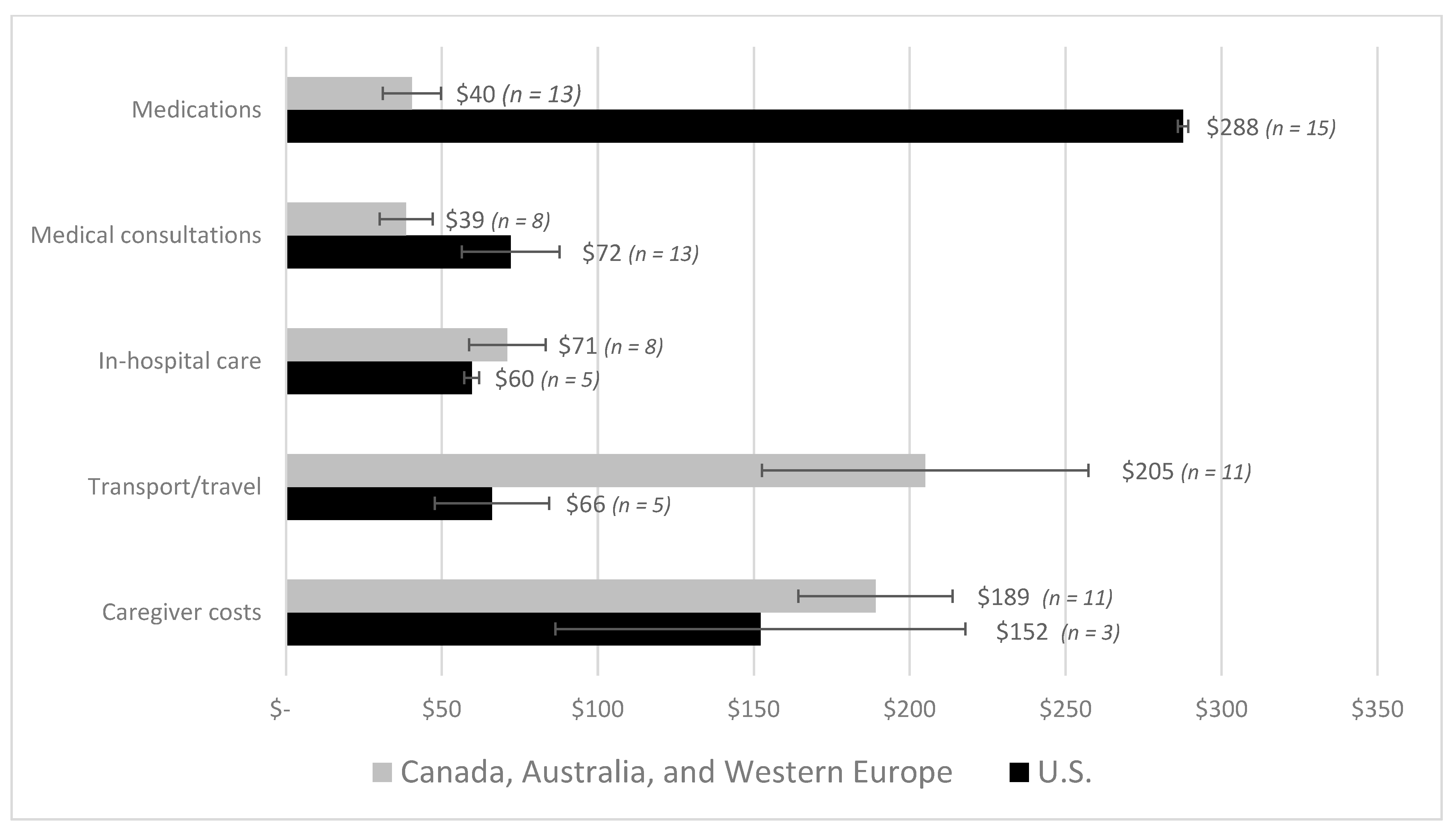
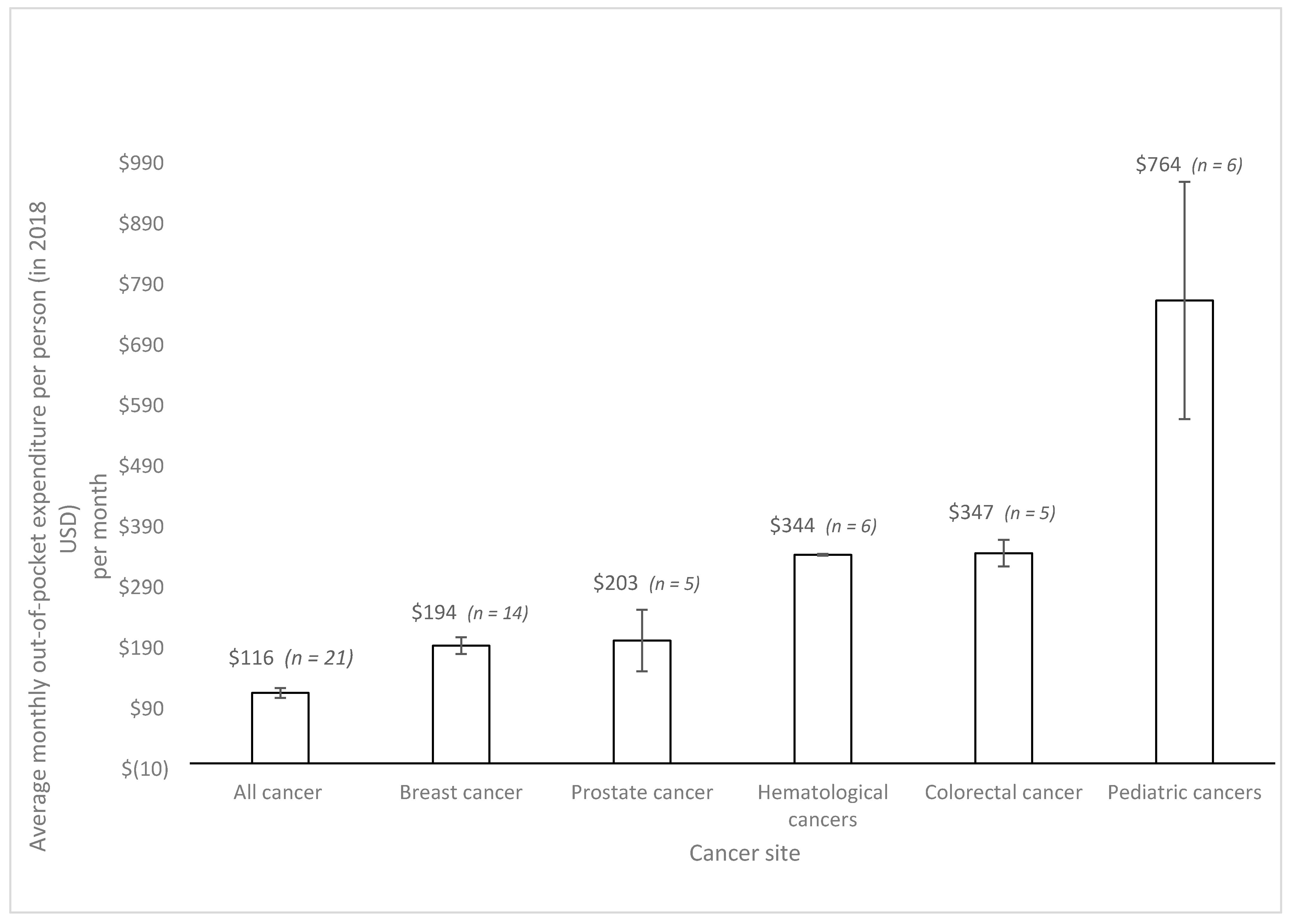
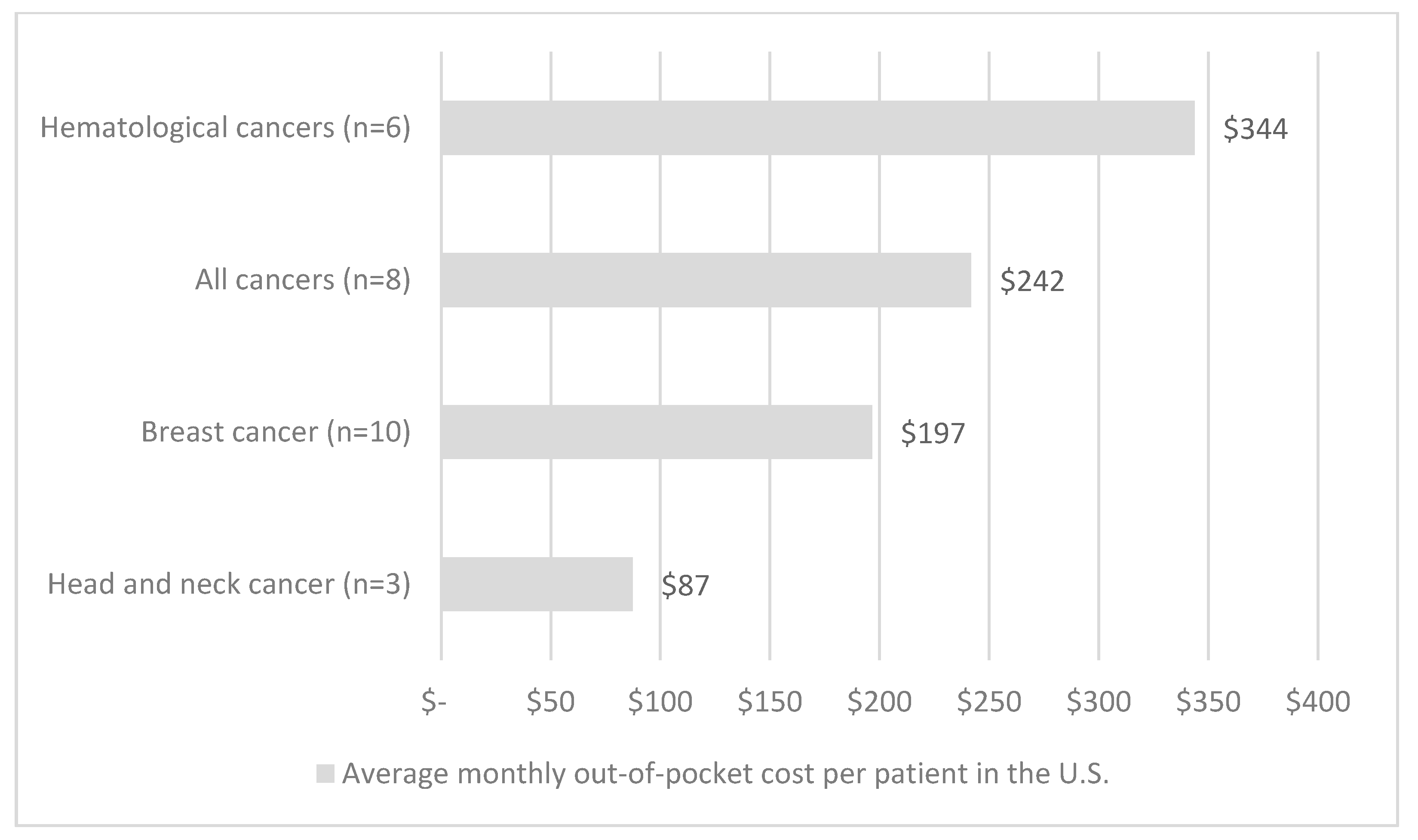
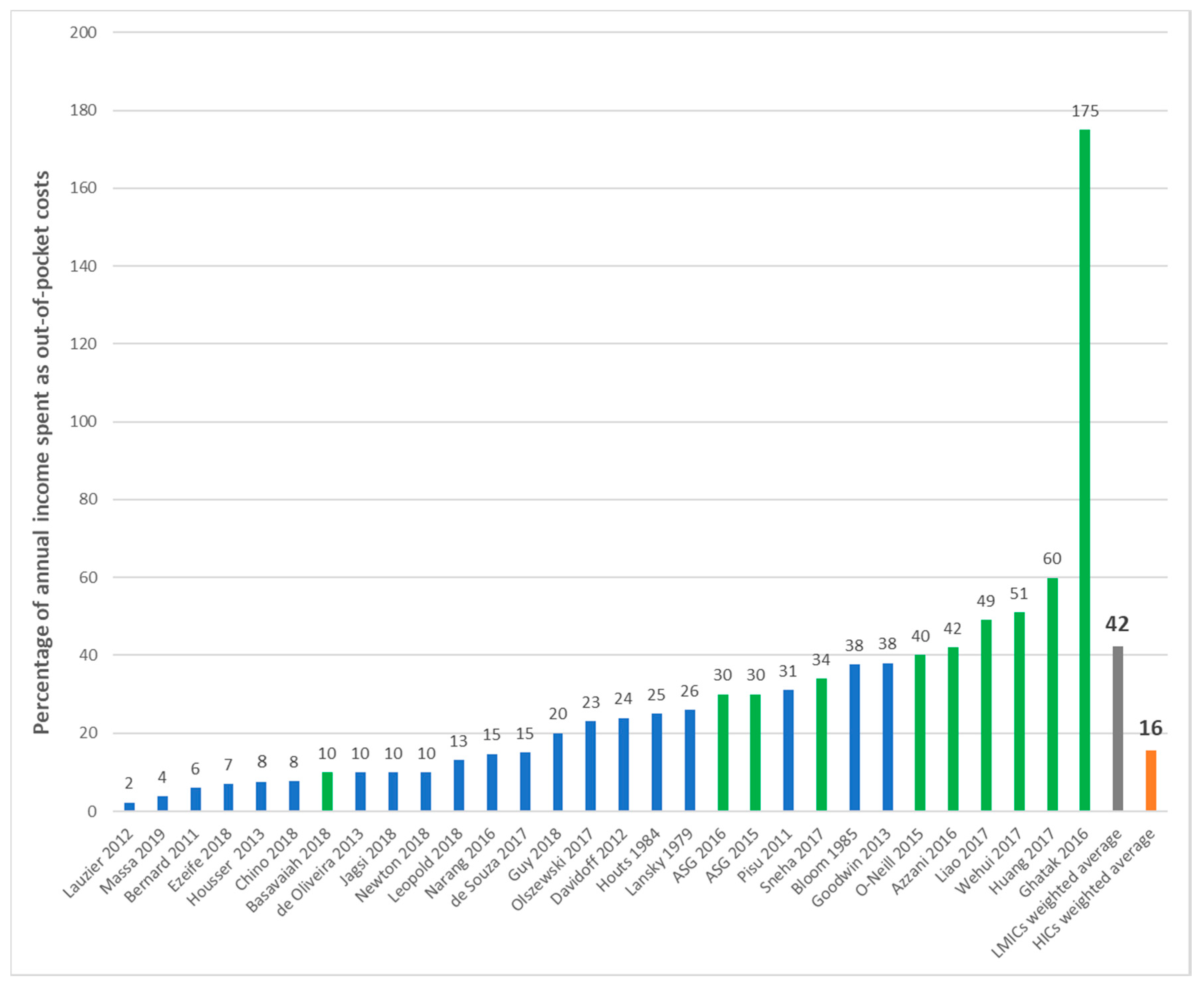
| First Author | Year | Country | Cancer Care Continuum | Sample Size | Mean Age (SD) | % over 60 Years | % Female | % Insured | Type of Insurance | Mean | Study Design | Study Population |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Income | ||||||||||||
| All cancer (adults) | ||||||||||||
| Bates | 2018 | Australia | Post diagnosis | 25,530 | NR | 57.20% | 44 | NR | NR | NR | Retrospective observational study | All cancer patients in Queensland |
| Callander | 2019 | Australia | Post diagnosis | 429 | 57.4 (15.4) | NR | 49 | 100% | Public | NR | Retrospective observational study | All cancer patients in Queensland |
| Gordon | 2009 | Australia | Diagnosis onwards | 439 | 57 (12) | NR | 61 | 47% | Private | 55% of households earned < AUD 40,000 per year | Cross-sectional | Adults diagnosed or treated for cancer at the Townsville Hospital Cancer Centre |
| Newton | 2018 | Australia | Diagnosis | 400 | 64 (11) | 53% over 65 | 49 | 100% | Any | AUD 919 weekly per household | Cross-sectional | Cancer patients who resided in rural regions of Western Australia |
| Action Study Group | 2015 | Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines, Thailand, Vietnam | Treatment (post-surgery) | 4584 | 51 | 13% over 65 | 72 | 44% | Any | NR | Prospective cohort study | All cancer patients with planned surgery |
| Yu | 2015 | Canada | Palliative care | 186 | NR | 61% over 70 | 54.84 | 100% | Public | NR | Cohort | End of life |
| Dumont | 2015 | Canada | Palliative care | 252 | 58 | NR | 73 | 100% | Public | NR | Longitudinal, prospective design with repeated measures | Patients enrolled in a regional palliative care program and their main informal caregivers |
| Longo | 2011 | Canada | Treatment onwards | 282 | 61.6 | NR | 47 | 100% | Public | 11% of households earned < USD 20,000 CAD per year | Cross-sectional design | Urban and rural patients in 5 of the 8 cancer clinics in the province of Ontario |
| Wenhui | 2017 | China | Treatment | 2091 | 63 | NR | NR | 100% | Any | NR | Cohort | NR |
| Koskinen | 2019 | Finland | Diagnosis onwards | 1978 | 66 (26–96 range) | NR | 45 | 100% | Public | NR | Cross-sectional registry and survey study | Patients having either prostate, breast or colorectal cancer |
| Buttner | 2018 | Germany | Treatment onwards | 502 | 46 (8) | 0% | 46.6 | 100% | Any | 33.3% households earned <USD 1000 Euros per year | Prospective cohort study | Working age cancer patients |
| Mahal | 2013 | India | Diagnosis onwards | 821 | NR | NR | NR | 3.41% | Private | NR | Cross-sectional survey | Household with at least one person living with cancer, or hospitalized due to cancer |
| Collins | 2017 | Ireland | Treatment onwards | 151 | Median 58 (range 20–79) | NR | 60 | NR | NR | NR | Retrospective cohort study | Cancer patients, 18 years or older |
| Baili | 2015 | Italy | Survivorship | 296 | NR | 17% over 80 | 59 | NR | NR | NR | Retrospective cohort study | Patients diagnosed between 2003 and 2007 |
| Isshiki | 2014 | Japan | Treatment | 521 | 63 | NR | NR | NR | NR | NR | Observational descriptive study | Cancer patients receiving anti-cancer treatment |
| Action Study Group | 2016 | Singapore, Brunei, Malaysia, Thailand, Indonesia, Philippines, Vietnam, Laos, Cambodia, Myanmar | Diagnosis onwards | 9513 | Median 52 (IQR = 26) | NR | 37 | 43 | Any | 41% of households earn 0–75% of mean national income | Prospective cohort | Newly diagnosed adult cancer patients recruited from 47 public and private hospitals |
| Marti | 2015 | UK | Survivorship | 298 | NR | 56% | 55 | 100% | Public | NR | Prospective cohort study | Patients diagnosed with potentially curable breast, colorectal or prostate cancer |
| Bernard | 2011 | USA | Treatment onwards | 4110 | NR | 43% over 55 | 62 | 94% | Any | USD 62,026 year in 2004 per household | Case–control | Persons 18 to 64 years of age who received treatment for cancer |
| Chino | 2018 | USA | Treatment | 245 | Median 60—Range 27–91 | NR | 55 | 100% | Any | NR | Retrospective cohort study | Patients with solid tumour cancers receiving chemotherapy or hormonal therapy |
| Colby | 2011 | USA | Treatment | 329 | NR | NR | 94.9 | 100% | Public | NR | Retrospective cohort study | Cancer patients who discontinued medication |
| Davidoff | 2012 | USA | Diagnosis onwards | 1868 | NR | 94% over 65 | 49 | 100% | Public | USD 35,356 per year per patient | Retrospective, observational study | Medicare beneficiaries with newly diagnosed cancer |
| Dusetzina | 2017 | USA | Treatment | 63,780 | NR | NR | 57.2 | 100% | Any | NR | Retrospective, observational study | Patients aged 18 through 64 years who had prescription drug coverage |
| Dusetzina | 2016 | USA | Treatment | 3344 | NR | NR | NR | 100% | Any | NR | Retrospective, observational study | Orally administered anticancer medications |
| Finkelstein | 2009 | USA | Treatment onwards | 679 | 50 (10.1) | NR | 69 | 79.80% | Any | USD 49,240 per year per household | Retrospective, observational study | Working age cancer patients (age 25–64) |
