Immune Checkpoint Inhibition as Primary Adjuvant Therapy for an IDH1-Mutant Anaplastic Astrocytoma in a Patient with CMMRD: A Case Report—Usage of Immune Checkpoint Inhibition in CMMRD
Abstract
1. Introduction
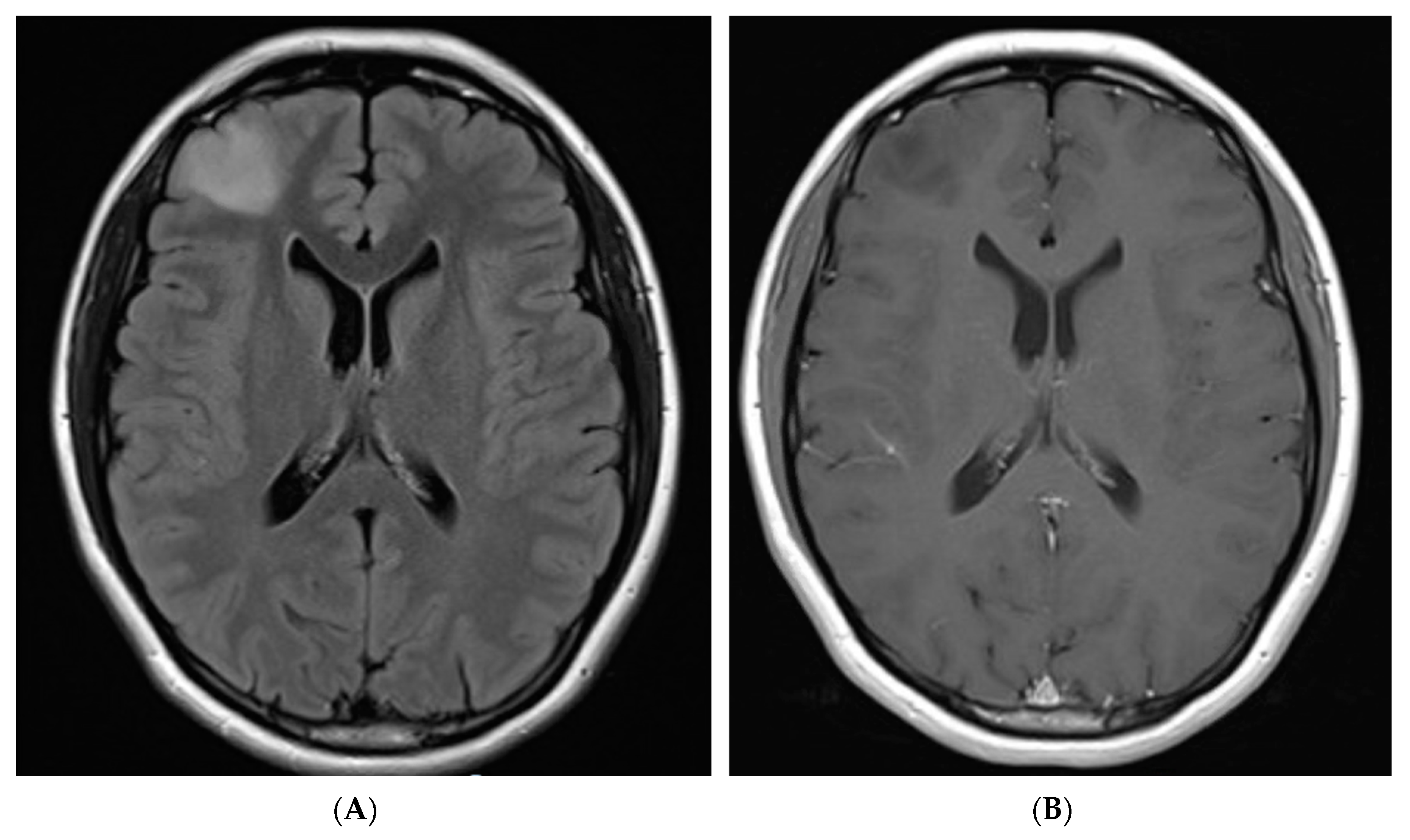
2. Case Description
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Lasset, C.; Desseigne, F.; Frappaz, D.; Bergeron, C.; Navarro, C.; Ruano, E.; Puisieux, A. Neurofibromatosis and early onset of cancers in hMLH1-deficient children. Cancer Res. 1999, 59, 294–297. [Google Scholar] [PubMed]
- Wimmer, K.; Rosenbaum, T.; Messiaen, L. Connections between constitutional mismatch repair deficiency syndrome and neurofibromatosis type 1. Clin. Genet. 2017, 91, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Bakry, D.; Aronson, M.; Durno, C.; Rimawi, H.; Farah, R.; Alharbi, Q.K.; Alharbi, M.; Shamvil, A.; Ben-Shachar, S.; Mistry, M.; et al. Genetic and clinical determinants of constitutional mismatch repair deficiency syndrome: Report from the constitutional mismatch repair deficiency consortium. Eur. J. Cancer 2014, 50, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Keijzers, G.; Rasmussen, L.J. DNA mismatch repair and its many roles in eukaryotic cells. Mutat. Res. Mutat. Res. 2017, 773, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Shiovitz, S.; Pritchard, C.C.; Jarvik, G.P. Lynch Syndrome: From Screening to Diagnosis to Treatment in the Era of Modern Molecular Oncology. Annu. Rev. Genom. Hum. Genet. 2019, 20, 293–307. [Google Scholar] [CrossRef]
- Tabori, U.; Hansford, J.R.; Achatz, M.I.; Kratz, C.P.; Plon, S.E.; Frebourg, T.; Brugières, L. Clinical Management and Tumor Surveillance Recommendations of Inherited Mismatch Repair Deficiency in Childhood. Clin. Cancer Res. 2017, 23, e32–e37. [Google Scholar] [CrossRef]
- Lavoine, N.; Colas, C.; Muleris, M.; Bodo, S.; Duval, A.; Entz-Werle, N.; Coulet, F.; Cabaret, O.; Andreiuolo, F.; Charpy, C.; et al. Constitutional mismatch repair deficiency syndrome: Clinical description in a French cohort. J. Med. Genet. 2015, 52, 770–778. [Google Scholar] [CrossRef]
- Kratz, C.P.; Holter, S.; Etzler, J.; Lauten, M.; Pollett, A.; Niemeyer, C.M.; Gallinger, S.; Wimmer, K. Rhabdomyosarcoma in patients with constitutional mismatch-repair-deficiency syndrome. J. Med. Genet. 2009, 46, 418–420. [Google Scholar] [CrossRef]
- Wimmer, K.; Kratz, C.P.; Vasen, H.F.; Caron, O.; Colas, C.; Entz-Werle, N.; Gerdes, A.-M.; Goldberg, Y.; Ilencikova, D.; Muleris, M.; et al. Diagnostic criteria for constitutional mismatch repair deficiency syndrome: Suggestions of the European consortium ‘Care for CMMRD’ (C4CMMRD). J. Med. Genet. 2014, 51, 355–365. [Google Scholar] [CrossRef]
- Suerink, M.; Potjer, T.; Versluijs, A.; Broeke, S.W.T.; Tops, C.; Wimmer, K.; Nielsen, M. Constitutional mismatch repair deficiency in a healthy child: On the spot diagnosis? Clin. Genet. 2017, 93, 134–137. [Google Scholar] [CrossRef]
