A Multidisciplinary Approach to Implement Personalized Breast Cancer Treatment and Care Plans
Abstract
1. Introduction
2. Materials and Methods
2.1. The Intervention
2.1.1. The Care Plan Application (CP App)
2.1.2. Personalized Treatment Plan (PTP)
2.1.3. Personalized Care Plan (PCP)
2.2. Implementation Process
2.2.1. Context of Initiative Implementation
2.2.2. Primary Outcome Measure
2.2.3. Primary Process Measure
2.2.4. Balancing Measures
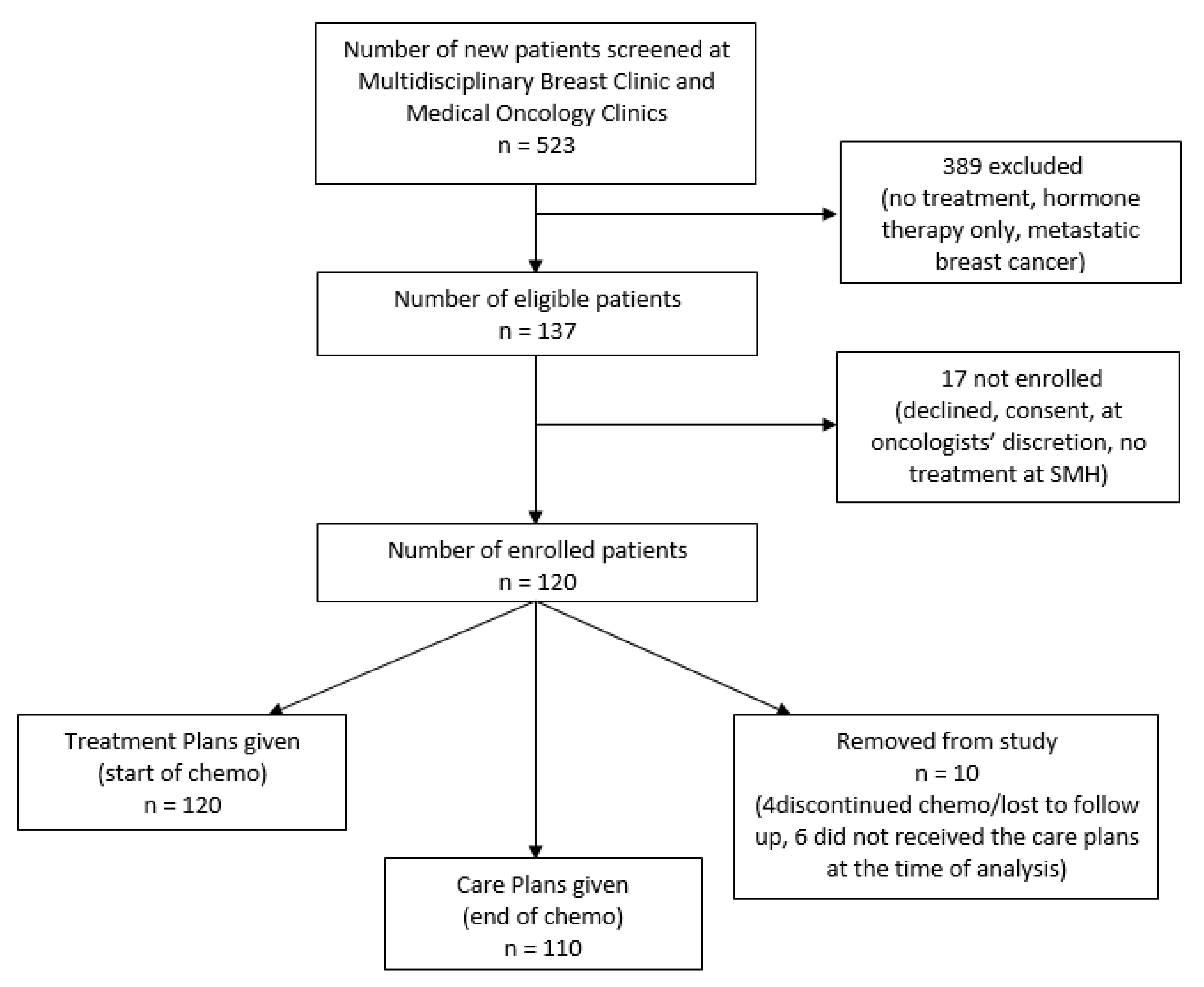
2.3. Participants
2.3.1. Patient Recruitment
2.3.2. Statistical Analysis
3. Results
- -
- PDSA 1: April–July 2017
- -
- PDSA 2: August–November 2017
- -
- PDSA 3: December 2017–March 2018
- -
- PDSA 4: April–July 2018
- -
- PDSA 5: August–November 2018
- -
- PDSA 6: December 2018–March 2019
- -
- PDSA 7: April–July 2019
3.1. Primary Outcome Measure Results
3.2. Process Measure Results
3.3. Balancing Measure Results
3.4. Collaboration with Institutional IT Department
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Mid-Chemotherapy | End of Chemotherapy | 3-Months Post Chemotherapy | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 1 | 2 | 3 | 4 | 5 | n | 1 | 2 | 3 | 4 | 5 | n | 1 | 2 | 3 | 4 | 5 | |
| Prepared to live as cancer survivor | 109 | 7 (6.5) | 6 (5.6) | 28 (26.2) | 45 (42.1) | 21 (19.6) | 117 | 2 (1.8) | 5 (4.5) | 27 (24.5) | 49 (44.5) | 27 (24.5) | 85 | 3 (3.8) | 3 (3.8) | 27 (33.8) | 32 (40.0) | 15 (18.8) |
| More easily communicate with other health care providers | 109 | 4 (3.7) | 5 (4.6) | 18 (16.7) | 58 (53.7) | 23 (21.3) | 117 | 1 (0.9) | 1 (0.9) | 18 (17.0) | 56 (52.8) | 30 (28.3) | 85 | 1 (1.2) | 3 (3.8) | 19 (23.8) | 38 (47.5) | 19 (23.8) |
| Better communicate with medical team | 109 | 2 (1.8) | 4 (3.7) | 13 (11.9) | 60 (55.0) | 30 (27.5) | 117 | 0 (0.0) | 3 (2.7) | 15 (13.3) | 55 (48.7) | 40 (35.4) | 85 | 0 (0.0) | 1 (1.2) | 17 (21.0) | 38 (46.9) | 25 (30.9) |
| Reduce risk of delayed complications for treatment | 109 | 2 (1.9) | 7 (6.7) | 34 (32.4) | 40 (38.1) | 22 (21.0) | 117 | 0 (0.0) | 2 (1.8) | 39 (35.8) | 43 (39.4) | 25 (22.9) | 85 | 1 (1.3) | 2 (2.5) | 34 (43.0) | 30 (38.0) | 12 (15.2) |