| Guy | 2018 | USA | Diagnosis onwards | 4271 | NR | 65% over 50 | 65 | 89.30% | Any | NR | Retrospective observational study | Adults with a cancer diagnosis |
| Houts | 1984 | USA | Treatment | 139 | 57 | NR | 66 | NR | NR | NR | Prospective observational study | Patients receiving outpatient chemotherapy treatments in seven oncology practices |
| John | 2016 | USA | Survivorship | 2977 | 61.9 (0.8) | 44.7% over 65 | 48 | 94% | Any | NR | Cross-sectional | Adults who self-reported ever having received any cancer diagnosis |
| Kircher | 2014 | USA | Treatment | 6607 | 70.1 (0.2) | NR | 48 | 100% | Any | NR | Case–control | Individuals aged over 55 years with cancer coded in the condition file in MEPS |
| Langa | 2004 | USA | Treatment onwards | 988 | 80 (0.3) | 100% | 54 | 100% | Public | NR | Observational descriptive study | Cancer patients over 70 years old |
| Narang | 2016 | USA | Diagnosis onwards | 1409 | Median 73 (IQR 69–79) | NR | 46.4 | 100% | Any | 25% of households earned < USD 22,380 per year | Prospective cohort study | US residents older than 50 years |
| Raborn | 2012 | USA | Treatment | 6094 | 53 (13) | NR | 54.4 | 100% | Any | NR | A retrospective claims-based analysis | Patients over 18 years with at least one claim of an oral oncolytic therapy |
| Shih | 2015 | USA | Treatment | 200,168 | 52 | NR | NR | 100% | Private | NR | Cohort | Patients undergoing chemotherapy, in Lifelink Health Plan Claims Database |
| Shih | 2017 | USA | Treatment | 42,111 | 72.17 (9.93) | 100% over 65 | 50.9 | 100% | Private | NR | Cohort | Medicare beneficiaries, insured |
| Stommel | 1992 | USA | Treatment | 192 | 58.7 (12.2) | NR | 49.5 | NR | NR | USD 34,473 per household per year | Cross-sectional | Study sample had at least one dependent in an activity of daily living and caregiver |
| Tangka | 2010 | USA | NR | 24,654 | NR | NR | NR | 100% | Any | NR | Cohort | Panel survey population |
| Tomic | 2013 | USA | Treatment | 28,979 | 59 (12) | 29% over 65 | 71 | 100% | Any | NR | Cohort | Adult patients who received chemotherapy and granulocyte colony-stimulating factors in the outpatient setting in the United States |
| Kaisaeng | 2014 | USA | Treatment | 3781 | 75 (7) | NR | 97 | 100% | Public | NR | Cross-sectional | Medicare beneficiaries who filled a prescription for imatinib, erlotinib, anastrozole, letrozole, or thalidomide during 2008. |
| Markman | 2010 | USA | NR | 1767 | NR | 42% | 58 | NR | NR | 7% of households earned < USD 20,000 per year | Observational descriptive study | Breast, colon, lung, and prostate cancer who joined the NexCura program |
| Jung | 2018 | USA | Treatment | 148,265 | 76 (7.3) | NR | 51 | 100% | Public | USD 61,317 per year | Natural experimental design | Elderly Medicare beneficiaries with cancer |
| Chang | 2004 | USA | Diagnosis onwards | 58 | 67 (12) | NR | 30 | 100% | Any | NR | Retrospective matched-cohort control | Individuals insured by private or Medicare supplemental health plans |
| All cancer (pediatric) | ||||||||||||
| Cohn | 2003 | Australia | Diagnosis onwards | 100 | 8.9 (range 0.8–18) | 0% | 50 | 100% | Any | NR | Cross-sectional | Children with cancer and their families |
| Tsimicalis | 2013 | Canada | Treatment | 78 | 37.38 (parents) | NR | NR | 100% | Public | Assumed: USD 73,500 per household per year | Cohort, cost of illness | Convenience sample |
| Tsimicalis | 2012 | Canada | Treatment | 99 | 7.85 (5.28) | NR | NR | 100% | Public | Assumed: USD 73,500 per household per year | Cohort, cost of illness | Convenience sample |
| Ahuja | 2019 | India | Diagnosis onwards | 11 | NR | NR | NR | NR | NR | NR | Prospective cohort | Children with cancer and their families |
| Sneha | 2017 | India | Treatment | 70 | 7.8 (2.2) | 0% | 31 | 0% | Private | 15% of households earned< 60,000 Rs. per year | Cross-sectional | Clinical setting |
| Ghatak | 2016 | India | Treatment onwards | 50 | 5.6 (2.9) | 0 | 24 | NR | NR | 239 USD per month per household | Prospective observational study | Families with children with acute lymphoblastic leukemia |
| Bloom | 1985 | USA | Diagnosis onwards | 569 | NR | NR | NR | NR | NR | USD 25,790 annual family income | Retrospective observational study | Children with malignant neoplasms |
| Lansky | 1979 | USA | Treatment | 70 | 7 (4.5) | 0 | 34 | NR | NR | USD 13,500 | Prospective cohort study | Parents of children in treatment for cancer by the pediatric hematology department |
| Breast | ||||||||||||
| Boyages | 2016 | Australia | Treatment | 361 | NR | 56% over 55 | 100 | NR | NR | 20.6% households earned < USD 45,000 (AUD 2016) per year | Cross-sectional | Females with primary stage I, II, or III breast cancer; had completed treatment at least 1 year prior to recruitment; and fluent in English |
| Gordon | 2007 | Australia | Diagnosis onwards | 287 | 57 (9.6) | 62% over 50 | 100 | 70% | Private | 29% of patients earned < USD 26,000 AUD per year | Longitudinal, population-based study | Women with breast cancer 0–18 months post-diagnosis |
