A Randomized Controlled Trial Comparing Alloderm-RTU with DermACELL in Immediate Subpectoral Implant-Based Breast Reconstruction
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. The REthinking Clinical Trials (REaCT) Program and Integrated Consent Process
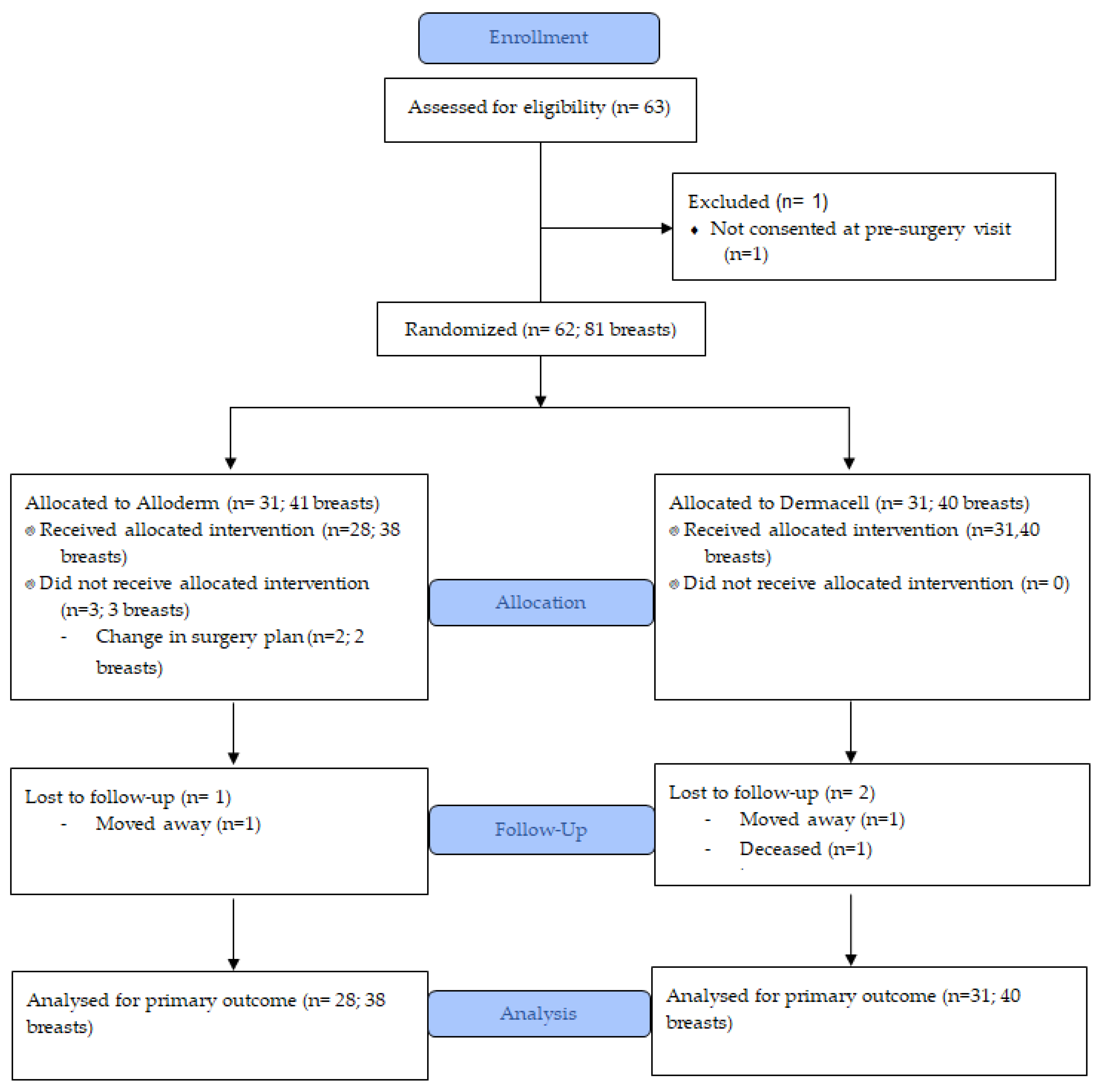
2.3. Randomization
2.4. Surgical Technique and Drain Management
2.5. Data Collection
2.6. Outcomes
2.6.1. Primary Outcomes
2.6.2. Secondary Outcomes
2.7. Sample Size and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Surgical Characteristics
3.3. Primary Outcome Measure
3.4. Secondary Outcomes Measures
3.5. Post-Surgical Treatment and Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albornoz, C.R.; Bach, P.B.; Mehrara, B.J.; Disa, J.J.; Pusic, A.L.; McCarthy, C.M.; Cordeiro, P.G.; Matros, E. A paradigm shift in U.S. breast reconstruction. Plast. Reconstr. Surg. 2013, 131, 15–23. [Google Scholar] [CrossRef]
- Kocak, E.; Nagel, T.W.; Hulsen, J.H.; Carruthers, K.H.; Povoski, S.P.; Salgado, C.J.; Chao, A.H. Biologic matrices in oncologic breast reconstruction after mastectomy. Expert Rev. Med. Devices 2013, 11, 65–75. [Google Scholar] [CrossRef]
- Chao, A.H. A Review of the use of acellular dermal matrices in postmastectomy immediate breast reconstruction. Plast. Surg. Nurs. 2015, 35, 131–134. [Google Scholar] [CrossRef]
- Lardi, A.M.; Ho-Asjoe, M.; Mohanna, P.-N.; Farhadi, J. Immediate breast reconstruction with acellular dermal matrix: Factors affecting outcome. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1098–1105. [Google Scholar] [CrossRef]
- Kalus, R.; Swartz, J.D.; Metzger, S.C. Optimizing safety, predictability, and aesthetics in direct to implant immediate breast reconstruction. Ann. Plast. Surg. 2016, 76, 320–327. [Google Scholar] [CrossRef]
- Hunsicker, L.M.; Ashikari, A.Y.; Berry, C.; Koch, R.M.; Salzberg, C.A. Short-term complications associated with acellular dermal matrix-assisted direct-to-implant breast reconstruction. Ann. Plast. Surg. 2017, 78, 35–40. [Google Scholar] [CrossRef]
- Headon, H.; Kasem, A.; Manson, A.; Choy, M.C.; Carmichael, A.R.; Mokbel, K. Clinical outcome and patient satisfaction with the use of bovine-derived acellular dermal matrix (SurgiMend™) in implant based immediate reconstruction following skin sparing mastectomy: A prospective observational study in a single centre. Surg. Oncol. 2016, 25, 104–110. [Google Scholar] [CrossRef]
- Martin, L.; O’Donoghue, J.M.; Horgan, K.; Thrush, S.; Johnson, R.; Gandhi, A. Acellular dermal matrix (ADM) assisted breast reconstruction procedures. Eur. J. Surg. Oncol. 2013, 39, 425–429. [Google Scholar] [CrossRef]