- Vasen, H.F.; Ghorbanoghli, Z.; Bourdeaut, F.; Cabaret, O.; Caron, O.; Duval, A.; Entz-Werle, N.; Goldberg, Y.; Ilencikova, D.; Kratz, C.P.; et al. Guidelines for surveillance of individuals with constitutional mismatch repair-deficiency proposed by the European Consortium “Care for CMMR-D” (C4CMMR-D). J. Med. Genet. 2014, 51, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Durno, C.; Aronson, M.; Tabori, U.; Malkin, D.; Gallinger, S.; Chan, H.S.L. Oncologic surveillance for subjects with biallelic mismatch repair gene mutations: 10 year follow-up of a kindred. Pediatr. Blood Cancer 2011, 59, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Durno, C.; Boland, C.R.; Cohen, S.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J.; et al. Recommendations on Surveillance and Management of Biallelic Mismatch Repair Deficiency (BMMRD) Syndrome: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 152, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Aronson, M.; Gallinger, S.; Cohen, Z.; Cohen, S.; Dvir, R.; Elhasid, R.; Baris, H.N.; Kariv, R.; Druker, H.; Chan, H.; et al. Gastrointestinal Findings in the Largest Series of Patients with Hereditary Biallelic Mismatch Repair Deficiency Syndrome: Report from the International Consortium. Am. J. Gastroenterol. 2016, 111, 275–284. [Google Scholar] [CrossRef]
- Ercan, A.; Durno, C.; Bianchi, V.; Edwards, M.; Aronson, M.; Scheers, I. Survival Benefit for Individuals with Constitutional Mismatch Repair Deficiency Syndrome Who Undergo a Surveillance Protocol: A Report from the International Replication Repair Deficiency Consortium. SIOP Abstr. Pediatr. Blood Cancer 2020, 67, e28742. [Google Scholar]
- Amayiri, N.; Tabori, U.; Campbell, B.; Bakry, D.; Aronson, M.; Durno, C.; Rakopoulos, P.; Malkin, D.; Qaddoumi, I.; Musharbash, A.; et al. High frequency of mismatch repair deficiency among pediatric high grade gliomas in Jordan. Int. J. Cancer 2015, 138, 380–385. [Google Scholar] [CrossRef]
- Li, L.; Hamel, N.; Baker, K.; McGuffin, M.J.; Couillard, M.; Gologan, A.; Marcus, V.; Chodirker, B.; Chudley, A.; Stefanovici, C.; et al. A homozygous PMS2 founder mutation with an attenuated constitutional mismatch repair deficiency phenotype. J. Med. Genet. 2015, 52, 348–352. [Google Scholar] [CrossRef]
- Campbell, B.B.; Light, N.; Fabrizio, D.; Zatzman, M.; Fuligni, F.; De Borja, R.; Davidson, S.; Edwards, M.; Elvin, J.A.; Hodel, K.P.; et al. Comprehensive Analysis of Hypermutation in Human Cancer. Cell 2017, 171, 1042–1056.e10. [Google Scholar] [CrossRef]
- Bouffet, E.; Larouche, V.; Campbell, B.B.; Merico, D.; De Borja, R.; Aronson, M.; Durno, C.; Krueger, J.; Cabric, V.; Ramaswamy, V.; et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting from Germline Biallelic Mismatch Repair Deficiency. J. Clin. Oncol. 2016, 34, 2206–2211. [Google Scholar] [CrossRef]