| Better deal with depression or depressed mood | 109 | 1 (0.9) | 7 (6.5) | 44 (41.1) | 42 (39.3) | 13 (12.1) | 117 | 2 (1.9) | 3 (2.8) | 40 (37.0) | 46 (42.6) | 17 (15.7) | 85 | 0 (0.0) | 7 (8.6) | 37 (45.7) | 28 (34.6) | 9 (11.1) |
| Enlist family’s support | 109 | 1 (0.9) | 5 (4.6) | 19 (17.4) | 54 (49.5) | 30 (27.5) | 117 | 0 (0.0) | 3 (2.7) | 24 (21.6) | 51 (45.9) | 33 (29.7) | 85 | 1 (1.2) | 1 (1.2) | 24 (29.6) | 38 (46.9) | 17 (21.0) |
| Better understand lifestyle changes that reduce recurrence risk | 109 | 6 (5.6) | 10 (9.3) | 30 (28.0) | 42 (39.3) | 19 (17.8) | 117 | 3 (2.7) | 4 (3.6) | 23 (20.9) | 56 (50.9) | 24 (21.8) | 85 | 0 (0.0) | 7 (8.6) | 25 (30.9) | 37 (45.7) | 12 (14.8) |
| Better understand lifestyle factors leading to cancer onset | 109 | 7 (6.5) | 7 (6.5) | 34 (31.5) | 45 (41.7) | 15 (13.9) | 117 | 3 (2.8) | 7 (6.4) | 28 (25.7) | 48 (44.0) | 23 (21.1) | 85 | 0 (0.0) | 6 (7.4) | 23 (28.4) | 40 (49.4) | 12 (14.8) |
| Actively participate in medical management of cancer | 109 | 1 (0.9) | 1 (0.9) | 19 (17.4) | 62 (56.9) | 26 (23.9) | 117 | 2 (1.9) | 1 (0.9) | 26 (24.1) | 57 (52.8) | 22 (20.4) | 85 | 0 (0.0) | 2 (2.5) | 20 (25.0) | 38 (47.5) | 20 (25.0) |
| Make nutritional changes which aid recovery | 109 | 4 (3.7) | 8 (7.4) | 23 (21.3) | 52 (48.1) | 21 (19.4) | 117 | 2 (1.8) | 1 (0.9) | 31 (28.2) | 51 (46.4) | 25 (22.7) | 85 | 0 (0.0) | 4 (4.9) | 26 (32.1) | 39 (48.1) | 12 (14.8) |
| Deal more effectively with pain | 109 | 2 (1.9) | 7 (6.5) | 36 (33.6) | 45 (42.1) | 17 (15.9) | 117 | 0 (0.0) | 7 (6.4) | 32 (29.4) | 44 (40.4) | 26 (23.9) | 85 | 0 (0.0) | 4 (4.9) | 39 (48.1) | 26 (32.1) | 12 (14.8) |
| Make physical activity changes to aid recovery | 109 | 5 (4.7) | 4 (3.7) | 27 (25.2) | 52 (48.6) | 19 (17.8) | 117 | 2 (1.9) | 3 (2.8) | 32 (29.6) | 53 (49.1) | 18 (16.7) | 85 | 0 (0.0) | 1 (1.2) | 28 (34.6) | 38 (46.9) | 14 (17.3) |
| Ask right questions about treatment | 109 | 1 (0.9) | 3 (2.8) | 8 (7.3) | 71 (65.1) | 26 (23.9) | 117 | 0 (0.0) | 1 (0.9) | 19 (16.8) | 59 (52.2) | 34 (30.1) | 85 | 0 (0.0) | 2 (2.5) | 14 (17.3) | 43 (53.1) | 22 (27.2) |