- Alderman, A.; Gutowski, K.; Ahuja, A.; Gray, D. ASPS Clinical practice guideline summary on breast reconstruction with expanders and implants. Plast. Reconstr. Surg. 2014, 134, 648e–655e. [Google Scholar] [CrossRef]
- Cheng, A.; Saint-Cyr, M. Comparison of different ADM materials in breast surgery. Clin. Plast. Surg. 2012, 39, 167–175. [Google Scholar] [CrossRef]
- Bullocks, J.M. DermACELL: A novel and biocompatible acellular dermal matrix in tissue expander and implant-based breast reconstruction. Eur. J. Plast. Surg. 2014, 37, 529–538. [Google Scholar] [CrossRef]
- Capito, A.E.; Tholpady, S.S.; Agrawal, H.; Drake, D.B.; Katz, A.J. Evaluation of host tissue integration, revascularization, and cellular infiltration within various dermal substrates. Ann. Plast. Surg. 2012, 68, 495–500. [Google Scholar] [CrossRef]
- Agrawal, H.; Tholpady, S.S.; Capito, A.E.; Drake, D.B.; Katz, A.J. Macrophage phenotypes correspond with remodeling outcomes of various acellular dermal matrices. Open J. Regen. Med. 2012, 1, 51–59. [Google Scholar] [CrossRef]
- Vashi, C. Clinical outcomes for breast cancer patients undergoing mastectomy and reconstruction with use of DermACELL, a sterile, room temperature acellular dermal matrix. Plast. Surg. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Hilton, J.; Mazzarello, S.; Fergusson, D.; Joy, A.A.; Robinson, A.; Arnaout, A.; Hutton, B.; VanderMeer, L.; Clemons, M. Novel methodology for comparing standard-of-care interventions in patients with cancer. J. Oncol. Pr. 2016, 12, e1016–e1024. [Google Scholar] [CrossRef]
- Basulaiman, B.; Awan, A.A.; Fergusson, D.; VanderMeer, L.; Arnaout, A.; Hilton, J.; Hutton, B.; Joy, A.A.; Robinson, A.; Califaretti, N.; et al. Creating a pragmatic trials program for breast cancer patients: Rethinking Clinical Trials (REaCT). Breast Cancer Res. Treat. 2019, 177, 93–101. [Google Scholar] [CrossRef]
- Delong, M.R.; Tandon, V.J.; Bertrand, A.A.; MacEachern, M.; Goldberg, M.; Salibian, A.; Pusic, A.L.; Festekjian, J.H.; Wilkins, E.G. Review of outcomes in pre-pectoral prosthetic breast reconstruction with and without surgical mesh assistance. Plast. Reconstr. Surg. 2020. [Google Scholar] [CrossRef]
- Ho, G.; Nguyen, T.J.; Shahabi, A.; Hwang, B.H.; Chan, L.S.; Wong, A.K. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann. Plast. Surg. 2012, 68, 346–356. [Google Scholar] [CrossRef]
- Jordan, S.W.; Khavanin, N.; Kim, J.Y.S. Seroma in prosthetic breast reconstruction. Plast. Reconstr. Surg. 2016, 137, 1104–1116. [Google Scholar] [CrossRef]
- Delong, M.R.; Tandon, V.J.; Farajzadeh, M.; Berlin, N.L.; MacEachern, M.P.; Rudkin, G.H.; Da Lio, A.L.; Cederna, P.S. Systematic review of the impact of acellular dermal matrix on aesthetics and patient satisfaction in tissue expander-to-implant breast reconstructions. Plast. Reconstr. Surg. 2019, 144, 967e–974e. [Google Scholar] [CrossRef]
