A Contemporary Report of Clinical Outcomes in Patients with Melanoma Brain Metastases
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Selection
2.3. Data Collection
2.4. Patient Outcomes
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Detection of Melanoma Brain Metastases
3.3. Predictors of Overall Survival
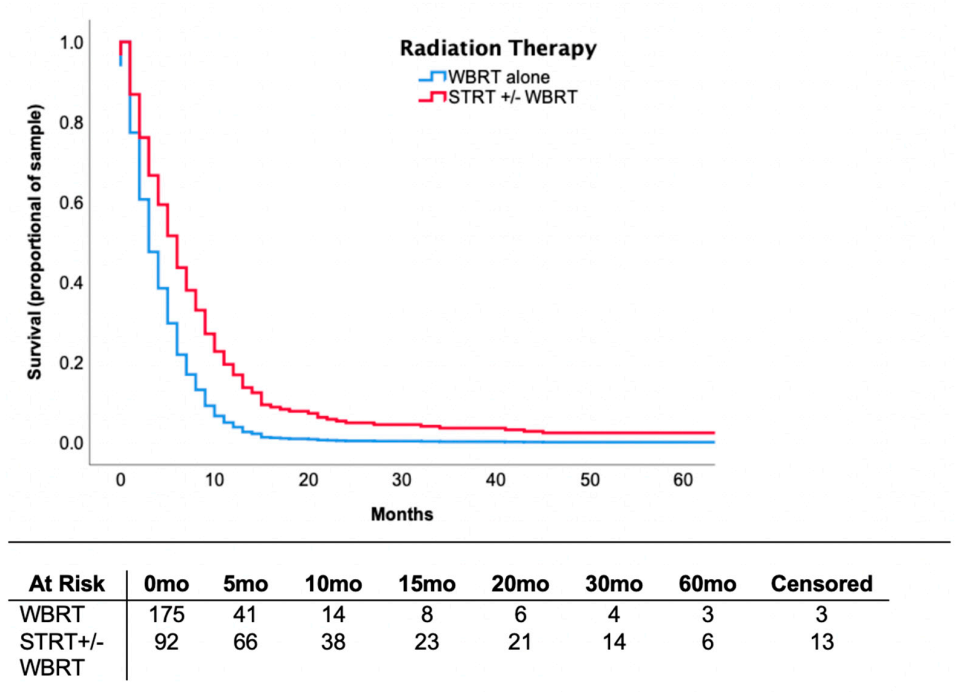
3.4. Locoregional Therapy
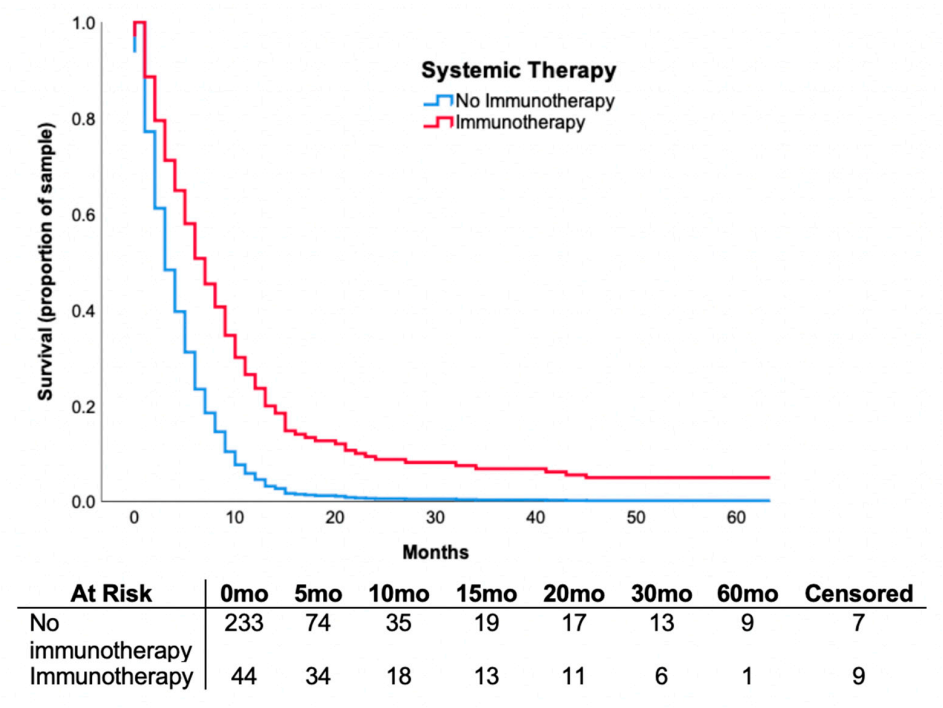
3.5. Systemic Therapy
3.6. Outcomes in Patients Receiving Combination of Immunotherapy and Radiotherapy
4. Discussion
4.1. Stereotactic Radiotherapy
4.2. Immunotherapy
4.3. Combination Radiation and Immunotherapy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samlowski, W.E.; Moon, J.; Witter, M.; Atkins, M.B.; Kirkwood, J.M.; Othus, M.; Ribas, A.; Sondak, V.K.; Flaherty, L.E. High frequency of brain metastases after adjuvant therapy for high-risk melanoma. Cancer Med. 2017, 6, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- Barnholtz-Sloan, J.S.; Nock, C.J.; Einstein, D.B. Diagnosis and treatment of mela-noma brain metastasis: A literature review. Cancer Control 2009, 16, 248–255. [Google Scholar] [CrossRef]
- Sampson, J.H.; Carter, J.H.; Friedman, A.H.; Seigler, H.F. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J. Neurosurg. 1998, 88, 11–20. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. The biology of brain metastasis: Challenges for therapy. Clin. Chem. 2013, 59, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.S.; Kirkwood, J.M.; Gore, M.; Dreno, B.; Thatcher, N.; Czarnetski, B.; Atkins, M.; Buzaid, A.; Skarlos, D.; Rankin, E.M. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: A phase II study. J. Clin. Oncol. 2004, 22, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.-J.; Flaherty, K.T.; Arance, A.; Chiarion-Sileni, V.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. (COMBI-MB)_Dabrafenib plus trametinib in patients with BRAF V600–mutant melanoma brain metastases. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef]
- McArthur, G.A.; Maio, M.; Arance, A.; Nathan, P.; Blank, C.; Avril, M.-F.; Garbe, C.; Hauschild, A.; Schadendorf, D.; Hamid, O.; et al. Vemurafenib in metastatic melanoma patients with brain metastases: An open-label, single-arm, phase 2, multicentre study. Ann. Oncol. 2017, 28, 634–641. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.A.; Scolyer, R.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Rulli, E.; Legramandi, L.; Salvati, L.; Mandala, M. The impact of targeted therapies and immunotherapy in melanoma brain metastases: A systematic review and meta-analysis. Cancer 2019, 125, 3776–3789. [Google Scholar] [CrossRef]
- Margolin, K.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef]
