A Population-Based Retrospective Study of Biliary Tract Cancers in Alberta, Canada
Abstract
1. Introduction
2. Methods
3. Results
3.1. Patient Demographics
3.2. Treatment
3.3. Recurrence
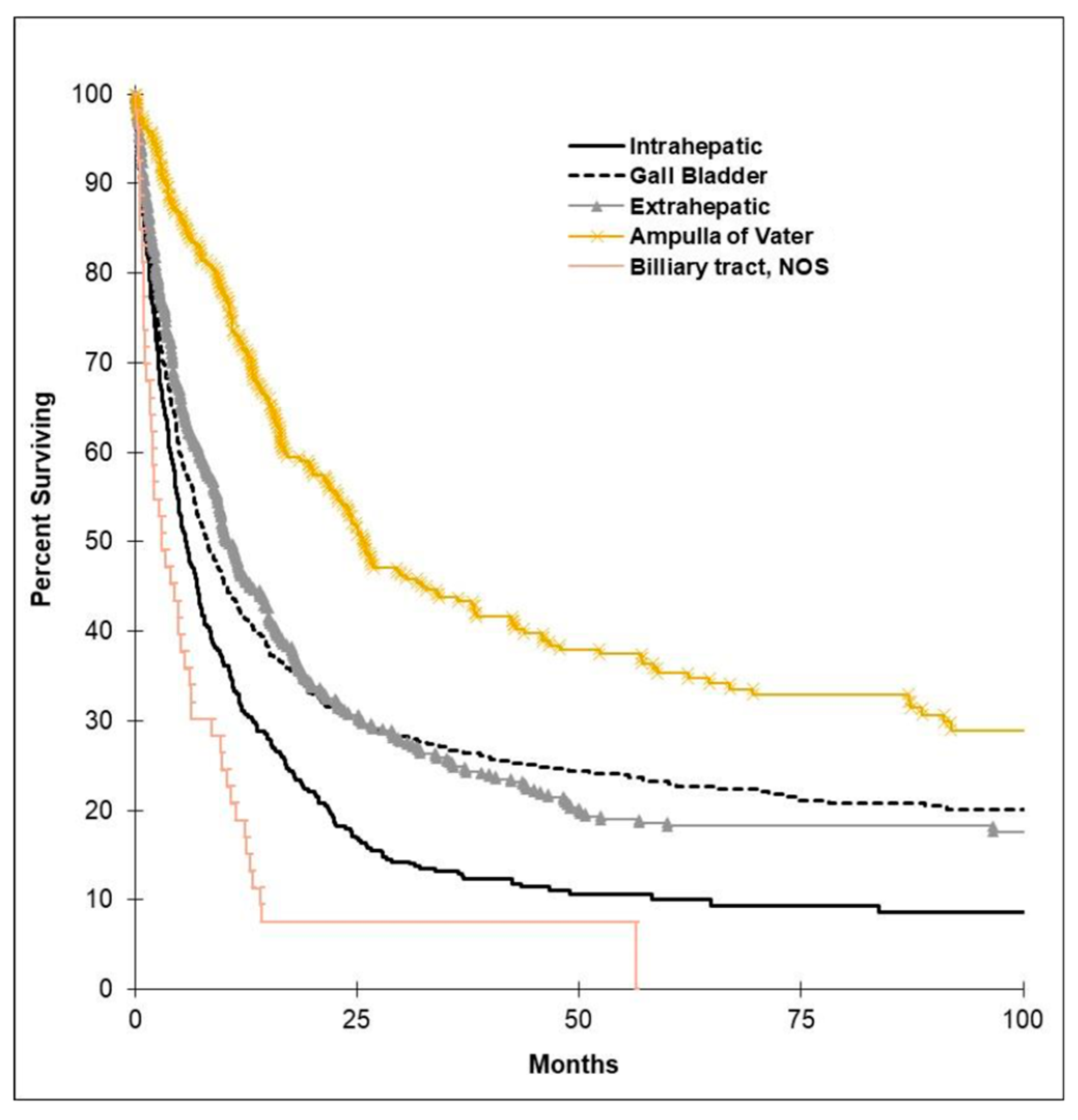
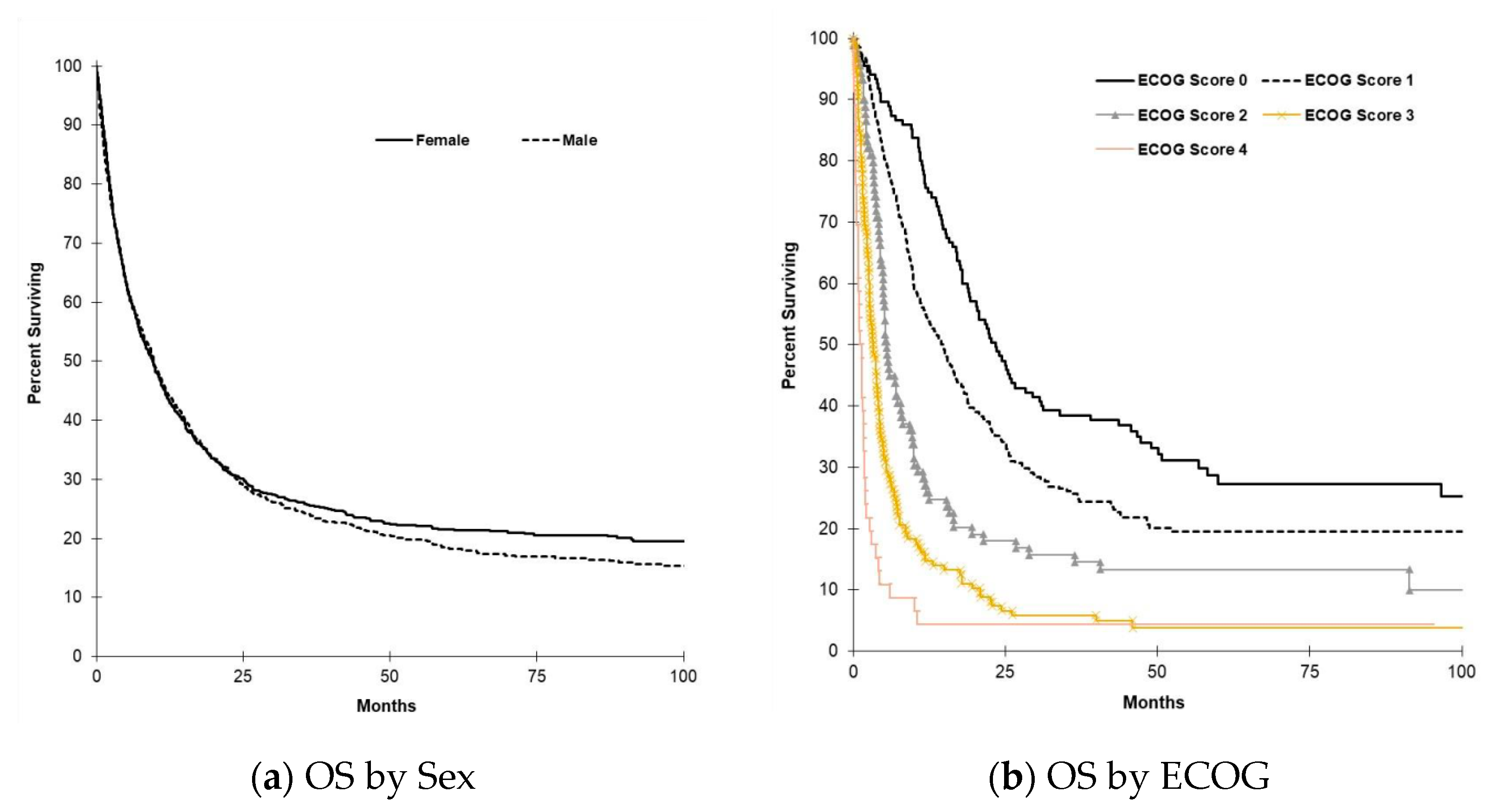
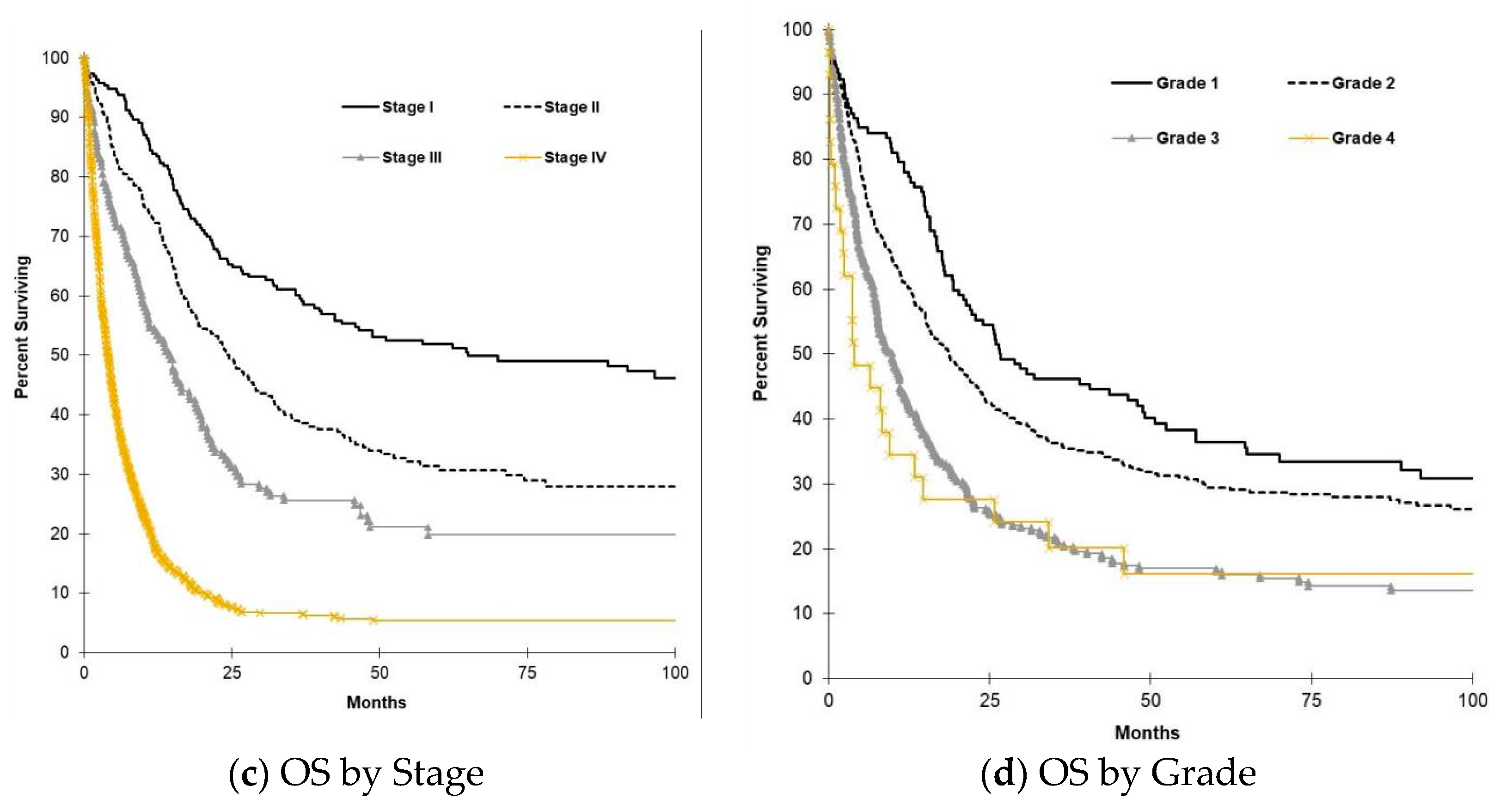
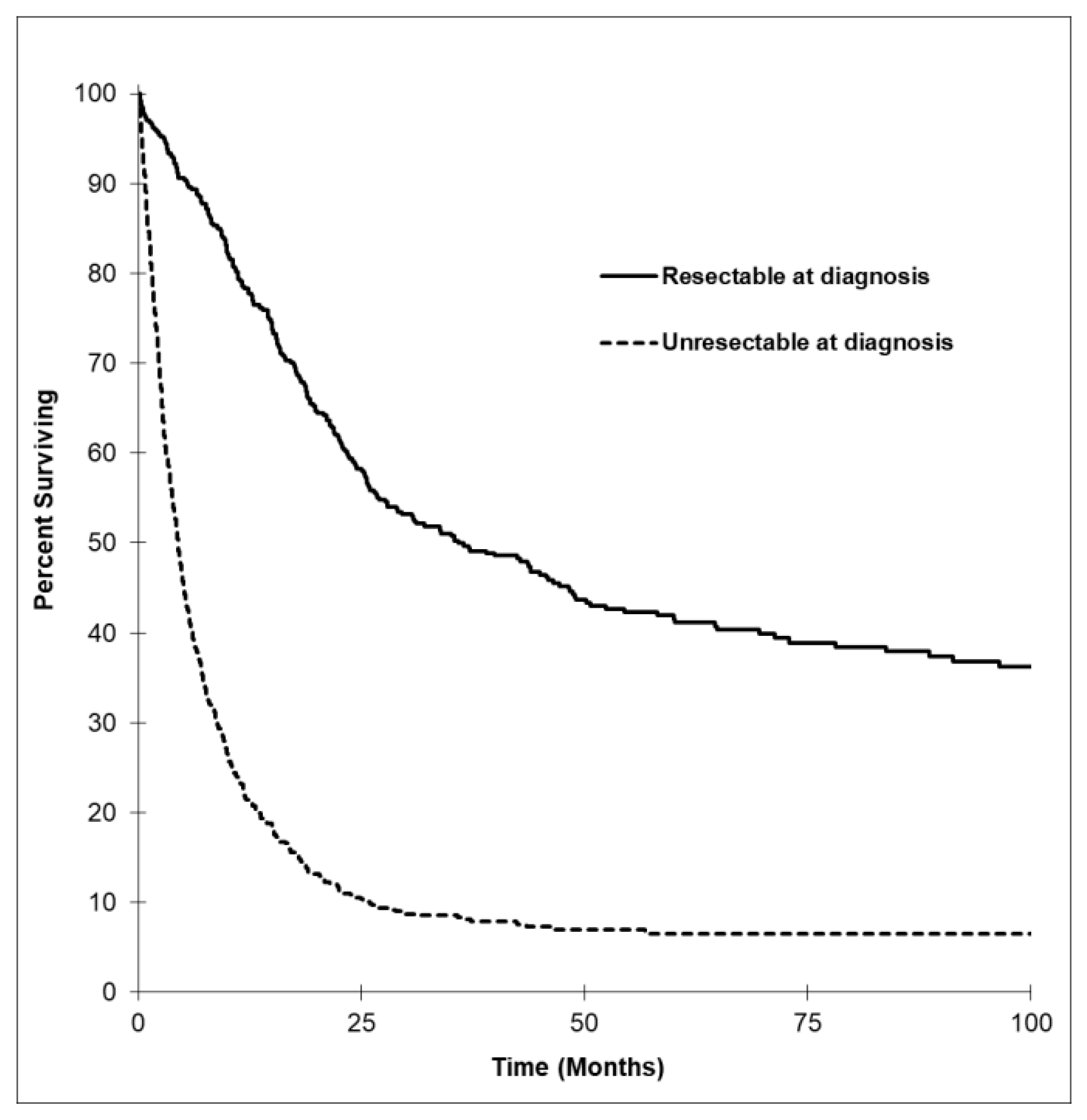
3.4. Survival Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTC | biliary tract cancers |
| CT | chemotherapy |
| OS | overall survival |
| ECOG | Eastern Cooperative Oncology Group |
| GBC | gallbladder cancer |
| IHC | intrahepatic cholangiocarcinoma |
| EHC | extrahepatic cholangiocarcinoma |
| AVT | ampulla of Vater tumors |
References
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Malka, D.; Boige, V.; Dromain, C.; Debaere, T.; Pocard, M.; Ducreux, M. Biliary tract neoplasms: Update 2003. Curr. Opin. Oncol. 2004, 16, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Thomas, H.C.; Davidson, B.R.; Taylor-Robinson, S.D. Cholangiocarcinoma. Lancet Lond. Engl. 2005, 366, 1303–1314. [Google Scholar] [CrossRef]