| Housser | 2013 | Canada | Treatment onwards | 301 | NR | 47% over 65 | 43 | 64.60% | Private | 14.1% of patients earned less than CAD 20,000 per year | Observational descriptive study | 19 years of age or older, residents of Newfoundland, and diagnosed with breast or prostate cancer |
| Lauzier | 2012 | Canada | Diagnosis-treatment | 1191 | NR | 31.60% | 67 | 100% | Public | 58% of households earned < USD 50,000 per year | Prospective cohort study | Women with breast cancer and their spouses |
| Liao | 2017 | China | Diagnosis-treatment | 2746 | 49.6 | 7% over 65 | 100 | 100% | Any | USD 8722 | Multicentre cross-sectional study | Patients with breast cancer diagnosis at a hospital affiliated with the CanSPUC project |
| O’Neill | 2015 | Haiti | Diagnosis onwards | 61 | 49 (9.8) | NR | 98 | NR | NR | USD 1333 per year per patient | Cross-sectional | Patients from Hopital Universitaire de Mirebalais |
| Bargallo-Rocha | 2017 | Mexico | Treatment | 69 | Median 56 (IQR 11.5) | NR | 100 | NR | NR | USD 548 in Mexico/ month | Cross-sectional | Female patients who underwent breast cancer surgery |
| Bekelman | 2014 | USA | Treatment | 15,643 | NR | 34.2 | 100 | 100% | Private | 13.5% households earned <USD 40,000/year | Retrospective observational study | Women with breast cancer with breast conserving surgery |
| Chin | 2018 | USA | Treatment | 6900 | NR | 21% | 100 | 100% | Public | NR | Retrospective cohort study | Female patients aged 18 to 64 years |
| Dean | 2018 | USA | Survivorship | 129 | 65 (8) | NR | 100 | 98% | Any | 13% patients earned <USD 30,000 per year | Prospective, longitudinal study | Women with stages I–III invasive breast cancer, completion of active breast cancer treatment, > 1 lymph node removed |
| Farias | 2018 | USA | Treatment | 6863 | NR | 17.30% | 100 | 100% | Private | NR | Retrospective, observational study | Women under the age of 64 with at least 1 prescription claim |
| Giordano | 2016 | USA | Diagnosis onwards | 14,643 | Median 54 | 12.2% over 65 | 100 | 100% | Any | NR | Observational Cohort Study | Women aged over 18 years with breast cancer diagnosed between 2008 and 2012 |
| Jagsi | 2014 | USA | Diagnosis onwards | 1502 | NR | 28% over 65 | 100 | NR | NR | USD 50,000 per year | Longitudinal cohort study | Patients age 20 to 79 years diagnosed with stage 0 to III breast cancer |
| Jagsi | 2018 | USA | Diagnosis onwards | 2502 | NR | 57% | 100 | 95% | Any | 37% of households earned < USD 40,000 per year | Cross-sectional survey | Patients with early stage breast cancer |
| Leopold | 2018 | USA | Treatment—end of life | 5364 | NR | 58% over 50 | 100 | 100% | Any | USD 50,054 | Longitudinal time series | Insured women with metastatic breast cancer |
| Pisu | 2016 | USA | Survivorship | 432 | NR | 47.7% over 65 | 100 | 94% | Public | 19.3% lowest income (<20,000 per year) | Prospective cohort study | Stage 0–III breast cancer, within the first three years after completing primary cancer treatment |
| Pisu | 2011 | USA | Survivorship | 261 | NR | 16% over 65 | 100 | NR | NR | 11.5% lowest income (<20,000 per year) | Cross-sectional | Patients diagnosed with stage I–II breast cancer, with a minimum of 1 month after treatment completion |
| Roberts | 2015 | USA | Treatment | 18,575 | 53.6 (7.5) | NR | 100 | 100% | Private | NR | A retrospective claims-based analysis | Women (ages 18–64) with at least two health encounter claims for breast cancer |
| Leukemia | ||||||||||||
| Kodama | 2012 | Japan | Treatment | 577 | Median 61 (15–94 range) | NR | 35 | 100% | Public | USD 36,731 USD per year | Observational descriptive study | Patients with CML who were prescribed imatinib |
| Wang | 2014 | Singapore | Treatment | 367 | NR | 8% over 61 | 62.1 | NR | NR | NR | Cohort | Secondary analysis of a prospective study |
| Darkow | 2012 | USA | Treatment | 995 | 62 | NR | 47 | 100% | Any | NR | Retrospective cohort study | Adult patients (aged >18 years) with an initial diagnosis of CML during 1997 to 2009 |
| Doshi | 2016 | USA | Diagnosis onwards | 1053 | 73 (8) | 96% over 65 | 47 | 100% | Public | NR | A retrospective claims-based analysis | Medicare patients with newly diagnosed CML |
| Dusetzina | 2014 | USA | Treatment | 1541 | 48(11) | NR | 45 | 100% | Any | NR | Retrospective, observational study | Adults (age 18 to 64 years) with CML who initiated imatinib therapy |
| Goodwin | 2013 | USA | Treatment onwards | 1015 | 61 (9.2) | NR | 39 | 97% | Any | NR | Observational descriptive study | Patients who had received intensive treatment for MM at the study site |
| Gupta | 2018 | USA | Treatment | 162 | 56 (13) | NR | 49 | 97% | Any | 42% patients earned less than USD 50,000 USD per year | Cross-sectional | Adult patients with MM taking medication |
| Shen | 2017 | USA | Treatment | 898 | 70 (12) | NR | 47 | 38% | Public | NR | Retrospective cohort study | Patients with Chronic Myeloid Leukemia Taking Targeted Oral Anticancer Medications |
| Olszewski | 2017 | USA | Treatment | 3038 | Median76 (IQR 71–82) | NR | 50 | 100% | Public | USD 29,700 per year | Observational descriptive study | Patients with Part D coverage at diagnosis |
| Colorectal | ||||||||||||