| Be informed of community resources available | 109 | 6 (5.6) | 3 (2.8) | 26 (24.1) | 53 (49.1) | 20 (18.5) | 117 | 0 (0.0) | 5 (4.4) | 26 (23.0) | 61 (54.0) | 21 (18.6) | 85 | 2 (2.5) | 4 (4.9) | 24 (29.6) | 37 (45.7) | 14 (17.3) |
| Provided helpful advice on issues related to return to work | 109 | 10 (11.2) | 10 (11.2) | 30 (33.7) | 27 (30.3) | 12 (13.5) | 117 | 6 (6.4) | 5 (5.3) | 40 (42.6) | 30 (31.9) | 13 (13.8) | 85 | 4 (6.2) | 5 (7.7) | 32 (49.2) | 19 (29.2) | 5 (7.7) |
| Deal more effectively with side effects of treatment | 109 | 1 (0.9) | 7 (6.4) | 25 (22.9) | 51 (46.8) | 25 (22.9) | 117 | 0 (0.0) | 4 (3.5) | 23 (20.4) | 54 (47.8) | 32 (28.3) | 85 | 0 (0.0) | 2 (2.5) | 27 (33.3) | 37 (45.7) | 15 (18.5) |
| Better deal with stressful emotions and anxiety | 109 | 2 (1.8) | 8 (7.3) | 36 (33.0) | 47 (43.1) | 16 (14.7) | 117 | 1 (0.9) | 2 (1.8) | 30 (26.5) | 56 (49.6) | 24 (21.2 | 85 | 0 (0.0) | 6 (7.4) | 31 (38.3) | 34 (42.0) | 10 (12.3) |
References
- Breast Cancer Statistics—Canadian Cancer Society [Internet]. Available online: https://www.cancer.ca:443/en/cancer-information/cancer-type/breast/statistics/?region=on (accessed on 12 July 2020).
- Playdon, M.; Ferrucci, L.M.; McCorkle, R.; Stein, K.D.; Cannady, R.; Sanft, T.; Cartmel, B. Health information needs and preferences in relation to survivorship care plans of long-term cancer survivors in the American Cancer Society’s Study of Cancer Survivors-I. J. Cancer Surviv. 2016, 10, 674–685. [Google Scholar] [CrossRef]
- Husson, O.; Holterhues, C.; Mols, F.; Nijsten, T.; van de Poll-Franse, L.V. Melanoma survivors are dissatisfied with perceived information about their diagnosis, treatment and follow-up care. Br. J. Dermatol. 2010, 163, 879–881. [Google Scholar] [CrossRef]
- Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition; The National Academies Press: Washington, DC, USA, 2006; Available online: https://www.nap.edu/catalog/11468/from-cancer-patient-to-cancer-survivor-lost-in-transition (accessed on 15 July 2020).