- Blechacz, B.R.A.; Gores, G.J. Cholangiocarcinoma. Clin. Liver Dis. 2008, 12, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Patel, T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001, 33, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Pairojkul, C. Cholangiocarcinoma: Lessons from Thailand. Curr. Opin. Gastroenterol. 2008, 24, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Lazcano-Ponce, E.C.; Miquel, J.F.; Muñoz, N.; Herrero, R.; Ferrecio, C.; Wistuba, I.I.; De Ruiz, P.A.; Urista, G.A.; Nervi, F. Epidemiology and Molecular Pathology of Gallbladder Cancer. CA A Cancer J. Clin. 2001, 51, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.M.; La Medina, A.R.-D.; Donohue, J.H. Diagnosis and Surgical Management of Gallbladder Cancer: A Review. J. Gastrointest. Surg. 2007, 11, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Randi, G.; Malvezzi, M.; Levi, F.; Ferlay, J.; Negri, E.; Franceschi, S.; La Vecchia, C. Epidemiology of biliary tract cancers: An update. Ann. Oncol. 2008, 20, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Hezel, A.F.; Zhu, A.X. Systemic Therapy for Biliary Tract Cancers. Oncologist 2008, 13, 415–423. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Burke, E.C.; Bodniewicz, B.J.; Youssef, B.M.; Klimstra, D.; Blumgart, L.H. Staging, Resectability, and Outcome in 225 Patients with Hilar Cholangiocarcinoma. Ann. Surg. 2001, 234, 507–519. [Google Scholar] [CrossRef]