- Woo, K.-J.; Paik, J.M.; Mun, G.-H.; Pyon, J.-K.; Jeon, B.J.; Bang, S.-I. Analysis of factors influencing drain amount, time to drain removal, and seroma formation in patients undergoing immediate expander-implant breast reconstruction. J. Plast. Surg. Hand Surg. 2017, 52, 53–59. [Google Scholar] [CrossRef]
- Hansson, E.; Rn, A.E.; Hallberg, H. Drain secretion and seroma formation after immediate breast reconstruction with a biological and a synthetic mesh, respectively: A randomized controlled study. Breast J. 2020. [Google Scholar] [CrossRef]
- Suga, H.; Shiraishi, T.; Shibasaki, Y.; Takushima, A.; Harii, K. Predictive factors for drainage volume after expander-based breast reconstruction. Plast. Reconstr. Surg. Glob. Open 2016, 4, e727. [Google Scholar] [CrossRef]
- Nahabedian, M.Y. Implant-based breast reconstruction following conservative mastectomy: One-stage vs. two-stage approach. Gland. Surg. 2016, 5, 47–54. [Google Scholar]
- Al-Ghazal, S.; Fallowfield, L.; Blamey, R. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur. J. Cancer 2000, 36, 1938–1943. [Google Scholar] [CrossRef]
- Wilkins, E.G.; Cederna, P.S.; Lowery, J.C.; Davis, J.A.; Kim, H.M.; Roth, R.S.; Goldfarb, S.; Izenberg, P.H.; Houin, H.P.; Shaheen, K.W. Prospective analysis of psychosocial outcomes in breast reconstruction: One-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast. Reconstr. Surg. 2000, 106, 1014–1025. [Google Scholar] [CrossRef]
- Bank, J.; Phillips, N.A.; Park, J.E.; Song, D.H. Economic analysis and review of the literature on implant-based breast reconstruction with and without the use of the acellular dermal matrix. Aesthet. Plast. Surg. 2013, 37, 1194–1201. [Google Scholar] [CrossRef]
- Jansen, L.A.; Macadam, S.A. The Use of AlloDerm in postmastectomy alloplastic breast reconstruction: Part II. A cost analysis. Plast. Reconstr. Surg. 2011, 127, 2245–2254. [Google Scholar] [CrossRef]
- Krishnan, N.M.; Chatterjee, A.; Rosenkranz, K.M.; Powell, S.G.; Nigriny, J.F.; Vidal, D.C. The cost effectiveness of acellular dermal matrix in expander–implant immediate breast reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 468–476. [Google Scholar] [CrossRef]
- Ibrahim, A.M.S.; Koolen, P.G.L.; Ganor, O.; Markarian, M.K.; Tobias, A.M.; Lee, B.T.; Lin, S.J.; Mureau, M.A. Does acellular dermal matrix really improve aesthetic outcome in tissue expander/implant-based breast reconstruction? Aesthet. Plast. Surg. 2015, 39, 359–368. [Google Scholar] [CrossRef]
- Forsberg, C.G.; Kelly, D.A.; Wood, B.C.; Mastrangelo, S.L.; Defranzo, A.J.; Thompson, J.T.; David, L.R.; Marks, M.W. Aesthetic outcomes of acellular dermal matrix in tissue expander/implant-based breast reconstruction. Ann. Plast. Surg. 2014, 72, S116–S120. [Google Scholar] [CrossRef]