- Bouffet, E.; Sudhaman, S.; Chung, J.; Kelly, J.; Coblentz, A.; Edwards, M.; Lipman, T.; Zhang, C.; Ercan, A.B.; Sambira, L.; et al. IMMU-18. favorable outcome in replication repair deficient hypermutant brain tumors to immune checkpoint inhibition: An international rrd consortium registry study. Neuro Oncol. 2020, 22, iii363. [Google Scholar] [CrossRef]
- Bent, M.J.V.D.; Baumert, B.; Erridge, S.C.; Vogelbaum, M.; Nowak, A.K.; Sanson, M.; Brandes, A.A.; Clement, P.M.; Baurain, J.F.; Mason, W.P.; et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: A phase 3, randomised, open-label intergroup study. Lancet 2017, 390, 1645–1653. [Google Scholar] [CrossRef]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M.; et al. Integrated Molecular Meta-Analysis of 1000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017, 32, 520–537.e5. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Perryman, L.; Hargrave, D. Paediatric and adult malignant glioma: Close relatives or distant cousins? Nat. Rev. Clin. Oncol. 2012, 9, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.; Felton, E.; Allen, I.; Tahir, P.; Mueller, S. Survival outcomes of pediatric high-grade glioma: Results of a 20-year systematic review and meta-analysis. J. Neuro Oncol. 2017, 137, 103–110. [Google Scholar] [CrossRef]
- McFaline-Figueroa, J.L.; Braun, C.J.; Stanciu, M.; Nagel, Z.D.; Mazzucato, P.; Sangaraju, D.; Cerniauskas, E.; Barford, K.; Vargas, A.; Chen, Y.; et al. Minor Changes in Expression of the Mismatch Repair Protein MSH2 Exert a Major Impact on Glioblastoma Response to Temozolomide. Cancer Res. 2015, 75, 3127–3138. [Google Scholar] [CrossRef]
- Suwala, A.K.; Stichel, D.; Schrimpf, D.; Kloor, M.; Wefers, A.K.; Reinhardt, A.; Maas, S.L.N.; Kratz, C.P.; Schweizer, L.; Hasselblatt, M.; et al. Primary mismatch repair deficient IDH-mutant astrocytoma (PMMRDIA) is a distinct type with a poor prognosis. Acta Neuropathol. 2021, 141, 85–100. [Google Scholar] [CrossRef]
- Shlien, A.; Campbell, B.B.; De Borja, R.; Alexandrov, L.B.; Merico, D.; Wedge, D.; van Loo, P.; Tarpey, P.S.; Coupland, P.; Behjati, S.; et al. Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra-hypermutated cancers. Nat. Genet. 2015, 47, 257–262. [Google Scholar] [CrossRef]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Touat, M.; Li, Y.Y.; Boynton, A.N.; Spurr, L.F.; Iorgulescu, J.B.; Bohrson, C.L.; Cortes-Ciriano, I.; Birzu, C.; Geduldig, J.E.; Pelton, K.; et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nat. Cell Biol. 2020, 580, 517–523. [Google Scholar] [CrossRef]
- Lombardi, G.; Idbaih, A.; Le Rhun, E.; Preusser, M.; Zagonel, V.; French, P. A New Landscape for Systemic Pharmacotherapy of Recurrent Glioblastoma? Cancers 2020, 12, 3775. [Google Scholar] [CrossRef] [PubMed]