- Haq, R.; Heus, L.; Baker, N.A.; Dastur, D.; Leung, F.H.; Leung, E.; Li, B.; Vu, K.; Parsons, J.A. Designing a multifaceted survivorship care plan to meet the information and communication needs of breast cancer patients and their family physicians: Results of a qualitative pilot study. BMC Med. Inform. Decis. Mak. 2013, 13, 76. [Google Scholar] [CrossRef]
- Haq, R.; Kong, A.; Leung, Y.M.; Richter, S.; Vu, K.; Jovicic, A.; Soren, B.; Li, B.; Gulasingam, P.; Jainudeen, N. Personalized multifaceted care plans for breast cancer survivors: Results of a randomized study. Oncol. Exch. 2016, 15, 15–21. [Google Scholar]
- McCabe, M.S.; Bhatia, S.; Oeffinger, K.C.; Reaman, G.H.; Tyne, C.; Wollins, D.S.; Hudson, M.M. American Society of Clinical Oncology statement: Achieving high-quality cancer survivorship care. J. Clin. Oncol. 2013, 31, 631–640. [Google Scholar] [CrossRef]
- Commission on Cancer (CoC). The CoCstandard 3.3 Survivorship Care Plan Explained [Internet]. Available online: https://www.carevive.com/wp-content/uploads/2015/02/resource_coc-standard-explained.pdf (accessed on 12 July 2020).
- Cancer Care Ontario. Ontario Cancer Plan IV 2015–2019 [Internet]. Available online: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/CCOOntarioCancerPlan4.pdf (accessed on 15 July 2020).
- Jabson, J.M. Follow-up care instructions, treatment summaries, and cancer survivors’ receipt of follow-up health care and late/long term effects. Support. Care Cancer 2015, 23, 1851–1856. [Google Scholar] [CrossRef]
- Tevaarwerk, A.J.; Sesto, M.E. Continued Challenges to the Adoption and Implementation of Survivorship Care Plans. J. Oncol. Pract. 2018, 14, 573–576. [Google Scholar] [CrossRef]
- Birken, S.A.; Mayer, D.K.; Weiner, B.J. Survivorship care plans: Prevalence and barriers to use. J. Cancer Educ. 2013, 28, 290–296. [Google Scholar] [CrossRef]
- Mayer, D.K.; Birken, S.A.; Chen, R.C. Avoiding implementation errors in cancer survivorship care plan effectiveness studies. J. Clin. Oncol. 2015, 33, 3528–3530. [Google Scholar] [CrossRef]
- Grunfeld, E.; Julian, J.A.; Pond, G.; Maunsell, E.; Coyle, D.; Folkes, A.; Joy, A.A.; Provencher, L.; Rayson, D.; Rheaume, D.E.; et al. Evaluating survivorship care plans: Results of a randomized, clinical trial of patients with breast cancer. J. Clin. Oncol. 2011, 29, 4755–4762. [Google Scholar] [CrossRef]
- Brennan, M.E.; Gormally, J.F.; Butow, P.; Boyle, F.M.; Spillane, A.J. Survivorship care plans in cancer: A systematic review of care plan outcomes. Br. J. Cancer 2014, 111, 1899–1908. [Google Scholar] [CrossRef]
- Mayer, D.K.; Birken, S.A.; Check, D.K.; Chen, R.C. Summing it up: An integrative review of studies of cancer survivorship care plans (2006–2013). Cancer 2015, 121, 978–996. [Google Scholar] [CrossRef]
- Birken, S.A.; Clary, A.S.; Bernstein, S.; Bolton, J.; Tardif-Douglin, M.; Mayer, D.K.; Deal, A.M.; Jacobs, S.R. Strategies for successful survivorship care plan implementation: Results from a qualitative study. J. Oncol. Pract. 2018, 14, e462–e483. [Google Scholar] [CrossRef]
- Parry, C.; Kent, E.E.; Forsythe, L.P.; Alfano, C.M.; Rowland, J.H. Can’t see the forest for the care plan: A call to revisit the context of care planning. J. Clin. Oncol. 2013, 31, 2651–2653. [Google Scholar] [CrossRef]
- Hryniuk, W.; Simpson, R.; McGowan, A.; Carter, P. Patient perceptions of a comprehensive cancer navigation service. Curr. Oncol. 2014, 21, 69–76. [Google Scholar] [CrossRef]
- Wolf, M.S.; Chang, C.H.; Davis, T.; Makoul, G. Development and validation of the Communication and Attitudinal Self-Efficacy scale for cancer (CASE-cancer). Patient Educ. Couns. 2005, 57, 333–341. [Google Scholar] [CrossRef]
- Kidane, A.; McCrady, M.; Kacikanis, A.; Santiago, C.; Transfer of Accountability for Breast Cancer Patients. 2018 Nursing Week BPG Sustainability Virtual Poster Gallery [Internet]. Available online: https://issuu.com/stmichaelshospital/docs/bpg-2018 (accessed on 9 July 2020).
