Four Cycles of Docetaxel-Cyclophosphamide versus Anthracycline-Taxane as Adjuvant Chemotherapy for HER2-Negative, Axillary Lymph Node Negative Breast Cancer: A Real-World Comparison of Alberta Patients Treated 2008–2012
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Identification of Study Population
2.2. Outcomes
2.3. Statistical Analysis
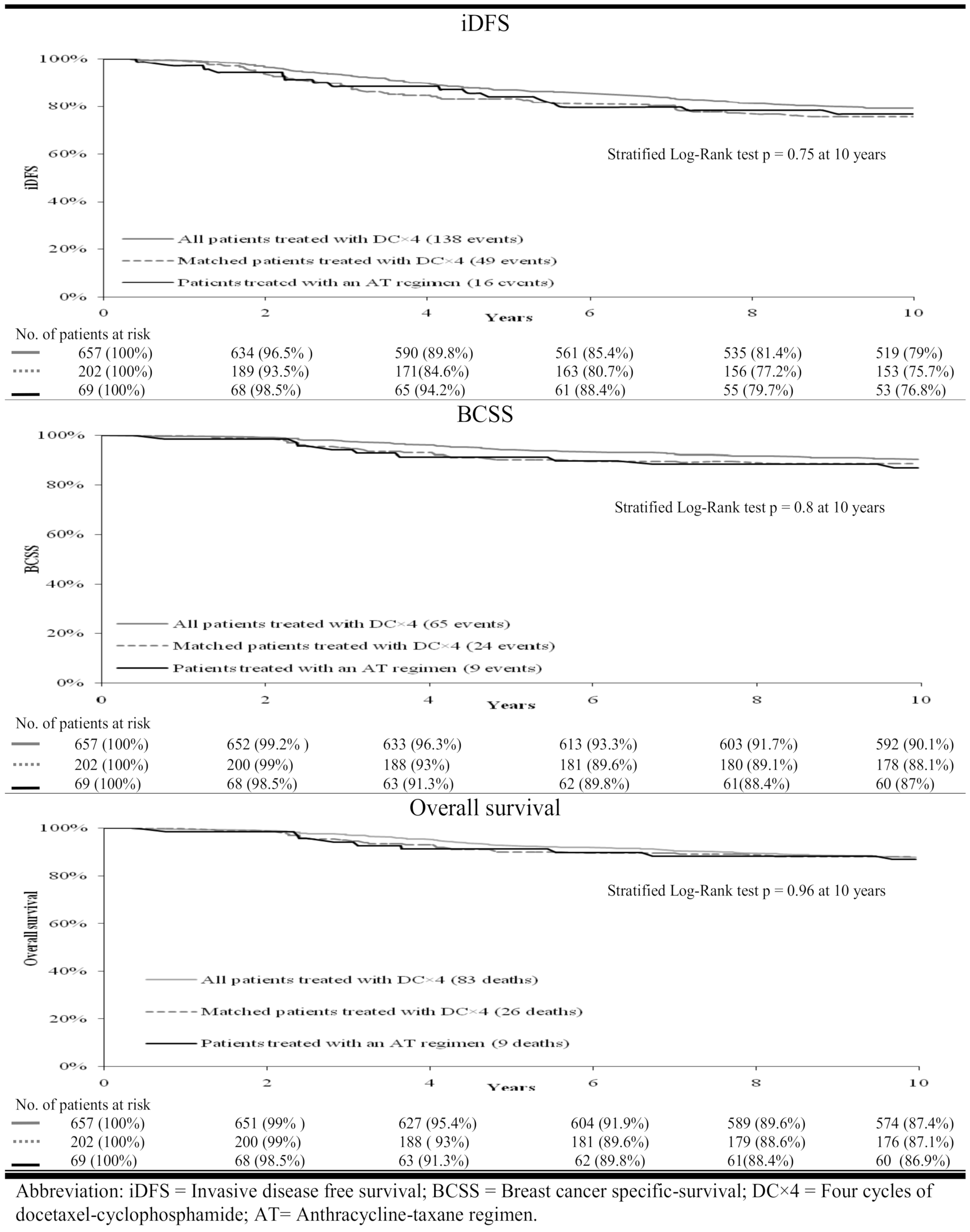
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar] [CrossRef]
- Eisen, A.; Fletcher, G.; Gandhi, S.; Mates, M.; Freedman, O.; Dent, S.; Trudeau, M. Optimal systemic therapy for early breast cancer in women: A clinical practice guideline. Curr. Oncol. 2015, 22, S67–S81. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Breast Cancer (Version 4.2020). Available online: https://www.nccn.org/professionals/physician_gls/default.aspx#breast (accessed on 21 May 2020).