- Khasraw, M.; Reardon, D.A.; Weller, M.; Sampson, J.H. PD-1 Inhibitors: Do they have a Future in the Treatment of Glioblastoma? Clin. Cancer Res. 2020, 26, 5287–5296. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.; Mobark, N.A.; Almubarak, L.; Aljelaify, R.; AlSaeed, M.; Almutairi, A.; Alqubaishi, F.; Hussain, M.E.; Balbaid, A.A.O.; Marie, A.S.; et al. Durable Response to Nivolumab in a Pediatric Patient with Refractory Glioblastoma and Constitutional Biallelic Mismatch Repair Deficiency. Oncologist 2018, 23, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Larouche, V.; Atkinson, J.; Albrecht, S.; LaFramboise, R.; Jabado, N.; Tabori, U.; Bouffet, E. Sustained complete response of recurrent glioblastoma to combined checkpoint inhibition in a young patient with constitutional mismatch repair deficiency. Pediatr. Blood Cancer 2018, 65, e27389. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Maruvka, Y.E.; Sudhaman, S.; Kelly, J.; Haradhvala, N.J.; Bianchi, V.; Edwards, M.; Forster, V.J.; Nunes, N.M.; Galati, M.A.; et al. DNA polymerase and mismatch repair exert distinct microsatellite instability signatures in normal and malignant human cells. Cancer Discov. 2020. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.; Miller, W.H.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Kamiya-Matsuoka, C.; Metrus, N.; Weathers, S.-P.; Ross, J.; Shaw, K.; Penas-Prado, M.; Loghin, M.; Alfaro-Munoz, K.; O’Brien, B.; Harrison, R.; et al. Is immuno-oncology therapy effective in hypermutator glioblastomas with somatic or germline mutations? Ann. Oncol. 2019, 30, v144. [Google Scholar] [CrossRef]
- Johnson, A.; Severson, E.; Gay, L.; Vergilio, J.; Elvin, J.; Suh, J.; Daniel, S.; Covert, M.; Frampton, G.M.; Hsu, S.; et al. Comprehensive Genomic Profiling of 282 Pediatric Low- and High-Grade Gliomas Reveals Genomic Drivers, Tumor Mutational Burden, and Hypermutation Signatures. Oncologist 2017, 22, 1478–1490. [Google Scholar] [CrossRef]
- Blank, C.U.; Haanen, J.B.; Ribas, A.; Schumacher, T.N. The “cancer immunogram”. Science 2016, 352, 658–660. [Google Scholar] [CrossRef]
- Lombardi, G.; Barresi, V.; Indraccolo, S.; Simbolo, M.; Fassan, M.; Mandruzzato, S.; Simonelli, M.; Caccese, M.; Pizzi, M.; Fassina, A.; et al. Pembrolizumab Activity in Recurrent High-Grade Gliomas with Partial or Complete Loss of Mismatch Repair Protein Expression: A Monocentric, Observational and Prospective Pilot Study. Cancers 2020, 12, 2283. [Google Scholar] [CrossRef] [PubMed]
- New Agent and Innovative Therapies Program (NAIT) Newsletter Early Phase Trials at SickKids. 2019. Available online: https://c17blog.files.wordpress.com/2019/09/nait_newsletter_september_2019.pdf (accessed on 15 January 2021).