- Halpern, M.T.; McCabe, M.S.; Burg, M.A. The Cancer Survivorship Journey: Models of Care, Disparities, Barriers, and Future Directions. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 231–239. [Google Scholar] [CrossRef]
- Dulko, D.; Pace, C.M.; Dittus, K.L.; Sprague, B.L.; Pollack, L.A.; Hawkins, N.A.; Geller, B.M. Barriers and facilitators to implementing cancer survivorship care plans. Oncol. Nurs. Forum. 2013, 40, 575–580. [Google Scholar] [CrossRef]
- Nicolaije, K.; Ezendam, N.; Vos, C.M.; Pijnenborg, J.M.; Boll, D.; Bos, E.A.; Hermans, R.H.; Engelhart, K.C.; Haartsen, J.E.; Pijlman, B.M.; et al. Impact of an automatically generated cancer survivorship care plan on patient-reported outcomes in routine clinical practice: Longitudinal outcomes of a pragmatic, cluster randomized trial. J. Clin. Oncol. 2015, 33, 3550–3559. [Google Scholar] [CrossRef]
- Ganz, P.A.; Casillas, J.; Hahn, E.E. Ensuring quality care for cancer survivors: Implementing the survivorship care plan. Semin. Oncol. Nurs. 2008, 24, 208–217. [Google Scholar] [CrossRef]
- Alfano, C.M.; Smith, T.; de Moor, J.S.; Glasgow, R.E.; Khoury, M.J.; Hawkins, N.A.; Stein, K.D.; Rechis, R.; Parry, C.; Leach, C.R.; et al. An action plan for translating cancer survivorship research into care. J. Natl. Cancer Inst. 2014, 106, dju287. [Google Scholar] [CrossRef]
- Richards, M.; Corner, J.; Maher, J. The National Cancer Survivorship Initiative: New and emerging evidence on the ongoing needs of cancer survivors. Br. J. Cancer 2011, 105 (Suppl. 1), S1–S4. [Google Scholar] [CrossRef]
- Creating and Sustaining Survivorship Care Plans in Practice [Internet]. ONS Voice. Available online: https://voice.ons.org/news-and-views/survivorship-care-plans-in-practice (accessed on 12 July 2020).
- National Academies of Sciences, Engineering, Health and Medicine Division, Board on Health Care Services, National Cancer Policy Forum. Long-Term Survivorship Care after Cancer Treatment: Proceedings of a Workshop; National Academies Press: Washington, DC, USA, 2018; 161p. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538366/ (accessed on 12 July 2020).
- Hahn, E.E.; Ganz, P.A. Survivorship programs and care plans in practice: Variations on a theme. J. Oncol. Prac. 2011, 7, 70–75. [Google Scholar] [CrossRef]
- Saulet, D.; Adam, S.; New Research Says Survivorship Care Plans Often Fall Short. Here Are 3 Ways to Do Better. [Internet]. Available online: http://www.advisory.com/research/oncology-roundtable/oncology-rounds/2018/09/survivorship-care-plans (accessed on 12 July 2020).