- Azim, H.A.; De Azambuja, E.; Colozza, M.; Bines, J.; Piccart, M.J. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann. Oncol. 2011, 22, 1939–1947. [Google Scholar] [CrossRef]
- Jones, S.E.; Savin, M.A.; Holmes, F.A.; O’Shaughnessy, J.A.; Blum, J.L.; Vukelja, S.; McIntyre, K.J.; Pippen, J.E.; Bordelon, J.H.; Kirby, R.; et al. Phase III Trial Comparing Doxorubicin Plus Cyclophosphamide with Docetaxel Plus Cyclophosphamide As Adjuvant Therapy for Operable Breast Cancer. J. Clin. Oncol. 2006, 24, 5381–5387. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.L.; Flynn, P.J.; Yothers, G.; Asmar, L.; Geyer, C.E., Jr.; Jacobs, S.A. Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J. Clin. Oncol. 2017, 35, 2647–2655. [Google Scholar] [CrossRef]
- Nitz, U.; Gluz, O.; Huober, J.; Kreipe, H.H.; Kates, R.E.; Hartmann, A.; Erber, R.; Moustafa, Z.; Scholz, M.; Lisboa, B.; et al. Final analysis of the prospective WSG-AGO EC-Doc versus FEC phase III trial in intermediate-risk (pN1) early breast cancer: Efficacy and predictive value of Ki67 expression. Ann. Oncol. 2017, 28, 2899. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, D.; Matikas, A.; Malamos, N.; Papakotoulas, P.; Kakolyris, S.; Boukovinas, I. Dose-dense FEC followed by docetaxel versus docetaxel plus cyclophosphamide as adjuvant chemotherapy in women with HER2-negative, axillary lymph node-positive early breast cancer: A multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann. Oncol. 2016, 27, 1873–1878. [Google Scholar] [PubMed]
- Ntellas, P.; Spathas, N.; Agelaki, S.; Zintzaras, E.; Saloustros, E. Taxane & cyclophosphamide vs anthracycline & taxane-based chemotherapy as adjuvant treatment for breast cancer: A pooled analysis of randomized controlled trials by the Hellenic Academy of Oncology. Oncotarget 2019, 10, 1209–1216. [Google Scholar]
- Batra, A.; Hannouf, M.B.; Alsafar, N.; Lupichuk, S. Four cycles of docetaxel and cyclophosphamide as adjuvant chemotherapy in node negative breast cancer: A real-world study. Breast 2020, 54, 1–7. [Google Scholar] [CrossRef]
- ARECCI-Ethics-Guideline-Tool.pdf. Available online: https://albertainnovates.ca/wp-content/uploads/2017/11/ARECCI-Ethics-Guideline-Tool.pdf (accessed on 3 June 2020).
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Stuart, E.A. Matching methods for causal inference: A review and a look forward. Stat. Sci. 2010, 25, 1–21. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | AT (n = 69) | DC4 (n = 657) | p-Value | Matched DC4 (n = 202) | p-Value |
|---|---|---|---|---|---|
| Age (years) | |||||
| Median | 46 | 53 | 47 | ||
| Mean (SD; range) | 45.8 (9.7; 25–69) | 52.6 (9.8; 23–78) | <0.0001 | 46 (9.4; 23–69) | 0.8 |
| Body Mass Index (kg/m2) | |||||
| Median | 25.3 | 27.5 | 26 | ||
| Mean (SD; range) | 27.1 (6.7;17.5–62) | 28.5 (6.6;15.6–64) | 0.1 | 27.1 (5.8;17.3– 49.4) | 0.9 |
| Year of diagnosis [n (%)] | |||||
| 2007–2008 | 11 (15.9%) | 78 (11.9%) | 0.24 | 26 (13%) | 0.8 |
| 2009–2010 | 22 (31.9%) | 275 (41.9%) | 68 (33.5%) | ||
| 2011–2012 | 36 (52.2%) | 304 (46.3%) | 108 (53%) | ||
| Charlson co-morbidity score [n (%)] | |||||
| Mean (SD, range) | 0.04 (0.26; 0–2) | 0.33 (0.67; 0–4) | <0.0001 | 0.04 (0.27; 0–2) | 0.9 |
| Score > 0–no. of patients (%) | 2 (2.9%) | 151(23%) | 0.0005 | 7 (3.4%) | 0.8 |