- Di Giacomo, A.M.; Ascierto, P.A.; Pilla, L.; Santinami, M.; Ferrucci, P.F.; Giannarelli, D.; Marasco, A.; Rivoltini, L.; Simeone, E.; Nicoletti, S.V.; et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): An open-label, single-arm phase 2 trial. Lancet Oncol. 2012, 13, 879–886. [Google Scholar] [CrossRef]
- Kluger, H.M.; Chiang, V.; Mahajan, A.; Zito, C.R.; Sznol, M.; Tran, T.; Weiss, S.A.; Cohen, J.V.; Yu, J.; Hegde, U.; et al. Long-Term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J. Clin. Oncol. 2019, 37, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.P.; Hamid, O.; Hodi, F.S.; Moschos, S.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef]
- Badiyan, S.N.; Regine, W.F.; Mehta, M.P. Stereotactic radiosurgery for treatment of brain metastases. J. Oncol. Pr. 2016, 12, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Carlino, M.S.; Fogarty, G.B.; Long, G.V. Treatment of melanoma brain metastases: A new paradigm. Cancer J. 2012, 18, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Chao, S.T.; Sneed, P.K.; Luo, X.; Suh, J.H.; Roberge, D.; Bhatt, A.; Jensen, A.W.; Brown, P.D.; Shih, H.; et al. Diagnosis-Specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4259 patients. Int. J. Radiat. Oncol. 2010, 77, 655–661. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef]
- Vosoughi, E.; Lee, J.M.; Miller, J.R.; Nosrati, M.; Minor, D.R.; Abendroth, R.; Lee, J.W.; Andrews, B.T.; Leng, L.Z.; Wu, M.; et al. Survival and clinical outcomes of patients with melanoma brain metastasis in the era of checkpoint inhibitors and targeted therapies. BMC Cancer 2018, 18, 490. [Google Scholar] [CrossRef]
- Staudt, M.; Lasithiotakis, K.; Leiter, U.; Meier, F.; Eigentler, T.K.; Bamberg, M.; Tatagiba, M.; Brossart, P.; Garbe, C. Determinants of survival in patients with brain metastases from cutaneous melanoma. Br. J. Cancer 2010, 102, 1213–1218. [Google Scholar] [CrossRef]
- Bottoni, U.; Clerico, R.; Paolino, G.; Ambrifi, M.; Corsetti, P.; Calvieri, S. Predictors and survival in patients with melanoma brain metastases. Med. Oncol. 2013, 30. [Google Scholar] [CrossRef] [PubMed]
- Bowden, G.; Faramand, A.; Niranjan, A.; Lunsford, L.D.; Monaco, E. Gamma knife radiosurgery for the management of more than 15 cerebral metastases. World Neurosurg. 2019, 126, e989–e997. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, R.; Corradini, S.; Gregucci, F.; Figlia, V.; Fiorentino, A.; Alongi, F. Role of radiosurgery/stereotactic radiotherapy in oligometastatic disease: Brain oligometastases. Front. Oncol. 2019, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Rava, P.; Leonard, K.; Sioshansi, S.; Curran, B.; Wazer, D.E.; Cosgrove, G.R.; Norén, G.; Hepel, J.T. Survival among patients with 10 or more brain metastases treated with stereotactic radiosurgery. J. Neurosurg. 2013, 119, 457–462. [Google Scholar] [CrossRef]