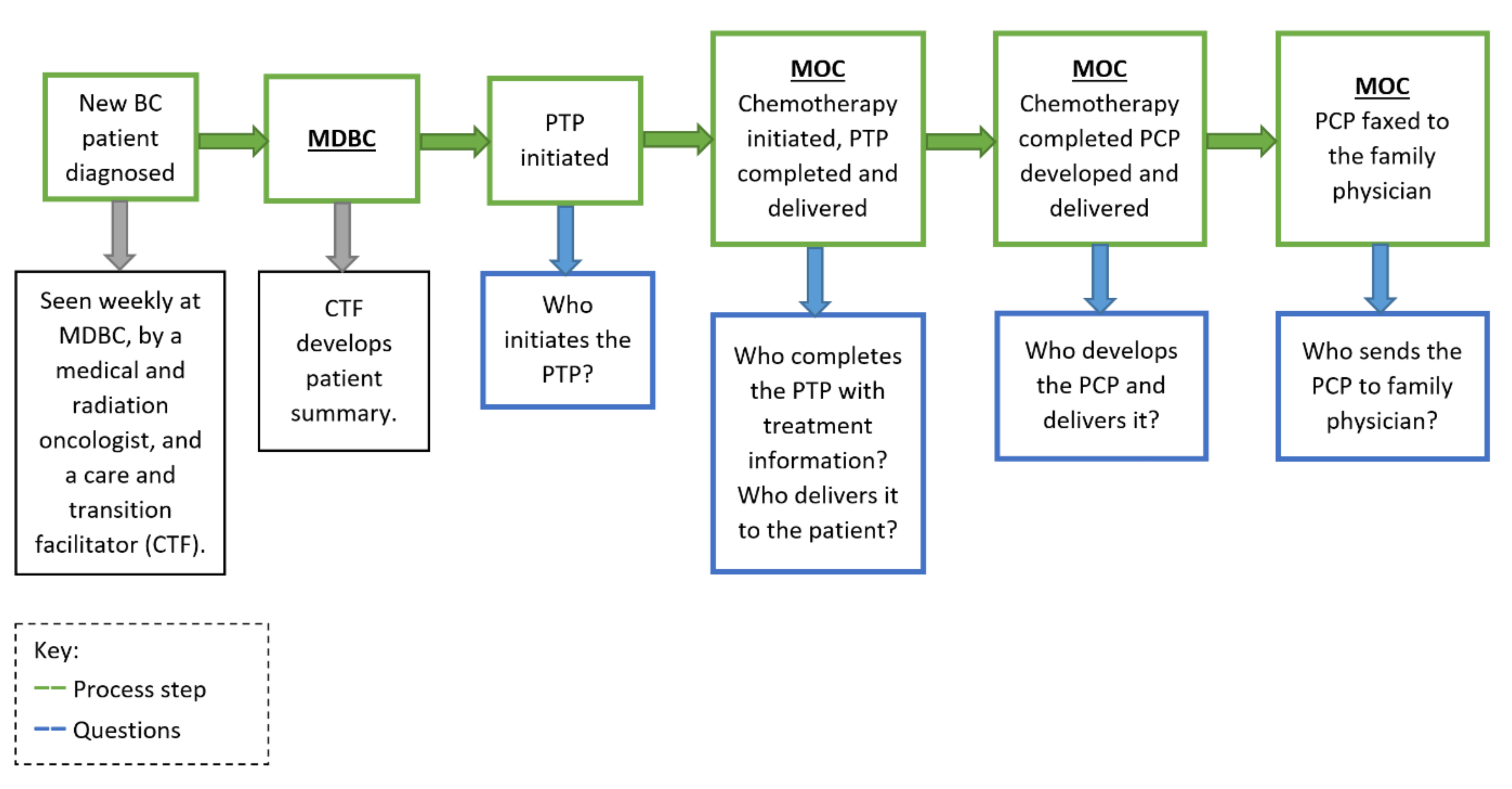
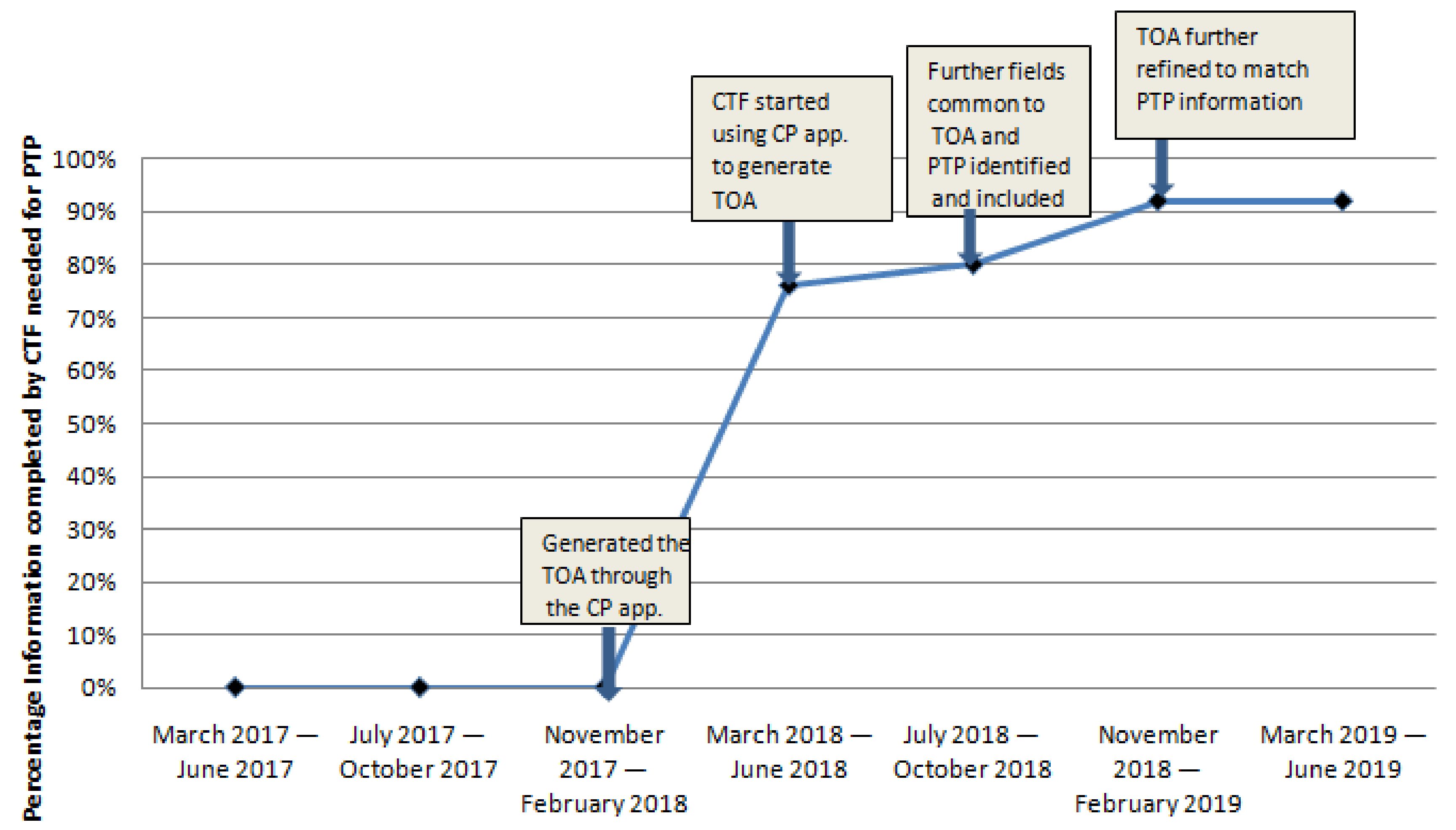
Each PDSA cycle started with:
| |
| PDSA 1 Apr–Jul 2017 |
|
| PDSA 2 Aug–Nov 2017 |
|
| PDSA 3 Dec 2017–Mar 2018 |
|
| PDSA 4 Apr–Jul 2018 |
|
| PDSA 5 Aug–Nov 2018 |
|
| PDSA 6 Dec 2018–Mar 2019 |
|
| PDSA 7 Apr–Jul 2019 |
|
| Mid Chemotherapy | End of Chemotherapy | 3-Months Post Chemotherapy | p-Value | |
|---|---|---|---|---|
| Number of patients who completed the form | 109 | 113 | 81 | NA |
| Prepared to live as cancer survivor (median (IQR)) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.07) | 4.00 (3.00, 4.00) | 0.55 |
| Better communicate with medical team (median (IQR)) | 4.00 (4.00, 4.00) | 4.00 (4.00, 5.00) | 4.00 (4.00, 5.00) | 0.13 |
| More easily communicate with other health care providers (median (IQR)) | 4.00 (4.00, 5.00) | 4.00 (4.00, 5.00) | 4.00 (4.00, 5.00) | 0.72 |
| Actively participate in medical management of cancer (median (IQR)) | 4.00 (4.00, 4.00) | 4.00 (3.50, 4.00) | 4.00 (3.00, 4.47) | 0.18 |
| Enlist family’s support (median (IQR)) | 4.00 (4.00, 5.00) | 4.00 (4.00, 5.00) | 4.00 (3.00, 4.00) | 0.06 |
| Deal more effectively with side effects of treatment (median (IQR)) | 4.00 (3.00, 4.00) | 4.00 (4.00, 5.00) | 4.00 (3.00, 4.00) | 0.23 |
| Deal more effectively with pain (median (IQR)) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 3.00 (3.00, 4.00) | 0.34 |
| Reduce risk of delayed complications for treatment (median (IQR)) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 0.18 |
| Better understand lifestyle factors leading to cancer onset (median (IQR)) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 0.12 |