- Primrose, J.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Tavolari, S.; Brandi, G. Circulating Tumor DNA in Biliary Tract Cancer: Current Evidence and Future Perspectives. Cancer Genom. Proteom. 2020, 17, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Futibatinib, an investigational agent for the treatment of intrahepatic cholangiocarcinoma: Evidence to date and future perspectives. Expert Opin. Investig. Drugs 2020, 1–8. [Google Scholar] [CrossRef]

| Variable | Number of Patients (%) |
|---|---|
| Gender | |
| Male | 766 (47.8%) |
| Female | 838 (52.2%) |
| Median age in years (Range) | 68 (19–99) |
| Tumor type | |
| Gallbladder | 530 (33.0%) |
| Extrahepatic bile duct | 394 (24.6%) |
| Intrahepatic bile duct | 385 (24.0%) |
| Ampulla of vader | 242 (15.1%) |
| Biliary tract NOS | 53 (3.3%) |
| Tumor grade | |
| Grade 1 | 132 (8.2%) |
| Grade 2 | 443 (27.6%) |
| Grade 3 | 293 (18.3%) |
| Grade 4 | 29 (1.8%) |
| Unknown | 707 (44.1%) |
| Lymphatic invasion | |
| Yes | 106 (6.6%) |
| No | 133 (8.3%) |
| Unknown | 1365 (85.1%) |
| Perineural invasion | |
| Yes | 142 (8.9%) |
| No | 86 (5.4%) |