| Huang | 2017 | China | Diagnosis onwards | 2356 | 57.4 | 28.3% over 65 | 43 | 100% | Any | CNY 54,525 per patient per year | Cross-sectional survey | Primary prevalent CRC patients undergoing treatment in hospitals |
| Hanly | 2013 | Ireland | Diagnosis and treatment | 154 | NR | 60% over 55 | 82 | NR | NR | NR | Retrospective observational study | Carers of colorectal cancer patients |
| O Ceilleachair | 2017 | Ireland | Survivorship | 497 | 67 | 46% over 70 | 38 | 52% | Private | NR | Case report | All cases of primary, invasive colorectal cancer in Ireland diagnosed October 2007–September 2009 |
| Shiroiwa | 2010 | Japan | Treatment | 1319 | NR | NR | NR | 100% | Public | NR | RCT, EE | Trial population, XELOX or XELOX plus bevacizumab and second-line therapy with XELOX |
| Azzani | 2016 | Malaysia | Diagnosis onwards | 138 | Median 63 (IQR = 19) | 35.5% over 70 | 49 | 9% | Private | 2000 RM/month | Prospective, longitudinal study | CRC patients seeking treatment at the University of Malaya Medical Centre (UMMC) in the first year following diagnosis |
| Sculpher | 2000 | UK | Treatment | 495 | 61 | NR | 36 | NR | NR | NR | Randomized-controlled trial | Colorectal cancer patients treated with Raltitrexed or Fluorouracil |
| Lung | ||||||||||||
| Ezeife | 2018 | Canada | Treatment—Palliative care | 200 | NR | 50% over 65 | 56 | 45.10% | Private | USD 41,000-USD 80,000 CAD | Cross-sectional | Patients with advanced lung cancer (stage IIIB/IV) |
| Andreas | 2018 | France, Germany and the United Kingdom | Treatment onwards | 831 | NR | 67% | 38 | 100% | Public | NR | Retrospective observational study | Patients ≥18 years of age that had undergone complete resection of stage IB-IIIA NSCLC |
| Wood | 2019 | France, Germany, Italy | Treatment | 1457 | 64.5 (10.1) | NR | 34.1 | NR | NR | NR | Cross-sectional | NR |
| Van Houtven | 2010 | USA | Initial treatment, Continuing, Terminal, overall | 1629 | NR | 42.1% over 65 | 75.8 | 100% | Any | USD 39,554 per year per household | Cross-sectional | Informal caregivers—Patients participating in the Share Thoughts on Care survey |
| Hess | 2017 | USA | Diagnosis onwards | 47,207 | 65 (10.4) | NR | 45 | 100% | Any | NR | Retrospective observational study | 18 years of age or older at the time of initial diagnosis of lung cancer |
| Head and neck | ||||||||||||
| Burns | 2017 | Australia | Survivorship-integrated care | 82 | 65 (7.4) | NR | 26 | NR | NR | NR | Randomized-controlled trial | Patients with head and neck cancer enrolled in speech pathology programs |
| Chauhan | 2018 | India | Treatment | 410 | NR | NR | NR | NR | NR | NR | Retrospective observational study | Head and cancer patients undergoing radiotherapy |
| de Souza | 2017 | USA | Treatment onwards | 73 | 60 (26–79) | NR | 21.9 | 100% | Any | USD 81,597 per year per household | Prospective observational study | Head and neck cancer patients with locally advanced stage |
| Massa | 2019 | USA | Diagnosis onwards | 16,771 | 65 (95CI 63.1–66.8) | NR | 35.5 | 97.40% | Any | USD 24,056 | Case control | Adult patients with cancer |
| Prostate | ||||||||||||
| Gordon | 2015 | Australia | Diagnosis onwards | 289 | 65 (8.4) | 78% | 0 | 71% | Private | 38% households had incomes between USD 37,000 and AUD 80,000 per year | Cross-sectional | Men who self-reported they had previously been diagnosed with prostate cancer |
| de Oliveira | 2013 | Canada | Survivorship | 585 | 73 | 92.50% | 0 | 100% | Public | 40% earned <USD 40,000 per year | Retrospective, observational study | All patients initially diagnosed with PC in 1993–1994, 1997–1998, and 2001–2002 |
| Geynisman | 2018 | USA | Treatment | 116 | 65 (range 27–88) | NR | 15 | 98% | Any | NR | Retrospective, observational study | Advanced renal and prostate cancer patients |
| Jayadevappa | 2010 | USA | Diagnosis—Treatment | 512 | 59 (6.3) | NR | 0 | NR | NR | 19% of patients earned < USD 40,000 per year | Prospective cohort study | 45 years of age, newly diagnosed with PCa within the prior 4 months and yet to initiate\ treatment |
| Skin | ||||||||||||
| Gordon | 2018 | Australia | Treatment onwards | 419 | 55 | NR | 54 | 74% | Private | NR | Retrospective, observational study | Consenting Qskin study participants |
| Gordon | 2018 | Australia | Treatment onwards | 539 | 56 | NR | 64 | NR | NR | NR | Retrospective, observational study | Consenting Qskin study participants |
| Thompson | 2019 | Australia | Treatment | 8613 | NR | NR | NR | 100% | Public | NR | Cohort | Admin data linked to study population |
| Grange | 2017 | France, Germany and the United Kingdom | Survivorship and Palliative care | 558 | NR | 54.50% | 44 | 100% | Public | NR | Retrospective observational study | Patients with advanced melanoma |
| Ovarian | ||||||||||||
| Bercow | 2018 | USA | Diagnosis onwards | 5031 | NR | 41.40% | 100 | 100% | Private | NR | Retrospective cohort study | Ovarian cancer patients enrolled in commercial insurance sponsored by over 100 employers in the United States |
| Calhoun | 2001 | USA | Treatment | 83 | NR | NR | 100 | NR | NR | NR | Prospective cohort study | Ovarian cancer patients who experienced chemotherapy-associated hematologic or neurologic toxicities |