- Colwell, A.S.; Damjanovic, B.; Zahedi, B.; Medford-Davis, L.; Hertl, C.; Austen, W.G. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix. Plast. Reconstr. Surg. 2011, 128, 1170–1178. [Google Scholar] [CrossRef]
- Govshievich, A.; Somogyi, R.B.; Brown, M.H. Conservative mastectomies and immediate reconstruction with the use of ADMs. Gland. Surg. 2015, 4, 453–462. [Google Scholar]
- Ibrahim, A.M.S.; Koolen, P.G.L.; Ashraf, M.M.A.A.; Kim, K.; Mureau, M.A.M.; Lee, M.M.B.T.; Lin, S.J. Acellular dermal matrix in reconstructive breast surgery. Plast. Reconstr. Surg. Glob. Open 2015, 3, e381. [Google Scholar] [CrossRef]
- Salzberg, C.A.; Ashikari, A.Y.; Koch, R.M.; Chabner-Thompson, E. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast. Reconstr. Surg. 2011, 127, 514–524. [Google Scholar] [CrossRef]
- Gabriel, A.; Maxwell, G.P. AlloDerm RTU Integration and clinical outcomes when used for reconstructive breast surgery. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1744. [Google Scholar] [CrossRef]
- Zenn, M.R.; Salzberg, C.A. A direct comparison of alloderm-ready to use (RTU) and DermACELL in immediate breast implant reconstruction. Eplasty 2016, 16, e23. [Google Scholar]
- Pittman, T.A.; Fan, K.L.; Knapp, A.; Frantz, S.; Spear, S.L. Comparison of different acellular dermal matrices in breast reconstruction. Plast. Reconstr. Surg. 2017, 139, 521–528. [Google Scholar] [CrossRef]
- Wu, L.-H.; Zhang, M.-X.; Chen, C.-Y.; Fang, Q.-Q.; Wang, X.-F.; Tan, W.-Q. Breast reconstruction with Alloderm Ready to Use: A meta-analysis of nine observational cohorts. Breast 2018, 39, 89–96. [Google Scholar] [CrossRef]
- Greig, H.; Roller, J.; Ziaziaris, W.; Van Laeken, N. A retrospective review of breast reconstruction outcomes comparing AlloDerm and DermaCELL. JPRAS Open 2019, 22, 19–26. [Google Scholar] [CrossRef]
- Hinchcliff, K.M.; Orbay, H.; Busse, B.K.; Charvet, H.; Kaur, M.; Sahar, D.E. Comparison of two cadaveric acellular dermal matrices for immediate breast reconstruction: A prospective randomized trial. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.P.; Tenenbaum, M.M.; Yan, Y.; Myckatyn, T.M. Cortiva versus AlloDerm ready-to-use in prepectoral and submuscular breast reconstruction. Plast. Reconstr. Surg. Glob. Open 2018, 6, e2013. [Google Scholar] [CrossRef] [PubMed]
- Ellis, H.L.; Asaolu, O.; Nebo, V.; Kasem, A. Biological and synthetic mesh use in breast reconstructive surgery: A literature review. World J. Surg. Oncol. 2016, 14, 121–130. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristic | All | Alloderm-RTU | DermACELL |