| Unknown | 1376 (85.8%) |
| Disease stage | |
| Stage 0 | 24 (1.5%) |
| Stage 1 | 193 (12.0%) |
| Stage 2 | 220 (13.7%) |
| Stage 3 | 148 (9.2%) |
| Stage 4 | 632 (39.4%) |
| Stage unknown | 387 (24.1%) |
| ECOG status | |
| ECOG 0 | 135 (8.4%) |
| ECOG 1 | 264 (16.5%) |
| ECOG 2 | 89 (5.5%) |
| ECOG 3 | 136 (8.5%) |
| ECOG 4 | 46 (2.9%) |
| ECOG unknown | 934 (58.2%) |
| Resectability at diagnosis | |
| Resectable | 374 (23.3%) |
| Not resectable | 597 (37.2%) |
| Undetermined/Unknown | 633 (39.5%) |
| Variable | GBC | EHC | IHC | AVT | BTC NOS |
|---|---|---|---|---|---|
| Number | 530 | 394 | 385 | 242 | 53 |
| Male (%) | 178 (33.6%) | 228 (57.9%) | 192 (49.9%) | 139 (57.4%) | 29 (54.7%) |
| Female (%) | 352 (66.4%) | 166 (42.1%) | 193 (50.1%) | 103 (42.6%) | 24 (45.3%) |
| Age at diagnosis (y) | 68 (26–99) | 68 (26–95) | 65 (19–89) | 67 (31–91) | 69 (29–88) |
| ECOG 0 (%) | 7 (1.3%) | 54 (13.7%) | 60 (15.6%) | 13 (5.4%) | 1 (1.9%) |
| ECOG 1 (%) | 27 (5.1%) | 113 (28.7%) | 104 (27.0%) | 16 (6.6%) | 4 (7.5%) |
| ECOG 2 (%) | 5 (0.9%) | 35 (8.9%) | 48 (12.5%) | 0 (0.0%) | 1 (1.9%) |
| ECOG 3 (%) | 12 (2.3%) | 49 (12.4%) | 66 (17.1%) | 7 (2.9%) | 2 (3.8%) |
| ECOG 4 (%) | 2 (0.4%) | 16 (4.1%) | 27 (7.0%) | 1 (0.4%) | 0 (0.0%) |