| Suidan | 2019 | USA | Treatment | 12,761 | NR | 44% | 100 | 100% | Private | NR | Cohort | All ovarian cancer patients in MartketScan database undergoing first line treatment |
| Pancreatic | ||||||||||||
| Basavaiah | 2018 | India | Treatment | 98 | 54.5 (10–87 range) | 41.8% over 60 | 33 | 29.60% | Any | NR | Prospective cohort | Patients undergoing pancreatic-duodenectomy |
| Bao | 2018 | USA | End-of-life | 3825 | NR | 100% | 55 | 100% | Public | NR | Retrospective cohort study | Patients 66 years or older when diagnosed with Stage IV pancreatic cancer in 2006–2011 |
| Anal | ||||||||||||
| Chin | 2017 | USA | Treatment | 1025 | NR | NR | 65 | 100% | Public | NR | Retrospective cohort study | Patients with anal cancer treated with Intensity-modulated radiotherapy |
| Brain | ||||||||||||
| Kumthekar | 2014 | USA | Treatment | 43 | Median 57 (range 24–73) | NR | 42 | 95% | Any | USD 75,000 per year | Prospective observational study | Patients within 6 months of diagnosis or tumor recurrence |
| First Author | Year | Definition of out-of-Pocket Cost | Total out-of-Pocket Cost Estimate (Mean—SD) | Time Frame of out-of-Pocket Estimate | Out-of-Pocket as % of Income | Currency | Currency Year |
|---|---|---|---|---|---|---|---|
| The Action Study Group | 2016 | Financial catastrophe was defined as OOP costs at 12 months exceeding 30% of annual household income | NR | 3- and 12-month follow-ups | 48% of cancer patients reported Financial catastrophe at 12 months | NR | NR |
| The Action Study Group | 2015 | Financial catastrophe (out-of-pocket costs of >30% of annual household income) | NR | 3 months | 31% of participants incurred Financial catastrophe | NR | 2015 |
| Ahuja | 2019 | Direct costs incurred by families of children being treated for cancer | 651 (356) | 14 weeks | NR | USD | 2013 |
| Andreas | 2018 | Cost of childcare, and non-reimbursed transportation costs incurred by the patient or their family/friends. | UK = 7 | 1 month | NR | Euro | 2013 |
| Germany = 6 | |||||||
| France = 0 | |||||||
| Azzani | 2016 | Payments for expenses such as hospital stays, tests, treatment, travel and food. | 8306 | 1 year | 42% of the median annual income in Malaysia. | RM | 2013 |
| Baili | 2015 | Direct expenses which were not entirely covered or only partially covered by the NHS | 160 (372) | 1 month | NR | Euro | 2015 |
| Bao | 2018 | Costs incurred by patients 30 days before death | 1930 (with chemotherapy) | 1 month | NR | USD | 2011 |
| Bargallo-Rocha | 2017 | Patient borne costs on transportation, housing, and salary due to breast cancer | 535 | 1 month | NR | USD | 2017 |
| Basavaiah | 2018 | Catastrophic expenditure was defined as the percentage of households in which OOP health payments exceeded 10% of the total household income | NR | From the first hospital visit to postoperative recovery | A total of 76.5% of the sample incurred catastrophic expenditure | USD | 2015 |
| Bates | 2018 | Patient co-payments for primary healthcare and prescription pharmaceuticals | 1000 (2000) | 1 year | NR | AUD | 2017 |
| Bekelman | 2014 | Summing deductible, co-payment, and coinsurance amounts. | 3421 (95% CI 3158–3706) | 1 year | NR | USD | 2013 |
| Bercow | 2018 | Out-of-pocket (OOP) payment was calculated as the sum of deductibles, copayments, and coinsurance | Median 2988 (IQR 1649–5088) | 1 year | NR | USD | 2013 |
| BerNRrd | 2011 | OOP expenditures on health insurance premiums in addition to OOP expenditures on healthcare services | 4772 | NR | 6% | USD | 2008 |
| Bloom | 1985 | Direct medical and nonmedical expenses borne by the family | 9787 | 1 year | 37.7% of family income | USD | 1981 |
| Boyages | 2016 | The financial cost of lymphedema care borne by women | 977 | 1 year | NR | AUD | 2014 |
| Burns | 2017 | Costs associated with return travel to the regional speech pathology service | 256 | NR | NR | AUD | 2015 |
| Buttner | 2018 | Direct payments for health services or treatments which are not covered by health insurance and need to be paid by the patients themselves | 205 (346) | 3 months | NR | Euro | 2018 |
| Calhoun | 2001 | Direct medical costs borne by patients | 3302 | 3 months | NR | USD | 2001 |
| Callander | 2019 | Patient co-payments for primary healthcare and prescription pharmaceuticals | 1191 (3099) | 1 year | NR | AUD | 2017 |
| Chang | 2004 | Copays and deductibles to caregivers | 302 (634) | 1 month | NR | USD | 2004 |
| Chauhan | 2018 | Only the direct OOP expenditure was assessed | 849 | NR | NR | USD | 2015 |
| Chin | 2018 | Copayments for oral anticancer medication | 19 | 1 month | NR | USD | 2014 |
| Chin | 2017 | The sum of Medicare Part A and Part B reimbursements, third-party payer reimbursements, and patient liability amounts | Median 6967 (5226–9076) | 1 year | NR | USD | 2011 |
| Chino | 2018 | Insurance premiums; medication copays; physician office charges; copays for procedures, tests, and studies; and costs related to travel for treatment | Median 393 (Range—0–26,586) | 1 month | 7.80% | USD | 2018 |
