Management of the Elderly Patients with High-Grade Serous Ovarian Cancer in the REAL-WORLD Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Surgical and Medical Treatment
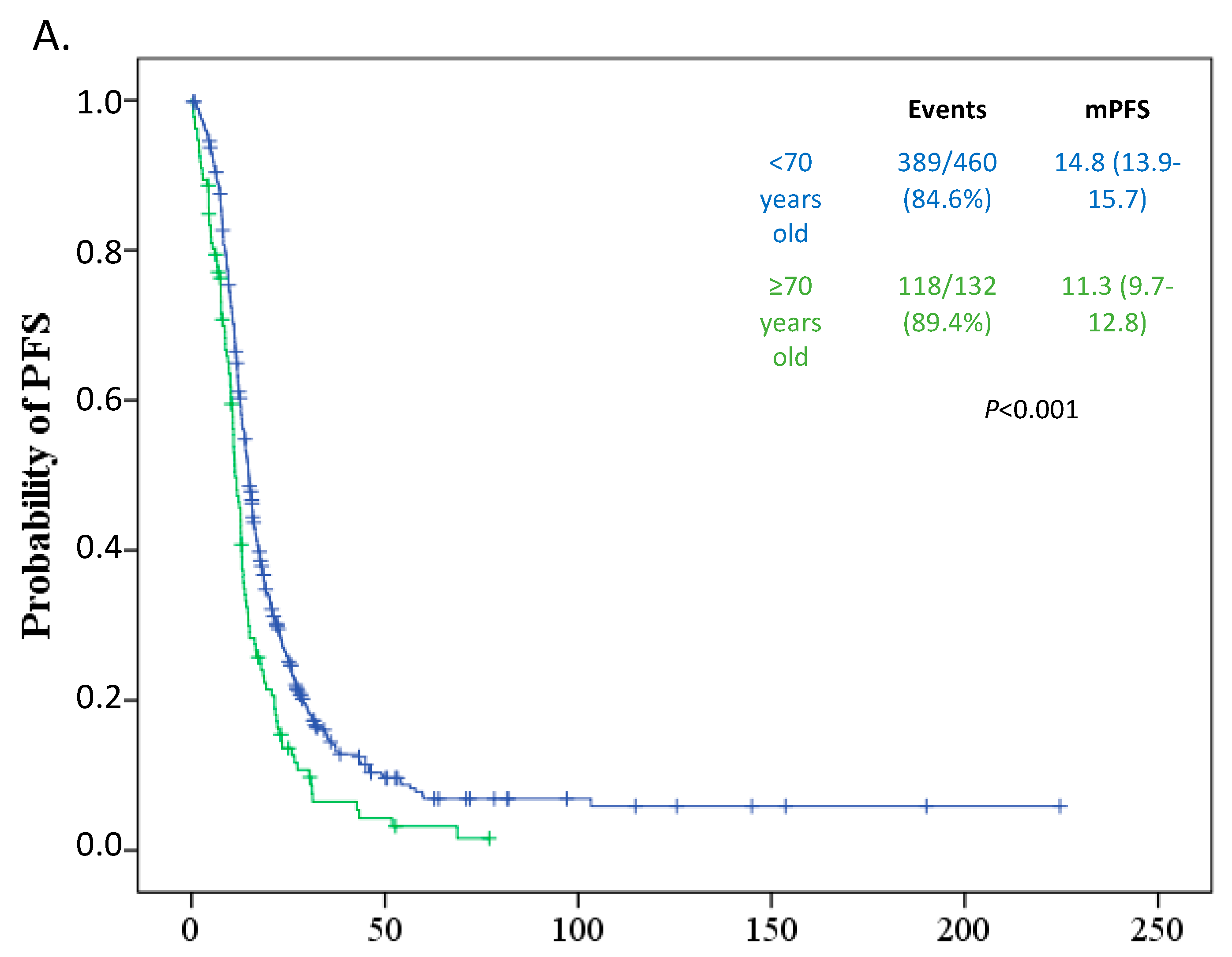
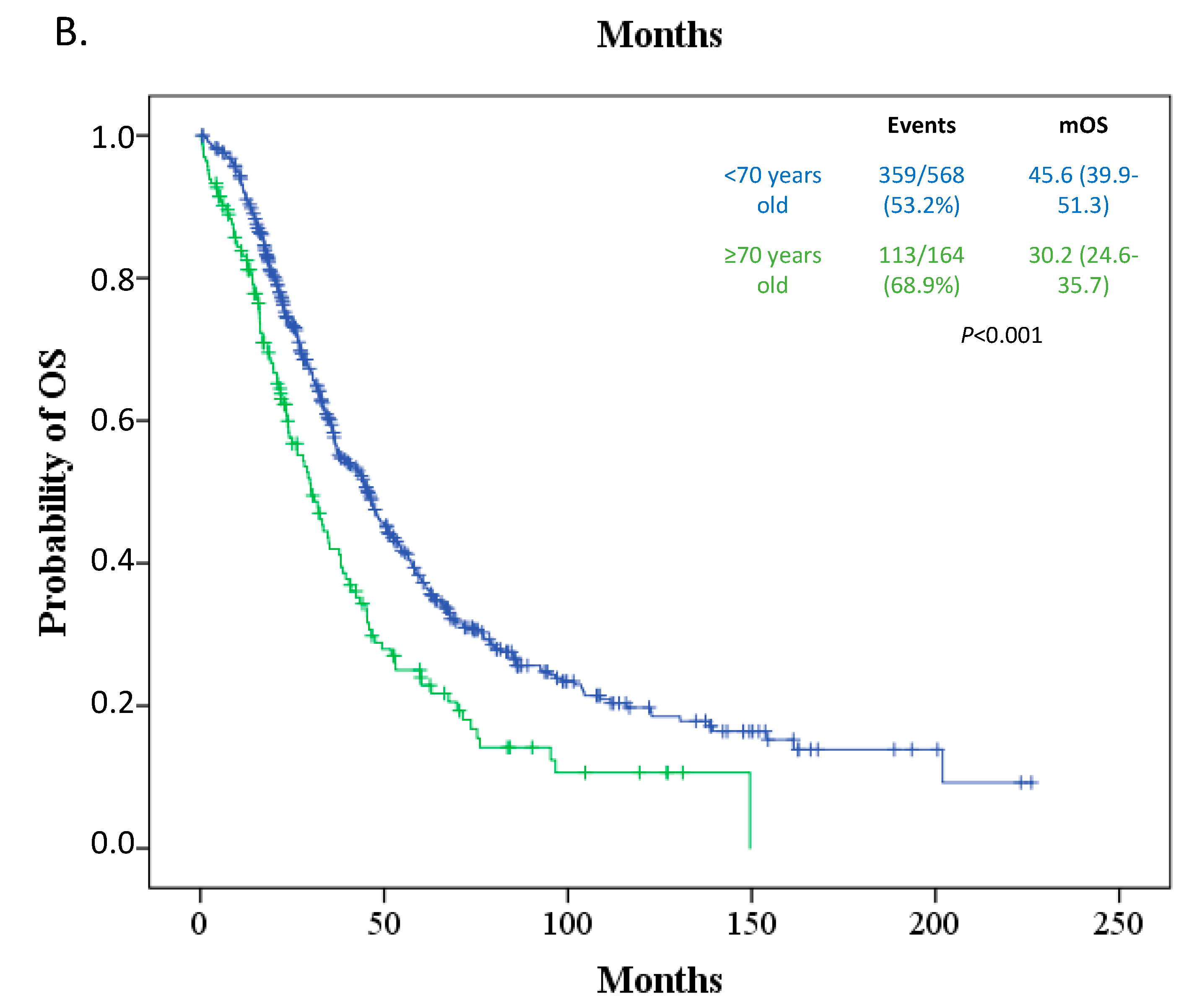
3.3. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Deng, F.; Xu, X.; Lv, M.; Ren, B.; Wang, Y.; Guo, W.; Feng, J.; Chen, X. Age is associated with prognosis in serous ovarian carcinoma. J. Ovarian Res. 2017, 10, 36. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.S.; Garshell, J.; Miller, D.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2008.
- Ries, L.G.; Young, J.; Keel, G.; Eisner, M.; Lin, Y.; Horner, M. SEER Survival Monograph: Cancer Survival among Adults: US SEER Program, 1988–2001, Patient and Tumor Characteristics; SEER Program, NIH Pub; National Cancer Institute: Bethesda, MD, USA, 2007; pp. 193–202.