| ECOG unknown (%) | 477 (90.0%) | 127 (32.2%) | 80 (20.8%) | 205 (84.7%) | 45 (84.9%) |
| Grade 1 (%) | 48 (9.1%) | 34 (8.6%) | 17 (4.4%) | 32 (13.2%) | 1 (1.9%) |
| Grade 2 (%) | 139 (26.2%) | 101 (25.2%) | 100 (26.0%) | 100 (41.3%) | 3 (5.7%) |
| Grade 3 (%) | 117 (22.1%) | 46 (11.7%) | 69 (17.9%) | 58 (24.0%) | 3 (5.7%) |
| Grade 4 (%) | 13 (2.5%) | 3 (0.8%) | 8 (2.1%) | 5 (2.1%) | 0 (0.0%) |
| Grade unknown (%) | 213 (40.2%) | 210 (53.3%) | 191 (49.6%) | 47 (19.4%) | 46 (86.8%) |
| Stage 0 (%) | 13 (2.5%) | 4 (1.0%) | 0 (0.0%) | 7 (2.9%) | 0 (0.0%) |
| Stage 1 (%) | 76 (14.3%) | 46 (11.7%) | 28 (7.3%) | 43 (17.8%) | 0 (0.0%) |
| Stage 2 (%) | 73 (13.8%) | 84 (21.3%) | 16 (4.2%) | 47 (19.4%) | 0 (0.0%) |
| Stage 3 (%) | 9 (1.7%) | 44 (11.2%) | 64 (16.6%) | 30 (12.4%) | 1 (1.9%) |
| Stage 4 (%) | 187 (35.3%) | 140 (35.3%) | 249 (64.7%) | 25 (10.3%) | 31 (58.5%) |
| Stage unknown (%) | 172 (32.5%) | 76 (19.3%) | 28 (7.3%) | 90 (37.2%) | 21 (39.6%) |
| Resectable at dx (%) | 77 (14.5%) | 174 (44.2%) | 59 (15.3%) | 64 (26.4%) | 0 (0.0%) |