|---|---|---|---|
| Number of Breasts (%) | 81 | 41 (50.6) | 40 (49.4) |
| Number of Patients (%) | 62 | 33 (50.0) | 33 (50.0) |
| Age, years, mean (SD) | 49.6 (11.1) | 47.8 (11.1) | 51.4 (11.0) |
| BMI, kg/m2, mean (SD) | 24.9 (4.7) | 24.9 (4.6) | 24.9 (4.9) |
| Smoking history | |||
| Current (%) | 4 (6.5) | 3 (9.7) | 1 (3.2) |
| Prior (≥1 month before) (%) | 19 (30.7) | 9 (29.0) | 10 (32.3) |
| Never (%) | 39 (62.9) | 19 (61.3) | 20 (64.5) |
| Diabetes (%) | 1 (1.6) | 0 (0) | 1 (3.2) |
| Heart disease a (%) | 8 (12.9) | 5 (16.1) | 3 (9.7) |
| Preoperative chemotherapy | 7 (11.3) | 4 (12.9) | 3 (9.7) |
| Prior radiotherapy (%) | 13 (16.1) | 5 (12.2) | 8 (20.0) |
| Breast size D cup or greater (%) | 11 (17.7) | 4 (12.9) | 7 (22.6) |
| Ptosis grade III or greater (%) | 4 (4.9) | 0 (0) | 4 (10.0) |
| Surgical Characteristic | All n = 78 | Alloderm-RTU n = 38 | DermACELL n = 40 |
|---|---|---|---|
| Therapeutic indication for surgery a | 53 (67.9) | 25 (65.8) | 28 (70.0) |
| Mastectomy Type | |||
| Nipple sparing | 40 (51.3) | 21 (55.3) | 19 (47.5) |
| Inframammary incision | 34 | 18 | 16 |
| Incision without vertical | 2 | 0 | 2 |
| Vertical or diagonal | 5 | 3 | 1 |
| Skin sparing | 38 (48.7) | 17 (44.7) | 21 (52.5) |
| Incision without vertical component | 13 | 5 | 8 |
| Vertical or diagonal | 21 | 12 | 9 |
| Wise pattern | 4 | 0 | 4 |
| Axillary surgery performed | 52 (66.7) | 25 (65.8) | 27 (67.5) |
| Sentinel node biopsy b | 49 | 23 | 26 |
| Mastectomy weight, g, median (IQR) | 467 (276, 665) | 600 (324, 714) | 387 (269, 542) |
| Clinical Outcomes | All n = 78 | Alloderm-RTU n = 38 | DermA-CELL n = 40 | p-Value | Risk Difference (95% CI) | |
|---|---|---|---|---|---|---|
| 1 * | 2 * | |||||
| Mean duration of drain (SD), days | 10.0 (5.0) | 10.8 (5.5) | 9.2 (4.5) | 0.16 | 0.15 | 1.6 (−0.7 to 3.9) |
| Minor complications | ||||||
| Seromas requiring aspiration post drain removal | 7 (9.0) | 2 (5.3) | 5 (12.5) | 0.43 | 0.28 | −7.2 (−19.7 to 5.2) |
| Red breast syndrome | 2 (2.6) | 1 (2.6) | 1 (2.5) | 1.00 | 0.97 | 0.1 (−6.9 to 7.2) |
| Wound dehiscence | 5 (6.4) | 3 (7.9) | 2 (5.0) | 0.67 | 0.68 | 2.9 (−8.0 to 13.8) |
| Requiring return to OR | 2 | 1 | 1 | |||
| Wound infection requiring antibiotics | 4 (5.1) | 3 (7.9) | 1 (2.5) | 0.35 | 0.32 | 5.4 (−4.5 to 15.2) |
| Requiring return to OR | 3 | 2 | 1 | |||
| Hematoma | 2 (2.6) | 2 (5.3) | 0 (0.0) | 0.23 | - | 5.3 (−1.8 to 12.4) |
| Requiring return to OR | 1 | 1 | 0 | |||
| Skin necrosis | 6 (7.7) | 2 (5.3) | 4 (10.0) | 0.68 | 0.77 | −4.7 (−16.4 to 7.0) |
| Requiring return to OR | 3 | 2 | 1 | |||
| Capsular contracture | 1 (1.3) | 1 (2.6) | 0 (0.0) | 0.49 | - | 2.6 (−2.5 to 7.7) |
| Major complications | ||||||