| Better understand lifestyle changes that reduce recurrence risk (IQR)) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 0.61 |
| Make nutritional changes which aid recovery (IQR)) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 0.51 |
| Make physical activity changes to aid recovery (median (IQR)) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 0.81 |
| Provided helpful advice on issues related to return to work (median (IQR)) | 3.44 (3.00, 4.00) | 3.67 (3.00, 4.00) | 3.00 (3.00, 4.00) | 0.77 |
| Be informed of community resources available (median (IQR)) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 4.00 (3.00, 4.00) | 0.39 |
| More easily communicate with other health care providers (median (IQR)) | 4.00 (3.53, 4.00) | 4.00 (4.00, 5.00) | 4.00 (3.00, 4.00) | 1 |
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Prepared to live their lives as cancer survivor (%) | 4 | 0.0 | 20.0 | 28.0 | 48.0 |
| Better communicate with their medical team (%) | 4.2 | 0.0 | 12.5 | 16.7 | 66.7 |
| Reduce risk of delayed complications for treatment (%) | 0.0 | 4.2 | 41.7 | 8.3 | 45.8 |
| Better deal with depression or depressed mood (%) | 4.2 | 0.0 | 50.0 | 20.8 | 25.0 |
| PTP and PCP helped to improve quality of patient care (%) | 4.0 | 0.0 | 16.0 | 16.0 | 64.0 |
| PTP and PCP should be incorporated into oncology clinic (%) | 4.2 | 0.0 | 4.2 | 16.7 | 75.0 |
| PTP and PCP has all the information needed (%) | 4.2 | 4.2 | 0.0 | 25.0 | 66.7 |
| Better understand lifestyle changes that reduce recurrence risk (%) | 4.5 | 9.1 | 22.7 | 22.7 | 40.9 |
| Better understand lifestyle factors leading to cancer onset (%) | 8.3 | 4.2 | 25.0 | 16.7 | 45.8 |