| Malignancies | European Consortium [11] | Canadian Surveillance Protocol [6,12] | US Multi-Society Task Force on Colorectal Cancer [13] |
|---|---|---|---|
| Brain tumors | MRI brain every 6 to 12 months, starting at the age of 2 | MRI brain at diagnosis then every 6 months | MRI brain every 6 months, starting at the age of 2 |
| Gastrointestinal tumors | Upper: annual video capsule endoscopy and gastroscopy, starting at the age of 10 | Upper: annual video capsule endoscopy and gastroscopy, starting between 4 to 6 years | Upper: annual video capsule endoscopy and gastroscopy, starting at the age of 8 |
| Lower: annual colonoscopy, starting at the age of 8 | Lower: annual colonoscopy, starting between 4 to 6 years | Lower: annual colonoscopy, starting at the age of 6 | |
| Hematologic malignancies | Annual clinical examination and CBC every 6 months, starting at the age of 1 | Abdominal ultrasound every 6 months, starting at the age of 1 | CBC every 6 months, starting at the age of 1 |
| Optional: abdominal ultrasound every 6 months, starting at the age of 1 | CBC every 6 months, starting at the age of 1 | ||
| Genitourinary tumors | Annual urine cytology and urine dipstick, starting at the age of 20 | Annual urine cytology and urine dipstick, starting at the age of 20 | Annual urinalysis, starting at the age of 10 |
| Gynecologic tumors | Annual gynecologic examination, transvaginal ultrasounds, and endometrial biopsy, starting at the age of 20 | Annual gynecologic examination, transvaginal ultrasounds, and endometrial biopsy, starting at the age of 20 | Annual gynecologic examination, transvaginal ultrasounds, and endometrial biopsy, starting at the age of 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rittberg, R.; Harlos, C.; Rothenmund, H.; Das, A.; Tabori, U.; Sinha, N.; Singh, H.; Chodirker, B.; Kim, C.A. Immune Checkpoint Inhibition as Primary Adjuvant Therapy for an IDH1-Mutant Anaplastic Astrocytoma in a Patient with CMMRD: A Case Report—Usage of Immune Checkpoint Inhibition in CMMRD. Curr. Oncol. 2021, 28, 757-766. https://doi.org/10.3390/curroncol28010074
Rittberg R, Harlos C, Rothenmund H, Das A, Tabori U, Sinha N, Singh H, Chodirker B, Kim CA. Immune Checkpoint Inhibition as Primary Adjuvant Therapy for an IDH1-Mutant Anaplastic Astrocytoma in a Patient with CMMRD: A Case Report—Usage of Immune Checkpoint Inhibition in CMMRD. Current Oncology. 2021; 28(1):757-766. https://doi.org/10.3390/curroncol28010074
Chicago/Turabian StyleRittberg, Rebekah, Craig Harlos, Heidi Rothenmund, Anirban Das, Uri Tabori, Namita Sinha, Harminder Singh, Bernie Chodirker, and Christina A. Kim. 2021. "Immune Checkpoint Inhibition as Primary Adjuvant Therapy for an IDH1-Mutant Anaplastic Astrocytoma in a Patient with CMMRD: A Case Report—Usage of Immune Checkpoint Inhibition in CMMRD" Current Oncology 28, no. 1: 757-766. https://doi.org/10.3390/curroncol28010074
APA StyleRittberg, R., Harlos, C., Rothenmund, H., Das, A., Tabori, U., Sinha, N., Singh, H., Chodirker, B., & Kim, C. A. (2021). Immune Checkpoint Inhibition as Primary Adjuvant Therapy for an IDH1-Mutant Anaplastic Astrocytoma in a Patient with CMMRD: A Case Report—Usage of Immune Checkpoint Inhibition in CMMRD. Current Oncology, 28(1), 757-766. https://doi.org/10.3390/curroncol28010074