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed]
- Norquist, B.M.; Harrell, M.I.; Brady, M.F.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Yi, Q.; Burger, R.A.; et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA Oncol. 2016, 2, 482–490. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Uyar, D.; Frasure, H.E.; Markman, M.; von Gruenigen, V.E. Treatment patterns by decade of life in elderly women (> or =70 years of age) with ovarian cancer. Gynecol. Oncol. 2005, 98, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Gerestein, C.G.; Damhuis, R.A.; de Vries, M.; Reedijk, A.; Burger, C.W.; Kooi, G.S. Causes of postoperative mortality after surgery for ovarian cancer. Eur. J. Cancer 2009, 45, 2799–2803. [Google Scholar] [CrossRef]
- Bun, S.; Yunokawa, M.; Ebata, T.; Shimomura, A.; Shimoi, T.; Kodaira, M.; Yonemori, K.; Shimizu, C.; Fujiwara, Y.; Kato, T.; et al. Feasibility of dose-dense paclitaxel/carboplatin therapy in elderly patients with ovarian, fallopian tube, or peritoneal cancer. Cancer Chemother. Pharmacol. 2016, 78, 745–752. [Google Scholar] [CrossRef]
- Joseph, N.; Clark, R.M.; Dizon, D.S.; Lee, M.S.; Goodman, A.; Boruta, D.; Schorge, J.O.; del Carmen, M.G.; Growdon, W.B. Delay in chemotherapy administration impacts survival in elderly patients with epithelial ovarian cancer. Gynecol. Oncol. 2015, 137, 401–405. [Google Scholar] [CrossRef]
- Fourcadier, E.; Tretarre, B.; Gras-Aygon, C.; Ecarnot, F.; Daures, J.P.; Bessaoud, F. Under-treatment of elderly patients with ovarian cancer: A population based study. BMC Cancer 2015, 15, 937. [Google Scholar] [CrossRef] [PubMed]
- Freyer, G.; Tinker, A.V. Clinical trials and treatment of the elderly diagnosed with ovarian cancer. Int. J. Gynecol. Cancer 2011, 21, 776–781. [Google Scholar] [CrossRef]
- Le Saux, O.; Falandry, C.; Gan, H.K.; You, B.; Freyer, G.; Peron, J. Inclusion of elderly patients in oncology clinical trials. Ann. Oncol. 2016, 27, 1799–1804. [Google Scholar] [CrossRef]
- Bhatla, N.; Denny, L. FIGO Cancer Report 2018. Int. J. Gynecol. Obstet. 2018, 143, 2–3. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Collett, D. Modelling Survival Data in Medical Research, 3rd ed.; Chapman and Hall/CRC: New York, NY, USA, 2014. [Google Scholar]
- Klein, J.; Moeschberger, M. Survival Analysis: Techniques for Censored and Truncated Data, 2nd ed.; Springer-Verlag: New York, NY, USA, 2003. [Google Scholar]
- Pectasides, D.; Fountzilas, G.; Aravantinos, G.; Kalofonos, C.; Efstathiou, H.; Farmakis, D.; Skarlos, D.; Pavlidis, N.; Economopoulos, T.; Dimopoulos, M.A. Advanced stage clear-cell epithelial ovarian cancer: The Hellenic cooperative oncology group experience. Gynecol. Oncol. 2006, 102, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.K.; Nayak, B. Management of Ovarian Cancer in Elderly. Rev. Recent Clin. Trials 2015, 10, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Reid, M.S.; Fong, D.N.; Myers, T.K.N.; Landrum, L.M.; Moxley, K.M.; Walker, J.L.; McMeekin, D.S.; Mannel, R.S. Ovarian cancer in the octogenarian: Does the paradigm of aggressive cytoreductive surgery and chemotherapy still apply? Gynecol. Oncol. 2008, 110, 133–139. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Langstraat, C.; Aletti, G.D.; Cliby, W.A. Morbidity, mortality and overall survival in elderly women undergoing primary surgical debulking for ovarian cancer: A delicate balance requiring individualization. Gynecol. Oncol. 2011, 123, 187–191. [Google Scholar] [CrossRef]