| Cohn | 2003 | Travel, accommodation, and communication costs | 19,604 (32,976) | 40 months | NR | AUD | 2003 |
| Colby | 2011 | Patient spending on ani cancer drugs | 645 | 3 months | NR | USD | 2011 |
| Collins | 2017 | Personal expenditure on regular and non-regular indirect costs during treatment. | 1138 (range 21–7089) | 1 month | NR | EUR | 2017 |
| Darkow | 2012 | Copayment for anti cancer medication | 124 | 1 month | NR | USD | 2012 |
| Davidoff | 2012 | Costs incurred by patients | 4727 (202) | 2 years | 23.90% | USD | 2007 |
| de Oliveira | 2013 | Medical costs associated with health Professional visits, and nonmedical costs such as travel, parking, food, and accommodation | 200 (95% CI USD 109–290) | 1 year | 10% | CAD | 2006 |
| de Souza | 2017 | Insurance premiums; deductibles; direct medical costs | 805 (range 6–10,156) | 1 month | 15.10% | USD | 2017 |
| Dean | 2018 | Co-payments for outpatient physician visits, physical and occupational therapy visits, complementary and integrative therapy visits | 2306 | 1 year | NR | USD | 2015 |
| Doshi | 2016 | Direct medical costs borne by patients | 2600 | 1 month | NR | NR | 2016 |
| Dumont | 2015 | NR | 576 (46) | 6 months | NR | CAD | 2015 |
| Dusetzina | 2017 | Copayment, coinsurance, and deductibles, adjusting to reflect spending on a median monthly dosage | 143 | 1 month | NR | USD | 2012 |
| Dusetzina | 2016 | Copayments for orally administered anticancer medications | 310 | 1 month | NR | USD | 2014 |
| Dusetzina | 2014 | Monthly copayments for imatinib | 108 (301) | 1 month | NR | USD | 2011 |
| Ezeife | 2018 | Expenses for prescription drugs, travel, childcare/babysitting, copayments, and deductibles | Median 1000–5000 | 1 year | From 2–12% (median) | CAD | 2018 |
| Farias | 2018 | Sum of the copayments, deductibles, and coinsurance paid for AET medication | 193 (97) | 1 month | NR | USD | 2018 |
| Finkelstein | 2009 | Copayments, deductibles, and payments for noncovered services | 1730 (2710) | 1 year | NR | USD | 2005 |
| Geynisman | 2018 | Co-pays for oral anti cancer medications | 81.26 | 1 month | NR | USD | 2018 |
| Ghatak | 2016 | Direct medical, living (rent, food, clothes), and transport costs | Median 524 (395–777 IQR) | 1 month | 3.5 times–7 times the monthly income | USD | 2013 |
| Giordano | 2016 | Drug and insurance-related costs borne by patients | 3226 | 18 months | NR | USD | 2013 |
| Goodwin | 2013 | Direct and indirect patient expenditure | NR | 1 year | 38% and 31% annually for patients receiving/not receiving chemotherapy, respectively. | NR | 2013 |
| Gordon | 2007 | Direct costs (garments and aids), health services (e.g., co-payments, pharmaceuticals) and paid home services | 1937 (3210) | 18 months | NR | USD | 2005 |
| Gordon | 2018 | Melanoma treatment costs borne by patients | 625 (575) | 3 years | NR | AUD | 2016 |
| Gordon | 2018 | Medical expenses for Medicare services borne by patients | 3514 (4325) | 2 years | NR | AUD | 2016 |
| Gordon | 2009 | Medical and non-medical costs borne by patients | 4826 (5852) | 16 months | NR | AUD | 2008 |
| Gordon | 2015 | Medical and non-medical costs borne by patients | 9205 (14,567) | 16 months | NR | AUD | 2012 |
| Grange | 2017 | Childcare and non-reimbursed transportation costs | France = 0 | 1 month | NR | EUR | 2013 |
| Germany = 332 (95% CI 271–401) | |||||||
| UK = 533 (477–594) | |||||||
| Gupta | 2018 | Costs for doctor visits, prescriptions, over-the-counter medications, transportation | 709 (1307) | 3 months | NR | USD | 2018 |
| Guy | 2018 | Expenditures toward any healthcare service, such as coinsurance, copayments, and deductibles | 2171 (95% CI 1970–2373) | 1 year | 4.3% had OOP > 20% of household income | USD | 2012 |
| Hanly | 2013 | Parking, meals and accommodation, domestic-related caring activities | 79.2 (151) | 1 week | NR | EUR | 2008 |
| Hess | 2017 | Copayments, deductibles and patient borne costs | 315 (95% CI 106–523) | 1 month | NR | USD | 2014 |
| Housser | 2013 | Costs not covered by insurance or assistance programs | Prostate: 910 (1025) Breast: 864 (1220) | 3 months | 17% had OOPC >7.5% of income (16% prostate, 19% breast) | CAD | 2008 |
| Houts | 1984 | Nonmedical expenses borne by patients | Median 21 (0–204 range) | 1 week | 28% of respondents were spending over 25% of their weekly incomes | USD | 1984 |
| Huang | 2017 | Overall medical and non-medical expenditure | 32,649 | 1 year | 59.9% of their previous-year household income | CNY | 2014 |
| Isshiki | 2014 | Travel/transport costs per outpatient treatment | 79 | 1 month | NR | USD | 2014 |
| Jagsi | 2014 | Medical expenses related to breast cancer, including copayments, hospital bills, and medication costs | Median <2000 | 4 years | NR | USD | 2014 |
| Jagsi | 2018 | Medical and non-medical expenses related to breast cancer (including copayments, hospital bills, and medication costs) | Median <2000 | NR | 17% of patients reported spending ≥10% of household income on out-of-pocket medical expenses | USD | 2018 |
| Jayadevappa | 2010 | Medication and non-medical costs paid by patients | 703 (2500) | 3 months | NR | USD | 2010 |