| Return to OR | 9 (11.5) | 6 (15.8) | 3 (7.5) | 0.30 | 0.28 | 8.3 (−5.9 to 22.5) |
| Suture removal | 1 | 1 | 0 | |||
| Wound dehiscence | 2 | 1 | 1 | |||
| Infection | 3 | 2 | 1 | |||
| Hematoma | 1 | 1 | 0 | |||
| Skin necrosis | 3 | 2 | 1 | |||
| Removal of implant | 4 | 2 | 2 | |||
| Loss of Implant | 4 (5.1) | 2 (5.3) | 2 (5.0) | 1.00 | 0.96 | 0.3 (−9.5 to 10.1) |
| Post-Surgical Outcome | All | Alloderm-RTU | DermACELL | p-Value | |
|---|---|---|---|---|---|
| 1 * | 2 * | ||||
| n Patients Evaluable | 59 | 28 | 31 | ||
| Final evaluation by plastic surgeon a | 49 (83.1) | 22 (78.6) | 27 (87.1) | 0.49 | - |
| Median number of days between surgery and first plastics assessment (IQR) | 7 (6, 9) | 7 (6, 9) | 7 (6, 9) | 0.81 | - |
| Median number of weeks between surgery and final assessment (IQR) | 9 (5, 17) | 13.5 (6, 19) | 8 (4, 14) | 0.10 | - |
| n Breasts Evaluable | 78 | 38 | 40 | ||
| Median number of plastic surgeon visits (IQR) | 4 (3, 5) | 4 (3, 5) | 3 (3, 4.5) | 0.13 | 0.80 |
| Indication for adjuvant radiotherapy | 13 (16.7) | 7 (18.4) | 6 (15.0) | 0.77 | 0.66 |
| Indication for adjuvant chemotherapy | 16 (27.1) | 9 (32.1) | 7 (22.6) | 0.56 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnaout, A.; Zhang, J.; Frank, S.; Momtazi, M.; Cordeiro, E.; Roberts, A.; Ghumman, A.; Fergusson, D.; Stober, C.; Pond, G.; et al. A Randomized Controlled Trial Comparing Alloderm-RTU with DermACELL in Immediate Subpectoral Implant-Based Breast Reconstruction. Curr. Oncol. 2021, 28, 184-195. https://doi.org/10.3390/curroncol28010020
Arnaout A, Zhang J, Frank S, Momtazi M, Cordeiro E, Roberts A, Ghumman A, Fergusson D, Stober C, Pond G, et al. A Randomized Controlled Trial Comparing Alloderm-RTU with DermACELL in Immediate Subpectoral Implant-Based Breast Reconstruction. Current Oncology. 2021; 28(1):184-195. https://doi.org/10.3390/curroncol28010020
Chicago/Turabian StyleArnaout, Angel, Jing Zhang, Simon Frank, Moein Momtazi, Erin Cordeiro, Amanda Roberts, Ammara Ghumman, Dean Fergusson, Carol Stober, Gregory Pond, and et al. 2021. "A Randomized Controlled Trial Comparing Alloderm-RTU with DermACELL in Immediate Subpectoral Implant-Based Breast Reconstruction" Current Oncology 28, no. 1: 184-195. https://doi.org/10.3390/curroncol28010020
APA StyleArnaout, A., Zhang, J., Frank, S., Momtazi, M., Cordeiro, E., Roberts, A., Ghumman, A., Fergusson, D., Stober, C., Pond, G., Jeong, A., Vandermeer, L., Hutton, B., Clemons, M., & on behalf of the REaCT Investigators. (2021). A Randomized Controlled Trial Comparing Alloderm-RTU with DermACELL in Immediate Subpectoral Implant-Based Breast Reconstruction. Current Oncology, 28(1), 184-195. https://doi.org/10.3390/curroncol28010020