- Trommer, M.; Marnitz, S.; Kocher, M.; Rueß, D.; Schlaak, M.; Theurich, S.; Von Bergwelt-Baildon, M.; Morgenthaler, J.; Jablonska, K.; Celik, E.; et al. Robotic stereotactic radiosurgery in melanoma patients with brain metastases under simultaneous Anti-PD-1 treatment. Int. J. Mol. Sci. 2018, 19, 2653. [Google Scholar] [CrossRef]
- Kamath, S.D.; Kumthekar, P.U. Immune checkpoint inhibitors for the treatment of central nervous system (CNS) metastatic disease. Front. Oncol. 2018, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef]
- Gabani, P.; Fischer-Valuck, B.W.; Johanns, T.M.; Hernandez-Aya, L.F.; Keller, J.W.; Rich, K.M.; Kim, A.H.; Dunn, G.P.; Robinson, C.G.; Chicoine, M.R.; et al. Stereotactic radiosurgery and immunotherapy in melanoma brain metastases: Patterns of care and treatment outcomes. Radiother. Oncol. 2018, 128, 266–273. [Google Scholar] [CrossRef]
- Knisely, J.P.S.; Yu, J.B.; Flanigan, J.; Sznol, M.; Kluger, H.M.; Chiang, V.L.S. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J. Neurosurg. 2012, 117, 227–233. [Google Scholar] [CrossRef]
| Characteristic | Frequency (%) |
|---|---|
| Age (median, IQR) | 61 (50–72.25) |
| Gender (male) | 185 (67.0) |
| Year of diagnosis ≥ 2012 | 103(37.3) |
| Location of primary tumor | |
| Head/neck | 52 (18.8) |
| Extremity | 63 (22.8) |
| Palms, soles, nails | 16 (5.8) |
| Trunk | 84 (30.4) |
| Unknown | 61 (22.1) |
| Stage at diagnosis | |
| 1 | 54 (19.6) |
| 2 | 75 (27.2) |
| 3 | 72 (26.1) |
| 4 | 63 (22.8) |
| Unknown | 12 (4.3) |
| BRAF mutation positive (n = 97) | 42 (43.3) |
| LDH (n = 228) | |
| Normal limit | 93 (33.7) |
| Upper limit | 89 (32.2) |
| 2× UNL | 11 (4.0) |
| 3× UNL | 35 (30.1) |
| Number of extracranial metastases | |
| 1 | 14 (5.1) |
| 2–3 | 158 (57.3) |
| 4–5 | 93 (33.7) |
| ≥6 | 11 (4.0) |
| Number of intracranial metastases | |
| 1 | 83 (30.1) |
| 2–4 | 81 (29.3) |
| >5 | 110 (39.9) |
| Presence of neurological symptoms | 191 (69.2) |
| Supratentorial lesions | 263 (95.3) |
| Infratentorial lesions | 77 (27.9) |
| Maximal diameter, cm (median, IQR) | 2.27 (1.2–3.0) |
| Hemorrhagic lesions | 101 (36.6) |
| Leptomeningeal lesions | 19 (6.9) |
| (a) | ||
|---|---|---|
| Parameter | Hazard Ratio (95% CI) | p-Value * |
| Age | 1.012 (1.004–1.021) | 0.005 |
| Gender (male) | 0.945 (0.730–1.223) | 0.667 |
| Stage | 0.958 (0.861–1.066) | 0.434 |
| LDH | 1.366 (1.240–1.504) | <0.001 |
| Number of BM | 1.493 (1.288–1.730) | <0.001 |
| Number of extracranial sites | 1.202 (1.097–1.317) | <0.001 |
| Neurological symptoms | 1.397 (1.071–1.823) | 0.014 |
| Diameter | 1.026 (0.945–1.114) | 0.542 |