| Jung | 2018 | Costs of specialty cancer drugs paid by patients | 3860 (1699) | 1 year | NR | USD | 2013 |
| John | 2016 | Alternative medicine costs borne by patients and not covered by insurance | 445 | 1 year | NR | USD | 2012 |
| Kaisaeng | 2014 | Copayments for oral anti cancer drugs | 154 (407) | 1 month | NR | USD | 2008 |
| Kircher | 2014 | Direct payment for all prescription drugs | 724 (42) | NR | NR | USD | 2010 |
| Kodama | 2012 | Copayment for medical expenses | Median 11,548 | 1 year | NR | USD | 2008 |
| Koskinen | 2019 | Out of pocket fees for outpatient visits, inpatient care, home care, and surgical procedures | 280 (603 for palliative care—383 metastatic disease—224 remission—264 rehabilitation—263 treatment) | 6 months | NR | Euro | 2010 |
| Kumthekar | 2014 | Medical and nonmedical expenses that were not reimbursed by insurance | 2451 (2521) | 1 month | NR | USD | 2014 |
| Langa | 2004 | Cost paid by patients on hospital services, outpatient care, home care, and medication | 4656 (3890) | 1 year | NR | USD | 1995 |
| Lansky | 1979 | Non-medical costs paid by the patient’s family | 56 (54) | 1 week | 26% of weekly income | USD | 1979 |
| Lauzier | 2012 | Costs for treatments and follow-up, consultations with other practitioners, home help, clothing, and natural health products | 1365 (1238) | 1 year | Out-of-pocket costs represented an average of 2.3% of annual family income | CAD | 2003 |
| Leopold | 2018 | Patient expenditures including coinsurance, copayment, and deductible amounts | 4247—95% CI (3956–4538) among low deductible health plan | 1 year | 13% of the 2011 real median income household | USD | 2012 |
| 6642—95% CI (6268–7016) among High deductible health plan | |||||||
| Liao | 2017 | Medical expenditure (self-pay and healthcare costs), non-medical expenditure (i.e., transportation, accommodation) | 8449 | Since diagnosis to treatment | 49% (overall OOP expenditure/annual income) | USD | 2014 |
| Longo | 2011 | Patient borne costs | Breast 392 (830) | 1 month | NR | CAD | 2001 |
| Other 149 (265) | |||||||
| Mahal | 2013 | Patient medical and non-medical spending | 5311 (4514–6108 95CI) | 1 year | NR | INR | 2004 |
| Markman | 2010 | Cancer related costs paid by patients | 12% spent between USD 10,000–25,000 | Since diagnosis | NR | USD | 2010 |
| 4% spent between USD 25,000–50,000 | |||||||
| 2% spent between USD 50,000–100,000 | |||||||
| Marti | 2015 | Medical and non-medical costs borne by patients, such as medications, travel, and childcare | Full Sample 39.8 (95% CI 14.5–65.3) | 3 months | NR | USD | 2012 |
| Colorectal 52 (22–126) | |||||||
| Breast 49 (12–86) | |||||||
| Prostate 11 (3–19) | |||||||
| Massa | 2019 | Total non-reimbursed cost of cancer patients | Median 929 (95% CI 775 to 1084) for HNC | 1 year | Median 3.93% of total income spent on OOP (95% CI 3.21 to 4.65) | USD | 2014 |
| 918 (885 to 951) for other cancer | |||||||
| Narang | 2016 | Costs paid by patients on inpatient hospitalization, nursing homes, clinic visits, outpatient surgery | 3737 average | 1 year | Uninsured: 23% | USD | 2012 |
| 2116 Medicaid | Medicaid: 8.5% | ||||||
| 5492 employer-sponsored insurance | Employer-sponsored insurance: 12.6% | ||||||
| 8115 uninsured | NR | ||||||
| Newton | 2018 | Direct medical and nonmedical expenses borne by the patient | 2179 (3077) (95% CI 1873–2518) | 21 weeks | 11% spent over 10% of household income | AUD | 2016 |
| O Ceilleachair | 2017 | OOPCs survivors had incurred as a result of their diagnosis, and which were not recouped from PHI or other sources | 1589 (3827) | 1 year | NR | Euro | 2008 |
| Olszewski | 2017 | Patient’s cost sharing on medication | No low-income subsidy: median 5623 (IQR 3882–9437) | 1 year | 23% of annual income among non-subsidized | USD | 2012 |
| Low-income subsidy: median 6 (IQR 3–10) | |||||||
| O-Neill | 2015 | Medical and nonmedical costs related to the hospital visit coinciding with the interview | 717 (95% CI 619–1171) | 1 year | >67% patients had catastrophic expenses (>40% of household income) | USD | 2014 |
| Pisu | 2016 | Out of pocket costs for medical care | Total at baseline: 232 (82) | 1 month | NR | USD | 2015 |
| Total at 3 months 186 (71) | |||||||
| Pisu | 2011 | Expenses since diagnosis, including monthly insurance premiums | Total: 316.1 (411.5) | 1 month | 31% for lowest income level (<20,000 per year) | USD | 2008 |
| Caucasian: 297 (296) | |||||||
| Minority: 204 (405) | |||||||
| Raborn | 2012 | Deductibles and co-payments for anticancer medication | Generic versions: 171 (652) | Per claim | NR | USD | 2009 |
| No available generic versions: 31 (130) | |||||||
| Roberts | 2015 | Deductibles, copayments, and coinsurance payments | 175 (484) | 1 year | NR | USD | 2012 |
| Sculpher | 2000 | Travel expenses for treatment appointments | Treated with Raltitrexed: 12.25 (41.87) Treated with Fluorouracil: 10.70 (20.16) | Per patient-journey | NR | GBP | 2000 |
| Shen | 2017 | Patient out of pocket expenses on targeted oral anti-cancer medications | Median 401 (IQR 1029) | 1 month | NR | USD | 2014 |