- Fanfani, F.; Fagotti, A.; Salerno, M.G.; Margariti, P.A.; Gagliardi, M.L.; Gallotta, V.; Vizzielli, G.; Panico, G.; Monterossi, G.; Scambia, G. Elderly and very elderly advanced ovarian cancer patients: Does the age influence the surgical management? Eur. J. Surg. Oncol. 2012, 38, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Ananth, C.V.; Tsui, J.; Glied, S.A.; Burke, W.M.; Lu, Y.-S.; Neugut, A.I.; Herzog, T.J.; Hershman, D.L. Comparative effectiveness of upfront treatment strategies in elderly women with ovarian cancer. Cancer 2014, 120, 1246–1254. [Google Scholar] [CrossRef]
- Chan, J.K.; Brady, M.F.; Penson, R.T.; Huang, H.; Birrer, M.J.; Walker, J.L.; DiSilvestro, P.A.; Rubin, S.C.; Martin, L.P.; Davidson, S.A.; et al. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. N. Engl. J. Med. 2016, 374, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Scambia, G.; Katsaros, D.; Gallo, C.; Pujade-Lauraine, E.; De Placido, S.; Bologna, A.; Weber, B.; Raspagliesi, F.; Panici, P.B.; et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 396–405. [Google Scholar] [CrossRef]
- Falandry, C.; Savoye, A.M.; Stefani, L.; Tinquaut, F.; Lorusso, D.; Herrstedt, J.; Bourbouloux, E.; Floquet, A.; Brachet, P.E.; Zannetti, A.; et al. EWOC-1: A randomized trial to evaluate the feasibility of three different first-line chemotherapy regimens for vulnerable elderly women with ovarian cancer (OC): A GCIG-ENGOT-GINECO study. J. Clin. Oncol. 2019, 37, 5508. [Google Scholar] [CrossRef]
- Selle, F.; Colombo, N.; Korach, J.; Mendiola, C.; Cardona, A.; Ghazi, Y.; Oza, A.M. Safety and Efficacy of Extended Bevacizumab Therapy in Elderly (≥70 Years) Versus Younger Patients Treated for Newly Diagnosed Ovarian Cancer in the International ROSiA Study. Int. J. Gynecol. Cancer 2018, 28, 729–737. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total Population | Age < 70 | Age ≥ 70 | |
|---|---|---|---|---|
| Median (Range) | Median (Range) | Median (Range) | ||
| Age | 61.5 (24.7–89.2) | 57.9 (24.7–69.9) | 74.2 (70.0–89.2) | |
| N (%) | N (%) | N (%) | p | |
| ECOG-PS | <0.001 | |||
| 0–1 | 575 (78.2) | 471 (82.6) | 104 (63.0) | |
| ≥2 | 111 (15.2) | 56 (9.8) | 55 (33.3) | |
| Missing | 49 (6.6) | 43 (7.6) | 6 (3.7) | |
| Stage | 0.906 | |||
| III | 605 (82.3) | 470 (82.4) | 135 (81.8) | |
| IV | 123 (16.7) | 95 (16.7) | 28 (17.0) | |
| Missing | 7 (1.0) | 5 (0.9) | 2 (1.2) | |
| Surgery | <0.001 | |||
| PDS | 578 (78.6) | 461 (80.9) | 117 (70.9) | |
| IDS | 110 (15.0) | 83 (14.6) | 27 (16.3) | |
| No surgery | 44 (6.0) | 23 (4.0) | 21 (12.7) | |
| Missing | 3 (0.4) | 3 (0.5) | 0 | |
| Surgical outcome | 0.047 | |||
| Optimal | 258 (35.1) | 212 (37.2) | 46 (27.9) | |
| Suboptimal | 447 (60.8) | 338 (59.3) | 109 (66.1) | |
| Missing | 30 (4.1) | 20 (3.5) | 10 (6.0) | |
| First-line chemotherapy | <0.001 | |||
| Platinum doublet | 596 (81.1) | 484 (84.9) | 112 (67.9) | |
| Carboplatin | 35 (4.8) | 9 (1.6) | 26 (15.7) | |
| Missing | 104 (14.1) | 77 (13.5) | 27 (16.4) | |