| Supratentorial | 0.998 (0.492–2.026) | 0.996 |
| Infratentorial | 1.480 (1.130–1.938) | 0.004 |
| Hemorrhagic lesions | 1.241 (0.964–1.599) | 0.094 |
| Leptomeningeal involvement | 1.245 (0.779–1.991) | 0.360 |
| Immunotherapy | 0.429 (0.300–0.615) | <0.001 |
| Targeted therapy | 0.764 (0.467–1.252) | 0.286 |
| Chemotherapy | 0.699 (0.531–0.920) | 0.011 |
| STRT | 0.369 (0.280–0.487) | <0.001 |
| WBRT | 1.555 (1.100–2.198) | 0.012 |
| Full craniotomy | 0.369 (0.256–0.530) | <0.001 |
| Partial craniotomy | 0.935 (0.638–1.369) | 0.729 |
| (b) | ||
| Parameter | Hazard Ratio (95% CI) | p-Value * |
| Age | 1.004 (0.995–1.013) | 0.362 |
| Number of BM | 1.105 (0.924–1.321) | 0.274 |
| Number of extracranial sites | 1.199 (1.084–1.327) | <0.001 |
| Neurological symptoms | 1.404 (1.052–1.872) | 0.021 |
| Infratentorial | 1.217 (0.915–1.618) | 0.177 |
| Immunotherapy | 0.467 (0.312–0.700) | <0.001 |
| Chemotherapy | 0.593 (0.442–0.796) | 0.001 |
| STRT | 0.456 (0.323–0.643) | <0.001 |
| WBRT | 0.721 (0.471–1.104) | 0.132 |
| Full craniotomy | 0.480 (0.308–0.750) | 0.001 |
| Treatment | Total (273) | Pre-2012 Era (173) * | Contemporary Era (103) * | Difference (%) | p-Value † |
|---|---|---|---|---|---|
| Radiation therapy | |||||
| STRT | 92 (33.3%) | 40 (23.1%) | 52 (50.5%) | +27.4% | <0.001 |
| WBRT | 230 (83.3%) | 165 (95.4%) | 65 (63.1%) | −32.3% | <0.001 |
| Systemic therapy | |||||
| Chemotherapy | 72 (26.1%) | 59 (34.1%) | 13 (12.6%) | −21.5% | <0.001 |
| Targeted therapy | 18 (6.5%) | 0 (0%) | 18 (17.5%) | +17.5% | - |
| Immunotherapy | 44 (15.9%) | 2 (1.2%) | 42 (40.8%) | +39.8% | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, W.J.; Baghai, T.; Ong, M.; Lo, B.; Ibrahim, A.M.; Smith, T.K.T.; Song, X. A Contemporary Report of Clinical Outcomes in Patients with Melanoma Brain Metastases. Curr. Oncol. 2021, 28, 428-439. https://doi.org/10.3390/curroncol28010045
Phillips WJ, Baghai T, Ong M, Lo B, Ibrahim AM, Smith TKT, Song X. A Contemporary Report of Clinical Outcomes in Patients with Melanoma Brain Metastases. Current Oncology. 2021; 28(1):428-439. https://doi.org/10.3390/curroncol28010045
Chicago/Turabian StylePhillips, William J., Tabassom Baghai, Michael Ong, Bryan Lo, Andrea M. Ibrahim, Tyler K.T. Smith, and Xinni Song. 2021. "A Contemporary Report of Clinical Outcomes in Patients with Melanoma Brain Metastases" Current Oncology 28, no. 1: 428-439. https://doi.org/10.3390/curroncol28010045
APA StylePhillips, W. J., Baghai, T., Ong, M., Lo, B., Ibrahim, A. M., Smith, T. K. T., & Song, X. (2021). A Contemporary Report of Clinical Outcomes in Patients with Melanoma Brain Metastases. Current Oncology, 28(1), 428-439. https://doi.org/10.3390/curroncol28010045