| Actively participate in medical management of cancer (%) | 4.2 | 0.0 | 20.8 | 37.5 | 37.5 |
| Make nutritional changes which aid recovery (%) | 4.2 | 0.0 | 20.8 | 37.5 | 37.5 |
| Deal more effectively with pain (%) | 0.0 | 0.0 | 33.3 | 29.2 | 37.5 |
| Make physical activity changes to aid recovery (%) | 8.3 | 0.0 | 29.2 | 37.5 | 25.0 |
| Ask right questions about treatment (%) | 4.2 | 4.2 | 12.5 | 33.3 | 45.8 |
| Be informed of community resources available (%) | 8.3 | 00.0 | 25.0 | 25.0) | 41.7 |
| Deal more effectively with side effects of treatment (%) | 4.2 | 00.0 | 20.8 | 25.0 | 50.0 |
| Better deal with stressful emotions and anxiety (%) | 4.2 | 00.0 | 33.3 | 33.3 | 29.2 |
| PTP and PCP is more time-consuming to use (%) | 36.0 | 16.0 | 40.0 | 4.0 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, R.; Kong, A.; Gulasingam, P. A Multidisciplinary Approach to Implement Personalized Breast Cancer Treatment and Care Plans. Curr. Oncol. 2021, 28, 767-782. https://doi.org/10.3390/curroncol28010075
Haq R, Kong A, Gulasingam P. A Multidisciplinary Approach to Implement Personalized Breast Cancer Treatment and Care Plans. Current Oncology. 2021; 28(1):767-782. https://doi.org/10.3390/curroncol28010075
Chicago/Turabian StyleHaq, Rashida, Amy Kong, and Pauline Gulasingam. 2021. "A Multidisciplinary Approach to Implement Personalized Breast Cancer Treatment and Care Plans" Current Oncology 28, no. 1: 767-782. https://doi.org/10.3390/curroncol28010075
APA StyleHaq, R., Kong, A., & Gulasingam, P. (2021). A Multidisciplinary Approach to Implement Personalized Breast Cancer Treatment and Care Plans. Current Oncology, 28(1), 767-782. https://doi.org/10.3390/curroncol28010075