| Bevacizumab administration | <0.001 | |||
| Yes | 121 (16.5) | 108 (18.9) | 13 (7.8) | |
| No | 614 (83.5) | 462 (81.1) | 152 (92.2) | |
| gBRCA testing | 0.108 | |||
| Yes | 110 (15.0) | 92 (16.1) | 18 (10.6) | |
| No | 625 (85.0) | 478 (83.9) | 147 (89.4) |
| PFS | OS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | 95% CI | p-Value * | HR | 95% CI | p-Value | HR | 95% CI | p-Value * | HR | 95% CI | p-Value | |
| Age | <0.001 | 0.075 | <0.001 | 0.294 | ||||||||
| <70 | 1 | 1 | 1 | 1 | ||||||||
| ≥70 | 1.54 | 1.25–1.89 | 1.26 | 0.98–1.62 | 1.60 | 1.29–1.98 | 1.15 | 0.89–1.48 | ||||
| ECOG-PS | <0.001 | 0.018 | <0.001 | <0.001 | ||||||||
| 0–1 | 1 | 1 | 1 | 1 | ||||||||
| ≥2 | 2.00 | 1.59–2.53 | 1.42 | 1.06–1.90 | 2.64 | 2.09–3.35 | 1.80 | 1.37–2.38 | ||||
| Stage | <0.001 | 0.182 | <0.001 | 0.311 | ||||||||
| III | 1 | 1 | 1 | 1 | ||||||||
| IV | 1.56 | 1.24–1.95 | 1.19 | 0.92–1.56 | 1.55 | 1.22–1.95 | 1.15 | 0.87–1.52 | ||||
| Surgery | <0.001 | 0.007 | <0.001 | <0.001 | ||||||||
| PDS | 1 | 1 | 1 | 1 | ||||||||
| IDS | 0.95 | 0.76–1.20 | 1.08 | 0.83–1.42 | 1.09 | 0.82–1.45 | 1.34 | 0.96–1.87 | ||||
| No surgery | 3.38 | 2.38–4.79 | 2.22 | 1.34–3.66 | 4.74 | 2.00–8.83 | 3.64 | 2.29–5.76 | ||||
| Surgical outcome | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| Complete | 1 | 1 | 1 | 1 | ||||||||
| Optimal/Suboptimal | 1.877 | 1.55–2.27 | 1.55 | 1.25–1.92 | 2.08 | 1.68–2.57 | 1.83 | 1.45–2.31 | ||||
| First-line chemotherapy | 0.004 | 0.035 | <0.001 | <0.001 | ||||||||
| Platinum doublet | 1 | 1 | 1 | 1 | ||||||||
| Carboplatin | 1.73 | 1.19–2.50 | 1.62 | 1.03–2.52 | 2.46 | 1.68–3.59 | 2.19 | 1.43–3.38 | ||||
| Bevacizumab administration | 0.001 | 0.717 | 0.010 | 0.332 | ||||||||
| Yes | 1 | 1 | 1 | 1 | ||||||||
| No | 1.45 | 1.16–1.82 | 1.19 | 0.92–1.56 | 1.47 | 1.09–1.98 | 1.21 | 0.83–1.76 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liontos, M.; Papatheodoridi, A.; Andrikopoulou, A.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A.; Zagouri, F.; Bamias, A.; Dimopoulos, M.-A. Management of the Elderly Patients with High-Grade Serous Ovarian Cancer in the REAL-WORLD Setting. Curr. Oncol. 2021, 28, 1143-1152. https://doi.org/10.3390/curroncol28020110
Liontos M, Papatheodoridi A, Andrikopoulou A, Thomakos N, Haidopoulos D, Rodolakis A, Zagouri F, Bamias A, Dimopoulos M-A. Management of the Elderly Patients with High-Grade Serous Ovarian Cancer in the REAL-WORLD Setting. Current Oncology. 2021; 28(2):1143-1152. https://doi.org/10.3390/curroncol28020110
Chicago/Turabian StyleLiontos, Michalis, Alkistis Papatheodoridi, Angeliki Andrikopoulou, Nikolaos Thomakos, Dimitrios Haidopoulos, Alexandros Rodolakis, Flora Zagouri, Aristotelis Bamias, and Meletios-Athanasios Dimopoulos. 2021. "Management of the Elderly Patients with High-Grade Serous Ovarian Cancer in the REAL-WORLD Setting" Current Oncology 28, no. 2: 1143-1152. https://doi.org/10.3390/curroncol28020110
APA StyleLiontos, M., Papatheodoridi, A., Andrikopoulou, A., Thomakos, N., Haidopoulos, D., Rodolakis, A., Zagouri, F., Bamias, A., & Dimopoulos, M.-A. (2021). Management of the Elderly Patients with High-Grade Serous Ovarian Cancer in the REAL-WORLD Setting. Current Oncology, 28(2), 1143-1152. https://doi.org/10.3390/curroncol28020110