| Overall Resected (%) | 99 (18.6%) | 153 (38.8%) | 52 (13.5%) | 64 (26.4%) | 0 (0.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beaulieu, C.; Lui, A.; Yusuf, D.; Abdelaziz, Z.; Randolph, B.; Batuyong, E.; Ghosh, S.; Bathe, O.F.; Tam, V.; Spratlin, J.L. A Population-Based Retrospective Study of Biliary Tract Cancers in Alberta, Canada. Curr. Oncol. 2021, 28, 417-427. https://doi.org/10.3390/curroncol28010044
Beaulieu C, Lui A, Yusuf D, Abdelaziz Z, Randolph B, Batuyong E, Ghosh S, Bathe OF, Tam V, Spratlin JL. A Population-Based Retrospective Study of Biliary Tract Cancers in Alberta, Canada. Current Oncology. 2021; 28(1):417-427. https://doi.org/10.3390/curroncol28010044
Chicago/Turabian StyleBeaulieu, Carissa, Arthur Lui, Dimas Yusuf, Zainab Abdelaziz, Brock Randolph, Eugene Batuyong, Sunita Ghosh, Oliver F. Bathe, Vincent Tam, and Jennifer L. Spratlin. 2021. "A Population-Based Retrospective Study of Biliary Tract Cancers in Alberta, Canada" Current Oncology 28, no. 1: 417-427. https://doi.org/10.3390/curroncol28010044
APA StyleBeaulieu, C., Lui, A., Yusuf, D., Abdelaziz, Z., Randolph, B., Batuyong, E., Ghosh, S., Bathe, O. F., Tam, V., & Spratlin, J. L. (2021). A Population-Based Retrospective Study of Biliary Tract Cancers in Alberta, Canada. Current Oncology, 28(1), 417-427. https://doi.org/10.3390/curroncol28010044