| Shih | 2015 | Patient OOP payments were calculated as allowed minus paid | USD 647 per month in 2011 | 1 month | NR | USD | 2011 |
| Shih | 2017 | Patient pay amount is the amount paid by beneficiaries that is not reimbursed by a third party; therefore, it captures the OOP payments for Medicare beneficiaries who are enrolled in the Part D program. | 850 | 1 month | NR | USD | 2012 |
| Shiroiwa | 2010 | Co-payment | Patients JPY 328,000 (95% CI: 323,000–334,000) | 11 months | NR | JPY | 2009 |
| Patients ≥ 70 years JPY 61,000 (95% CI: 60,000–63,000) | NR | ||||||
| Sneha | 2017 | Medical expenses and nonmedical out-of-pocket expenses incurred by the families in the course of care | NR | Per day | Non-medical expenses—Urban: 22% | INR | 2012 |
| Rural: 46% | |||||||
| Stommel | 1992 | Out-of-pocket payments for services: hospital and physician services, nursing homes, medications, visiting nurses, home health aides, and purchases of special equipment, supplies, and foods and supplements | 660 (624) | 3 months | NR | USD | 1993 |
| Suidan | 2019 | Patient out-of-pocket expenses, in addition to insurance payments made. | Neoadjuvant chemotherapy: USD 2519 | 8 months | NR | USD | 2017 |
| Primary debulking: USD 2977 | NR | ||||||
| Tangka | 2010 | OOP cost (inpatient, outpatient, other noninpatient (costs related to emergency room visits, home healthcare, vision aids, and other medical supplies), Rx) attributable to cancer = difference between expenditures for persons with cancer and persons without cancer, adjusted for sociodemographic and comorbidities | 3996 | 1 year | NR | USD | 2007 |
| Thompson | 2019 | Costs to items associated with the excision, including consultations, skin cancer treatment, Anatomical pathology, skin flaps and Anesthesia;Excluding bulk-billed patients were co-payment would be USD 0 | Private clinical rms: 80 (34, 170) | Treatment episode (up to 3 days post-discharge) | NR | AUD | 2018 |
| Public hospital: 35 (30, 104) | |||||||
| Private hospital: 350 (196, 596) | |||||||
| Tomic | 2013 | Out-of-pocket costs for G-CSF per patient | 100–150: pegfilgrastim 50–80: filgrastim | 3 months | NR | USD | 2010 |
| Tsimicalis | 2013 | Direct costs included health services, prescription medications, over-the-counter medications, complementary medicines, supplies, equipment, family medical fees and medications, as well as travel, food, communication, accommodations, moving or renovations, provider for the child with cancer, domestic labour (e.g., sibling child care), funeral, and other cost categories not yet captured in the literature | 730 (1520) | 3 months | NR | CDN | 2007 |
| Tsimicalis | 2012 | Direct costs as well as travel, food, communication, accommodations, moving or renovations, provider for the child with cancer, domestic labour (e.g., sibling childcare), funeral, and other costs | 5446 (6659) | 3 months | NR | CDN | 2007 |
| Van Houtven | 2010 | Out-of-pocket expenditures for the patient’s medical care as well as nonmedical expenditures | Overall: 1243 | By phase | NR | USD | 2005 |
| Initial: 921 | NR | ||||||
| Continuing: 1545 | NR | ||||||
| Terminal: 1015 | NR | ||||||
| Wang | 2014 | Ward charges, laboratory charges, radiology charges, prescription charges, surgical charges, and other charges | 2230 (95% CI: 1976–2483) | Per episode | NR | USD | 2012 |
| Wenhui | 2017 | NR | 1878 | NR | 51.6 | USD | 2008 |
| 1146 | NR | NR | |||||
| 348 | NR | NR | |||||
| Wood | 2019 | Direct out-of-pocket expenses were defined as wage losses (per week); non-medical expenses associated with general practitioner or hospital visits (in the last 3 months); costs of treatments for conditions linked to NSCLC (in the last week), such as those for pain or symptom relief; and other non-medical costs arising from the diagnosis (per week), including additional childcare costs, assistance at home (cleaner, housekeeper, gardener), and travel costs. | Patient: 823 Caregivers: 1019 | 3 months, reported as annual | NR | EUR | 2018 |
| Yu | 2015 | Out-of-pocket costs: costs paid by the patient/family for travel, supplies, medications, etc. | NR | Entire palliative trajectory | NR | CDN | 2012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iragorri, N.; de Oliveira, C.; Fitzgerald, N.; Essue, B. The Out-of-Pocket Cost Burden of Cancer Care—A Systematic Literature Review. Curr. Oncol. 2021, 28, 1216-1248. https://doi.org/10.3390/curroncol28020117
Iragorri N, de Oliveira C, Fitzgerald N, Essue B. The Out-of-Pocket Cost Burden of Cancer Care—A Systematic Literature Review. Current Oncology. 2021; 28(2):1216-1248. https://doi.org/10.3390/curroncol28020117
Chicago/Turabian StyleIragorri, Nicolas, Claire de Oliveira, Natalie Fitzgerald, and Beverley Essue. 2021. "The Out-of-Pocket Cost Burden of Cancer Care—A Systematic Literature Review" Current Oncology 28, no. 2: 1216-1248. https://doi.org/10.3390/curroncol28020117
APA StyleIragorri, N., de Oliveira, C., Fitzgerald, N., & Essue, B. (2021). The Out-of-Pocket Cost Burden of Cancer Care—A Systematic Literature Review. Current Oncology, 28(2), 1216-1248. https://doi.org/10.3390/curroncol28020117