| 0 | 67 (97.1%) | 506 (77%) | 195 (96.6%) | ||
| 1 | 1 (1.45%) | 97 (14.8%) | 4 (2%) | ||
| 2 | 1 (1.45%) | 44 (6.7%) | 3 (1.4%) | ||
| 3 | 0 | 9 (1.4%) | |||
| 4 | 0 | 1 (0.15%) | |||
| Histology [n (%)] | |||||
| Ductal | 58 (84%) | 479 (72.9%) | 0.12 | 165 (82%) | 0.8 |
| Mixed Ductal-Lobular | 5 (7.25%) | 96 (14.6%) | 20 (9.9%) | ||
| Others | 6 (8.7%) | 82 (12.5%) | 17 (8.4%) | ||
| Stage [n (%)] | |||||
| Stage I | 12 (17.4%) | 253 (38.5%) | 0.0005 | 37 (18.3%) | 0.9 |
| Stage II | 57 (82.6%) | 404 (61.5%) | 165 (81.6%) | ||
| Grade [n (%)] | |||||
| Well differentiated | 0 | 26 (4%) | <0.007 | 0 | 0.9 |
| Moderately differentiated | 6 (8.7%) | 139 (21.2%) | 20 (9.9%) | ||
| Poorly differentiated | 63 (91.3%) | 492 (74.9%) | 182 (90.1%) | ||
| Hormone receptor status [n (%)] | |||||
| Positive | 33 (47.8%) | 468 (71.2%) | <0.0001 | 95 (47%) | 0.9 |
| Negative | 36 (52.2%) | 189 (28.8%) | 107 (52.9%) | ||
| Definitive breast surgery [n (%)] | |||||
| Breast-conserving surgery | 30 (43.5%) | 390 (59.4%) | <0.01 | 90 (44.5%) | 0.9 |
| Mastectomy | 39 (56.5%) | 267 (40.6%) | 112 (55.5%) | ||
| Radiotherapy [n (%)] | 36 (52.2%) | 397 (60.4%) | 0.18 | 103 (51%) | 0.9 |
| Time interval to first cycle of chemotherapy (months) | |||||
| Median | 2.73 | 2.8 | 2.71 | ||
| Mean (SD; range) | 2.86 (0.77; 1.2–4.8) | 3.25 (1.38; 0–15) | 0.5 | 2.82 (0.8; 0–5.9) | 0.7 |
| No. of patients (%) | |||||
| ≥0 months–<3 months | 39 (56%) | 404 (61.5%) | 0.42 | 122 (60%) | 0.6 |
| ≥3 months–<6 months | 30 (43.4%) | 245 (37.2%) | 80 (40%) | ||
| ≥6 months | 0 | 8 (1.2%) | 0 | ||
| Type of adjuvant chemotherapy [n (%)] | |||||
| DC×4 cycles | 657 (100%) | 202 (100%) | |||
| FEC × 3 cycles → D × 3 cycles | 63 (91.3%) | ||||
| AC × 4 cycles → D × 4 cycles | 3 (4.35%) | ||||
| DAC × 6 cycles | 3 (4.35%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannouf, M.; Batra, A.; Lupichuk, S. Four Cycles of Docetaxel-Cyclophosphamide versus Anthracycline-Taxane as Adjuvant Chemotherapy for HER2-Negative, Axillary Lymph Node Negative Breast Cancer: A Real-World Comparison of Alberta Patients Treated 2008–2012. Curr. Oncol. 2021, 28, 1137-1142. https://doi.org/10.3390/curroncol28020109
Hannouf M, Batra A, Lupichuk S. Four Cycles of Docetaxel-Cyclophosphamide versus Anthracycline-Taxane as Adjuvant Chemotherapy for HER2-Negative, Axillary Lymph Node Negative Breast Cancer: A Real-World Comparison of Alberta Patients Treated 2008–2012. Current Oncology. 2021; 28(2):1137-1142. https://doi.org/10.3390/curroncol28020109
Chicago/Turabian StyleHannouf, Malek, Atul Batra, and Sasha Lupichuk. 2021. "Four Cycles of Docetaxel-Cyclophosphamide versus Anthracycline-Taxane as Adjuvant Chemotherapy for HER2-Negative, Axillary Lymph Node Negative Breast Cancer: A Real-World Comparison of Alberta Patients Treated 2008–2012" Current Oncology 28, no. 2: 1137-1142. https://doi.org/10.3390/curroncol28020109
APA StyleHannouf, M., Batra, A., & Lupichuk, S. (2021). Four Cycles of Docetaxel-Cyclophosphamide versus Anthracycline-Taxane as Adjuvant Chemotherapy for HER2-Negative, Axillary Lymph Node Negative Breast Cancer: A Real-World Comparison of Alberta Patients Treated 2008–2012. Current Oncology, 28(2), 1137-1142. https://doi.org/10.3390/curroncol28020109




